Abstract
We have previously shown that RhoA-mediated actin polymerization stimulates smooth muscle cell (SMC)-specific transcription by regulating the nuclear localization of the myocardin-related transcription factors (MRTFs). On the basis of the recent demonstration that nuclear G-actin regulates MRTF nuclear export and observations from our laboratory and others that the RhoA effector, mDia2, shuttles between the nucleus and cytoplasm, we investigated whether nuclear RhoA signaling plays a role in regulating MRTF activity. We identified sequences that control mDia2 nuclear-cytoplasmic shuttling and used mDia2 variants to demonstrate that the ability of mDia2 to fully stimulate MRTF nuclear accumulation and SMC-specific gene transcription was dependent on its localization to the nucleus. To test whether RhoA signaling promotes nuclear actin polymerization, we established a fluorescence recovery after photobleaching (FRAP)-based assay to measure green fluorescent protein-actin diffusion in the nuclear compartment. Nuclear actin FRAP was delayed in cells expressing nuclear-targeted constitutively active mDia1 and mDia2 variants and in cells treated with the polymerization inducer, jasplakinolide. In contrast, FRAP was enhanced in cells expressing a nuclear-targeted variant of mDia that inhibits both mDia1 and mDia2. Treatment of 10T1/2 cells with sphingosine 1-phosphate induced RhoA activity in the nucleus and forced nuclear localization of RhoA or the Rho-specific guanine nucleotide exchange factor (GEF), leukemia-associated RhoGEF, enhanced the ability of these proteins to stimulate MRTF activity. Taken together, these data support the emerging idea that RhoA-dependent nuclear actin polymerization has important effects on transcription and nuclear structure.
Keywords: smooth muscle, serum response factor, myocardin-related factors, RhoA, diaphanous formins
smooth muscle cell (SMC) differentiation is critical during vasculogenesis and angiogenesis, and it is well recognized that defective control of this process plays an important role in the progression of atherosclerosis and restenosis (26). Thus identification of mechanisms that control SMC differentiation will be important for our understanding of vascular development and the progression of vascular disease. Serum response factor (SRF) regulates the expression of a number of muscle-specific, cytoskeletal, and early response growth genes by binding to conserved CArG [CC(A/T)6GG] cis elements found within their promoters (for a review, see Ref. 37). The cell type- and gene-specific effects of SRF are mediated by direct interactions with additional cofactors, and extensive evidence indicates that the SRF cofactors of the myocardin family (myocardin and the myocardin-related transcription factors, MRTF-A/MKL-1 and MRTF-B/MKL-2) regulate SMC differentiation marker gene expression (46). Indeed, genetic deletion of myocardin or MRTF-B in the mouse resulted in embryonic lethality attributable to defects in SMC differentiation in the dorsal aorta and brachial arches, respectively (22, 24). Moreover, mice lacking MRTF-A fail to upregulate SMC differentiation marker gene expression in myoepithelial cells during lactation (23). The precise contributions of each myocardin factor to SMC differentiation are complicated by the high functional homology and overlapping expression patterns of this family, the well-known plasticity of SMCs, and the existence of multiple SMC lineages. However, it is clear that the identification of the molecular mechanisms that regulate myocardin factor activity will be critical for our understanding of the control of SMC phenotype.
Miralles et al. (30) were the first to demonstrate that MRTF-A activity was regulated by the small GTPase, RhoA, by a mechanism that involves G-actin binding to the RPEL domains within the MRTF-A NH2 terminus that masks the MRTF-A nuclear import sequence. Interestingly, Vartiainen et al. (45) showed that the rate-limiting determinant of MRTF-A nuclear accumulation was Crm-1-dependent nuclear export and that MRTF-A binding to G-actin in the nucleus was required for this export mechanism (45). These authors went on to show that the association between MRTF-A and G-actin in the nucleus also inhibited the transcriptional activity of MRTF-A without preventing its association with SRF target genes. Although these data indicate that nuclear G-actin is a critical determinant of MRTF-A nuclear accumulation and activity, very little is known about the regulation of G-actin levels within this compartment.
Actin has been detected in a variety of chromatin remodeling and transcription complexes, and its presence in the nucleus is now well accepted (for a review, see Ref. 36). However, because nuclear actin filaments are not easily visualized by phalloidin staining, the elucidation of the mechanisms by which actin mediates these effects has been difficult. Early studies in Xenopus oocytes identified short-actin-containing filaments at the inner surface of the nuclear envelope often originating at nuclear pore complexes (18, 20). In addition, using fluorescence recovery after photobleaching (FRAP) techniques to monitor green fluorescent protein (GFP)-β-actin mobility, McDonald et al. (27) estimated that 20% of nuclear actin was polymeric. The existence of polymerized nuclear actin has also been inferred by the ability of actin polymerization inhibitors such as latrunculin and cytochalasin to attenuate the activities of RNA polymerase and chromatin modifying enzymes (for a review, see Ref. 12). Importantly, very recent studies have directly visualized polymerized actin in the nucleus using high phalloidin concentrations and/or actin-binding domains fused to fluorescent probes (2, 3).
We have previously shown that three diaphanous-related formins (DRFs), mDia1, mDia2, and FHOD1, that are downstream of RhoA are highly expressed in SMCs and strongly stimulate SMC marker gene expression by enhancing actin polymerization and MRTF nuclear localization (42). The DRFs are characterized by two highly conserved formin homology (FH) domains, a GTPase-binding domain (GBD) that interacts with Rho family GTPases, an NH2-terminal diaphanous inhibitory domain (DID), and a COOH-terminal diaphanous auto-regulatory domain (DAD) (for a review, see Ref. 9). The molecular mechanisms that control DRF activity have been fairly well described. In their inactive state, the DRFs are inhibited by an intramolecular interaction between the COOH-terminal DAD and the NH2-terminal DID (38). High-affinity binding of activated RhoA to the GBD disrupts the DAD-DID interaction, resulting in exposure of the catalytically active FH1FH2 region and linear actin polymerization.
On the basis of observations from our laboratory and others that mDia2 shuttles through the nucleus (29), we hypothesized that this pathway promotes MRTF nuclear accumulation. In the present study, we directly measured agonist-induced nuclear RhoA activation and took advantage of nuclear localization variants of mDia2, RhoA, and the Rho guanine nucleotide exchange factor (GEF), leukemia-associated RhoGEF (LARG), to demonstrate that nuclear RhoA signaling enhances MRTF nuclear accumulation and SMC-specific gene expression. We also used GFP-actin FRAP to demonstrate that mDia signaling reduces nuclear actin mobility, suggesting that mDia signaling regulates nuclear actin dynamics.
MATERIALS AND METHODS
Cell culture.
SMCs were isolated from thoracic aortas of 8-wk-old mice by enzymatic digestion as described previously (42) and were maintained in DMEM/F12 (1:1) plus 10% fetal bovine serum and 0.5% penicillin-streptomycin. 10T1/2 cells were maintained as above but in DMEM.
Plasmids.
mDia2 constructs were subcloned into an NH2-terminal Flag-tagged pcDNA3.1 expression vector and/or into EGFP-C1 (Clontech). Point mutations in mDia2 were generated using the Quikchange Site-Directed Mutagenesis Kit (Stratagene). MRTF-A/B were obtained and cloned as described previously (42). GFP-FHOD1 and GFP-mDia1 were generous gifts from Michael Mendelsohn (Tufts University, Boston, MA) and Shuh Narumiya (Kyoto University, Japan), respectively.
siRNA knockdown.
The following short-interfering (si)RNAs were obtained from Invitrogen; nontargeted control siRNA (NTC) (to GFP) 5′GGUGCGCUCCUGGACGUAGCC-3′, mDia2 5′-GCAUGACAAGUUUGUGAUATT-3′, and mDia1 5′-GGACCUCUAUUGCCCUCAATT-3′. SMCs or 10T1/2 cells were transfected with siRNAs using Dharmafect (Dharmacon) and were harvested 96 h posttransfection for protein expression analysis.
Western blotting.
Cleared cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and then probed with the following antibodies: Flag M2 (Sigma), histone H3 (Abcam), vinculin (Sigma), GAPDH (Santa Cruz Biotechnology), tubulin (Sigma), SM α-actin (Sigma), calponin (Lifespan), SM22 (a generous gift from Mario Gimona; University of Salzburg, Austria), mDia1 and mDia2 (generous gifts of Henry Higgs, Dartmouth College, Hanover, NH), and FHOD1 (a generous gift from Michael Mendelsohn, Tufts University, Boston, MA).
Immunoflourescence and protein localization.
10T1/2 cells were plated in eight- or four-well chamber slides, maintained in 10% serum for 48 h, fixed in 4.0% paraformaldehyde, and permeabilized in 0.5% Triton X-100. Slides were incubated for 2–3 h in primary anti-flag antibody (Sigma) or anti-mDia2. Texas Red- or FITC-conjugated anti-mouse IgG (1:1,000; Jackson ImmunoResearch) and DAPI (90 nM) were added for 1 h. Leptomycin B treatments (5 ng/ml; Sigma) were administered for 3 h. For all localization experiments, GFP- or flag-tagged proteins were scored into three separate categories: nuclear, cytoplasmic, or diffuse (a nuclear:cytoplasmic ratio from approximately 0.75 to 1.25). Localization results are presented as averages of at least three separate experiments in which at least 100 cells per condition were scored.
Cell fractionation.
Cell fractionation was conducted as previously described (48). In short, 10T1/2 cells were gently scraped into cytoplasmic lysis buffer and incubated on ice for 15 min. Nuclei were pelleted by centrifugation, and supernatants from this spin were saved as the cytoplasmic fraction. The nuclear pellet was washed three times and resuspended in cytoplasmic lysis buffer containing 0.5 M NaCl. Five percent of the nuclear fraction volume and 2.5% of the cytoplasmic fraction volume were separated by SDS-PAGE.
Transient transfections and reporter gene assays.
Mouse aortic SMCs or 10T1/2 cells were seeded on 48-well plates and transfected at 70–80% confluency using TransIT-LT1 (Mirus), according to the manufacturer's protocol. Luciferase assays were conducted 24 h after transfection using the Steady-Glo system (Promega). The SM22, SM α-actin, c-fos, and TK promoters have been previously described (25). For sphingosine-1-phosphate (S1P) treatment, cells were serum starved in 0.5% FBS for 18 h and then treated with S1P (10 μm; Matreya) for 8 h.
GST pull-downs.
Cos7 cells expressing Flag-tagged mDia2 variants were scraped in lysis buffer containing protease and phosphatase inhibitors. Cleared lysate (500 μg) was incubated with 15 μg of glutathione S-transferase (GST) or GST-L63RhoA for 3 h. Precipitated complexes were washed three times in lysis buffer, eluted in sample buffer, and then separated by SDS-PAGE.
Nuclear Rho assays.
Our methods were based on a protocol previously described by Guilluy et al. (15). In brief, nearly confluent 10T1/2 cells were serum starved in 0.5% FBS for 18 h and then treated with 10 μM S1P for 7.5 min. Cells were scraped into hypotonic lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl and 0.5 mM DTT) and then passed eight times through a 21-gauge needle. Nuclei were pelleted by centrifugation at 1,000 g for 5 min, resuspended in 25% (wt/vol) solution of iodixanol (OptiPrep, Sigma), and repelleted at 10,000 g for 10 min. Purified nuclei were resuspended in Rho activity assay buffer [50 mM Tris·HCl (pH 7.6), 500 mM NaCl, 1% (vol/vol) Triton X-100, 0.1% (wt/vol) SDS, 0.5% (wt/vol) deoxycholate, and 10 mM MgCl2] and then sonicated for 10 s. RhoA activity assays on cleared nuclear lysates were then performed as previously described (25).
Detection of nuclear RhoA activity by fluorescence microscopy.
Mouse 10T1/2 cells were transfected with GFP or a GFP fusion protein containing the Rhotekin Rho-binding domain (RBD) that binds to active but not inactive RhoA. Cells were serum starved with 0.5% FBS for 18 h and stimulated with 10 μM S1P for 7.5 min. Cells were fixed with 4% paraformaldehyde, stained with DAPI, and then imaged by confocal microscopy. Fluorescence intensity within an 8-μm diameter circle at the center of the nucleus was quantified by Image J, and at least 100 cells were quantified from seven random fields from two separate experiments.
GFP-actin FRAP.
FRAP experiments were performed on HeLa cells expressing GFP-β-actin using a Zeiss LSM 710 fluorescence microscope with a ×63 oil objective. In brief, pre-bleach GFP fluorescence was determined by averaging three acquisition scans at low laser power. A - μm square was then bleached at full laser power (488 nM) for 250 ms. Total fluorescence of the bleached region was determined every 0.5 s for the first 10 s and 5 s thereafter for a total of 100 s. To correct for GFP bleaching that may have occurred during the recovery scans, fluorescence values at each time point were normalized to GFP fluorescence in an unbleached region. Some cells were treated with the actin polymerizing reagent jasplakinolide (1 μM) for 30 min before GFP-actin FRAP determinations.
Statistical analyses.
Results are presented as means ± SE from at least three independent experiments. For luciferase assays and GFP-based nuclear RhoA activity experiments, group differences were analyzed by paired Student's t-test. FRAP assays were analyzed by repeated-measures ANOVA followed by Bonferroni's post hoc test for significant differences between individual curves using GraphPad Prism 5.0. Protein localization experiments were analyzed using the Chi-squared test. For all experiments, significance was considered as P < 0.05.
RESULTS
mDia1 and mDia2 are required for SMC differentiation marker gene expression.
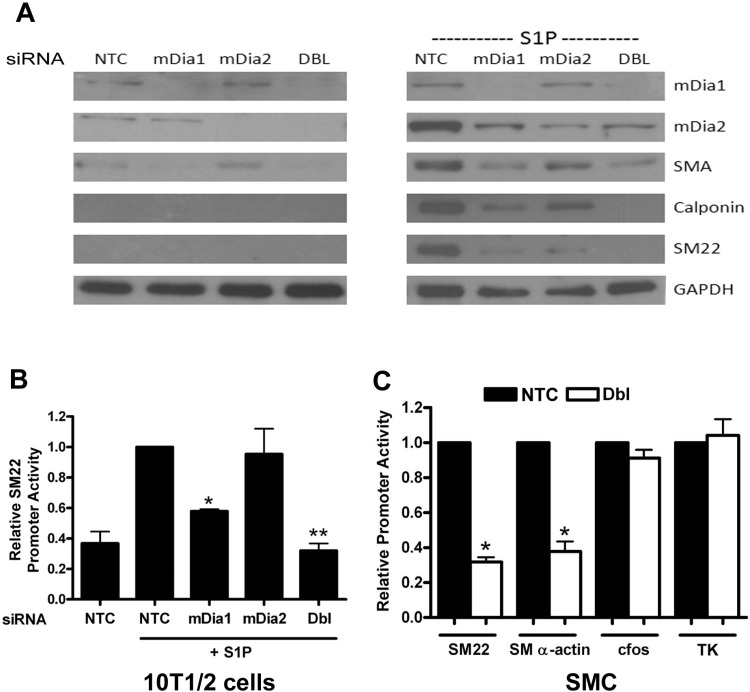
We have previously shown that S1P upregulates SMC differentiation marker gene expression in multipotential 10T1/2 cells through RhoA-dependent stimulation of MRTF nuclear localization. To examine the contributions of mDia1 and mDia2 in this model, we used an siRNA approach to knockdown mDia1 and mDia2 expression. Knockdown of mDia1 or mDia2 inhibited SM α-actin, SM22, and calponin expression in S1P-treated cells, and the combinatorial knockdown inhibited SMC marker gene expression more effectively than either knockdown alone, suggesting that each of these formins has an independent effect on SMC marker gene expression (Fig. 1A). Interestingly, mDia1 and mDia2 protein levels were strongly increased following S1P treatment, and mDia1 expression was slightly inhibited by mDia2 knockdown. These results suggest that mDia1/mDia2 expression may be important for SMC-specific gene expression and may be regulated by a positive feedback loop. The double knockdown also inhibited SMC-specific promoter activity in both 10T1/2 cells and primary rat aortic SMCs (Fig. 1, B and C), whereas overexpression of either mDia1 or mDia2 increased SMC-specific promoter activity (see Fig. 5 and Ref. 42). Taken together, these results indicate that mDia1 and mDia2 regulate SMC-specific transcription.
Fig. 1.
Knockdown of mDia1 and mDia2 inhibited smooth muscle cell (SMC)-specific transcription and gene expression. A: multipotential 10T1/2 cells were transfected with nontargeted control siRNA (NTC) or siRNA to mDia1, mDia2, or both mDia1 and mDia2 (Dbl). Following 18 h of serum starvation in 0.5% FBS and 24 h of exposure to 10 μM sphingosine-1-phosphate (S1P) or vehicle, cell lysates were subjected to Western analysis with the indicated antibody. B: control and mDia knockdown 10T1/2 cells were transiently transfected with luciferase constructs driven by the SM22 promoter. Luciferase activity was measured following 18 h of serum starvation (0.5% FBS) and 8-h treatment with 10 μM S1P and is expressed relative to NTC plus S1P set to 1. *P < 0.05 vs NTC. plus S1P; **P < 0.05 vs. mDia1 plus S1P. C: control and mDia double knockdown mouse aortic SMCs were transiently transfected with luciferase constructs driven by the SM22, SM α-actin, c-fos, and minimal thymidine kinase promoters. Luciferase activity was measured after 48 h and is expressed relative to NTC transfected cells set to 1. *P < 0.05 vs. NTC.
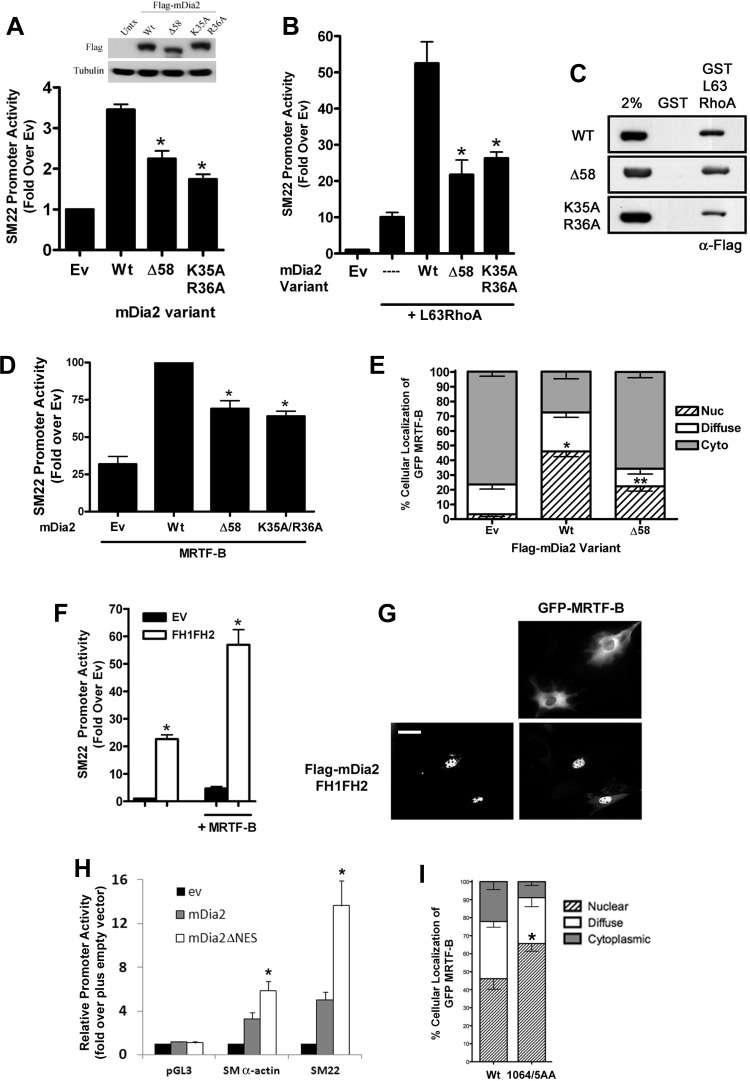
Fig. 5.
Nuclear localization of mDia2 was required for full activation of SMC-specific transcription. 10T1/2 cells were cotransfected with SM22-luciferase and the indicated mDia2 nuclear localization variant in the absence (A) or presence (B) of constitutively active L63RhoA. Luciferase activity was measured after 48 h and is expressed relative to empty expression vector (Ev) set to 1. *P < 0.05 vs. Wt. C: Cos7 cells were transfected with the indicated Flag-mDia2 variants. Lysates were incubated with glutathione S-transferase (GST) or GST-L63RhoA for 1 h. Following extensive washing, precipitates were run on an SDS page gel and probed with an anti-Flag Ab. D: 10T1/2 cells were cotransfected with SM22-luciferase and myocardin-related transcription factor (MRTF)-B in the presence or absence of the indicated mDia2 nuclear localization variants. E: quantification of GFP-MRTF-B localization in serum-starved 10T1/2 cells coexpressing the indicated mDia2 variants. At least 100 cells were counted under each condition from 3 independent experiments. *Nuclear localization P < 0.05 vs. empty vector. **Nuclear localization P < 0.05 vs. Wt. F: 10T1/2 cells were cotransfected with SM22-luciferase and FH1FH2 domain of mDia2 plus or minus MRTF-B. G: GFP-MRTF-B localization in serum-starved 10T1/2 cells in the absence (top) and presence (bottom) of the FH1FH2 domain of mDia2. Scale bar = 20 μm. H: 10T1/2 cells were cotransfected with the indicated luciferase reporter vector and either Wt mDia2 or an mDia2 variant in which the COOH-terminal NES was deleted (ΔNES). I: quantification of GFP-MRTF-B localization in 10T1/2 cells coexpressing Wt mDia2 or a mDia2 variant containing a mutation to the COOH-terminal NES (1064/5AA). At least 100 cells were counted under each condition from 3 independent experiments.
mDia2 shuttles between the nucleus and cytoplasm.
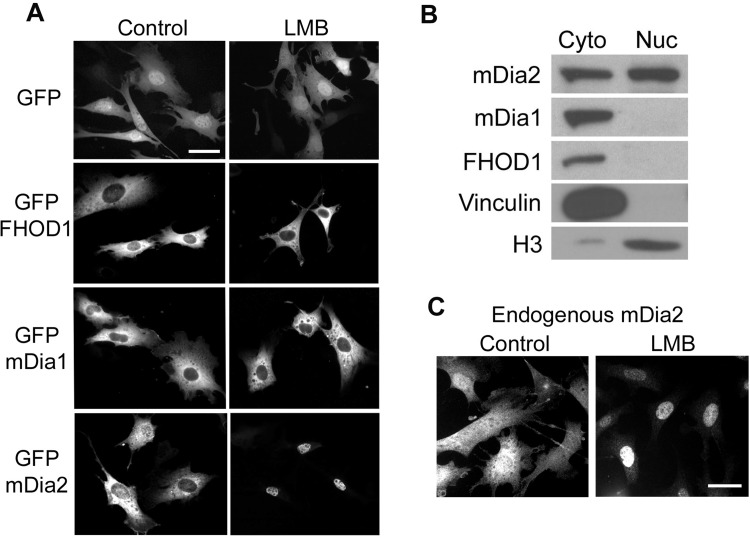
We and others originally attributed the transcriptional effects of mDia1, mDia2, and FHOD1 to a decrease in cytoplasmic G-actin levels that promoted MRTF nuclear import. However, given that the rate-limiting step in MRTF-A nuclear accumulation was shown to be nuclear export (45), we wanted to test whether these actin polymerization factors had a nuclear function. As shown in Fig. 2, all three DRFs were predominantly cytoplasmic in 10T1/2 cells under normal conditions. However, in excellent agreement with a previous study by Miki et al. (29), treatment with leptomycin B induced almost complete nuclear localization of mDia2, indicating that mDia2 shuttles through the nucleus and that its cytoplasmic localization is maintained by Crm-1-dependent nuclear export. We also performed cell fractionation experiments and probed cytoplasmic and nuclear fractions with antibodies specific to mDia1, mDia2, and FHOD1. Even in the absence of leptomycin B, a considerable amount of mDia2 was detected in the nuclear fraction (Fig. 2B). Importantly, we observed no significant differences in the localization of endogenous, Flag-tagged, or GFP-tagged proteins. Although we did not detect mDia1 or FHOD1 in the nucleus following leptomycin B treatment, it remains possible that cytoplasmic localization of these DRFs is maintained by a Crm-1-independent nuclear export mechanism. In fact, an mDia1 NH2-terminal deletion mutant was shown to be predominantly nuclear in NIH 3T3 cells (6), as was a similar caspase-3 cleavage fragment of FHOD1 in HeLa cells (28), suggesting that these DRFs may enter the nucleus under some conditions. In addition, mDia1 was previously identified in a multi-protein complex with exportin6, a nuclear envelope protein not inhibited by leptomycin B that mediates the export of an actin-profilin complex (43).
Fig. 2.
mDia2 shuttles between the nucleus and cytoplasm. A: localization of green fluorescent protein (GFP) or GFP-tagged FHOD1, mDia1, or mDia2 in 10T1/2 cells in the absence and presence of leptomycin B (LMB). Scale bar = 20 μm. B: nuclear and cytoplasmic lysates were prepared from untreated 10T1/2 cells by differential centrifugation and were subjected to Western analysis using the indicated antibodies. Note that vinculin and histone 3 serve as controls for the cytoplasmic and nuclear fractions, respectively. C: control and leptomycin B-treated 10T1/2 cells were fixed and stained with anti-mDia2 antibody. Scale bar = 20 μm.
Identification of mDia2 nuclear import and export sequences.
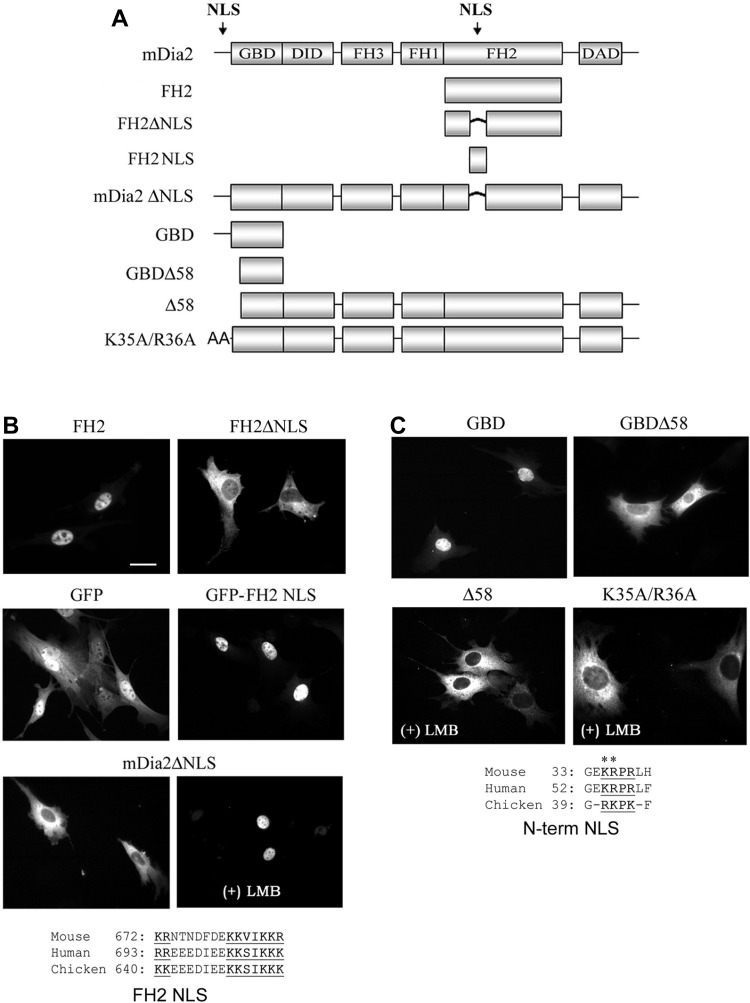
To begin to test whether nuclear mDia2 played an important role in regulating MRTF activity, we generated an mDia2 deletion series to identify the nuclear import and export signals that regulate its localization (Fig. 3A). Notably, the FH2 domain of mDia2 localized predominantly to the nucleus (Fig. 3B). Sequence analysis of this domain revealed a conserved consensus bipartite nuclear localization signal (NLS) from residues 673–692, and deletion of these amino acids (FH2ΔNLS) completely inhibited nuclear localization of the FH2 domain. Fusion of this sequence to GFP significantly enhanced GFP nuclear accumulation, providing strong evidence that this region functions as a true NLS. Deletion of the FH2 NLS from the full-length mDia2 molecule did not block leptomycin B-induced nuclear accumulation, suggesting that sequences outside the FH2 domain could mediate mDia2 nuclear import. A basic domain within the NH2 terminus was previously shown to mediate mDia2 nuclear import in HeLa cells (29), and, as shown in Fig. 3C, deletion (Δ58) or mutation (K35A/R36A) of this NLS completely blocked leptomycin B-induced nuclear accumulation in our 10T1/2 model.
Fig. 3.
mDia2 contains 2 nuclear import signals. A: schematic of the GFP-mDia2 variants examined. NLS, nuclear localization signal; GBD, GTPase-binding domain; DID, diaphanous inhibitory domain; DAD, diaphanous auto-regulatory domain. B and C: localization of the indicated mDia2 NLS variants in 10T1/2 cells 48 h posttransfection in the absence or presence of LMB as indicated. Note the conservation of the NLS sequences located in the FH2 and NH2-terminal regions. Scale bar = 20 μm.
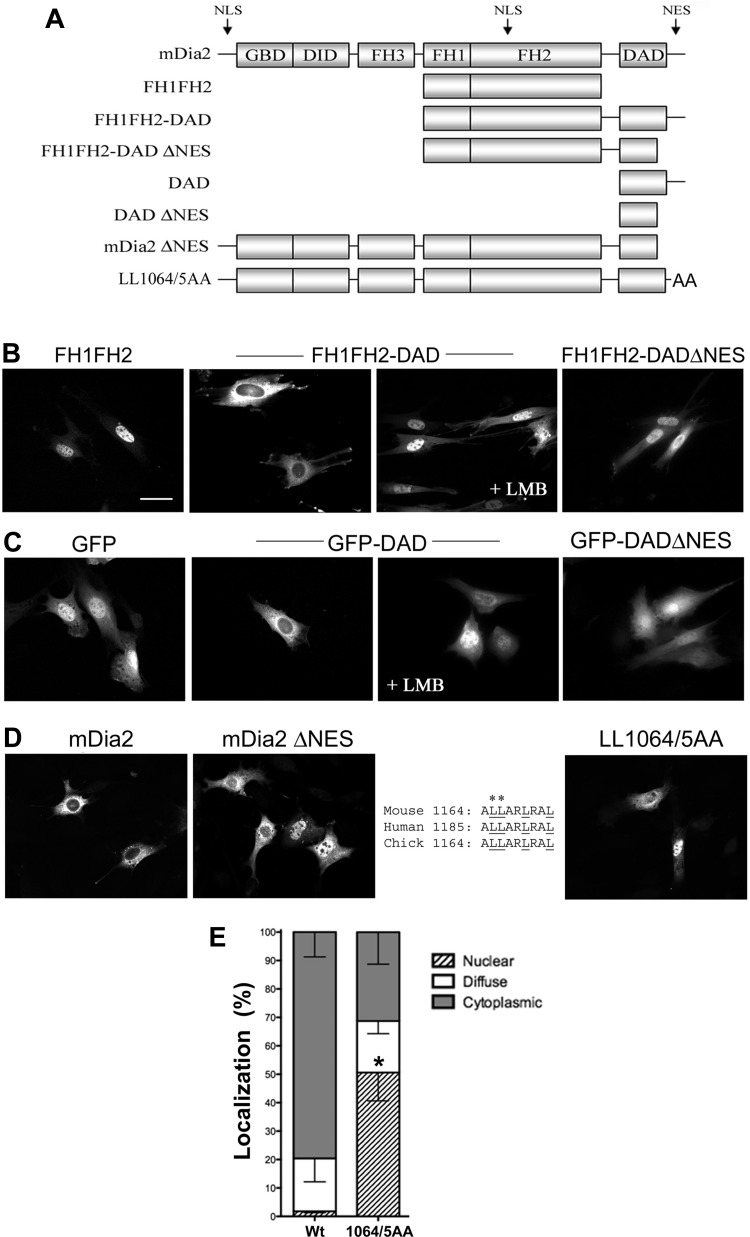
Although Miki et al. (29) identified several potential nuclear export sequences (NES) within mDia2, their mutation analyses failed to demonstrate that any of these were functional in the context of full-length mDia2. Our deletion analyses (Fig. 4A) demonstrated that nuclear localization of the FH1FH2 construct was inhibited by the addition of the COOH-terminal DAD motif (Fig. 4B). Importantly, this effect was completely reversed by leptomycin B treatment, strongly suggesting the presence of a Crm-1-dependent NES within this region. Fusion of the mDia2 DAD-containing domain to GFP also resulted in Crm-1-dependent cytoplasmic localization, further suggesting that this region functions as a true NES (Fig. 4C). Importantly, deletion (ΔNES) or mutation (L1064A/L1065A) of this sequence resulted in significant nuclear localization of full-length mDia2 in the absence of leptomycin B (Fig. 4, D and E).
Fig. 4.
mDia2 contains a nuclear export sequence (NES) at the extreme COOH terminus. A: schematic of the GFP-mDia2 variants examined. B and C: localization of GFP-mDia2 NES variants in 10T1/2 cells 48 h posttransfection in the absence or presence of LMB as indicated. Scale bar = 20 μm. D: immunohistochemical localization of Flag-mDia2 variants containing a deletion or mutation of the conserved COOH-terminal leucine-rich region (inset: sequences). E: quantification of wild-type (Wt) and LL1064/5AA mDia2 localization. *Nuclear localization P < 0.05 vs. Wt.
Nuclear localization of mDia2 is required for full MRTF activation.
The data presented so far indicate that mDia2 shuttles through the nucleus and is an important regulator of SMC differentiation marker gene expression. To determine whether the presence of mDia2 in the nucleus was required for its effects on MRTF-dependent SMC-specific transcription, we compared wild-type and nuclear localization-deficient mDia2 variants for their ability to stimulate SM22 promoter activity. As shown in Fig. 5A, the Δ58 and K35A/R36A variants that do not enter the nucleus did not stimulate SM22 promoter activity as strongly as wild-type mDia2, and these differences were further enhanced in the presence of constitutively active RhoA (Fig. 5B). Importantly, all three variants were expressed at similar levels, and neither mutation affected RhoA binding as measured by GST pull-down assays (Fig. 5C). The latter result is in excellent agreement with a previous report that the first 75 amino acids of mDia1 were dispensable for high-affinity RhoA binding (31) and strongly suggest that the decreased transcriptional effects observed were not due to deficient activation of the mDia2 variants. The Δ58 and K35A/R36A mutations inhibited the ability of mDia2 to stimulate MRTF-B-dependent transactivation (Fig. 5D), and the Δ58 mutation could not fully promote MRTF-B nuclear accumulation (Fig. 5E). The considerable transcriptional effects of the FH1FH2 deletion variant also support a role for nuclear mDia2 in the regulation of MRTF activity. Although this catalytically active region of mDia2 localized predominantly to the nucleus, it strongly increased SM22 promoter activity by enhancing MRTF-B activity and nuclear accumulation (Fig. 5, F and G). Finally, enhancing mDia2 nuclear localization by deletion and/or mutation of the mDia2 NES significantly increased the ability of mDia2 to activate SMC-specific promoter activity (Fig. 5H) and MRTF nuclear localization (Fig. 5I).
Structural features of mDia2 regulate its nuclear shuttling.
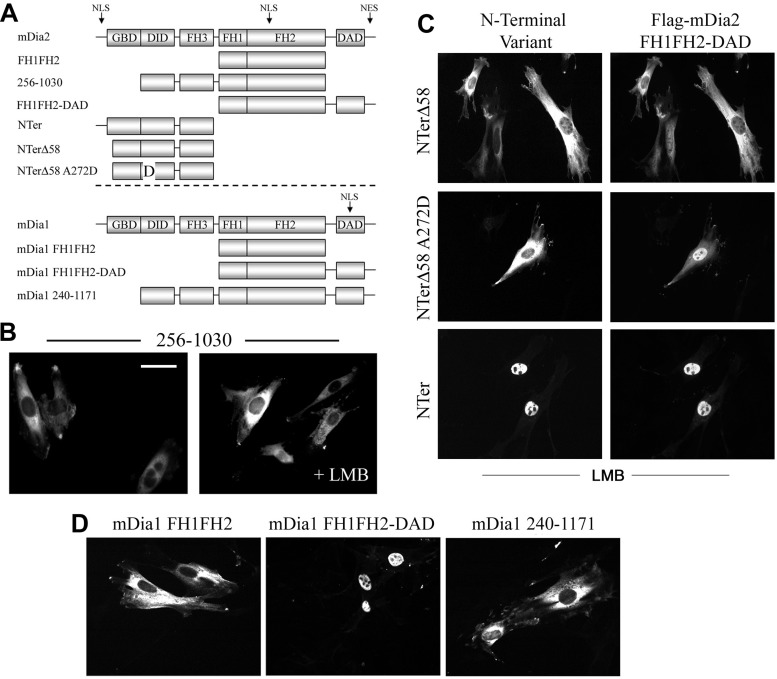
As shown in Fig. 3C, the NLS within the FH2 domain cannot drive nuclear localization of a full-length mDia2 construct in which the NH2-terminal NLS is mutated (even in the presence of leptomycin B). Given that the catalytically active FH1FH2 region is sequestered in the autoinhibitory state of mDia2, we hypothesized that the activity of the FH2 NLS is masked in the full-length molecule. Indeed, the addition of the DID and FH3 domains (AA 256–533) to the FH1FH2 region resulted in cytoplasmic localization of this fragment (Fig. 6B) and also inhibited the effects of the FH1FH2 domain on SMC-specific promoter activity and MRTF localization (data not shown). When fused to GFP, the DID-FH3 had no effect on GFP localization, suggesting that this region hinders the function of the FH2 NLS only in the context of the mDia2 protein. To further test this idea, we monitored FH1FH2-DAD localization in the presence of NH2-terminal fragments of mDia2, an approach that takes advantage of the ability of NH2- and COOH-terminal halves of mDia2 to interact even when expressed as separate molecules (6). As shown in Fig. 6C, FH1FH2-DAD nuclear accumulation (in the presence of leptomycin B) was inhibited in the presence of the NTerΔ58 fragment. Importantly, an NTerΔ58 variant that contained a mutation that inhibits DID-DAD binding (A272D) (21) did not inhibit FH1FH2-DAD nuclear localization, suggesting that a physical interaction between the NH2- and COOH-terminal halves was required for this effect. As expected, a full-length NH2-terminal fragment did not prevent nuclear accumulation of FH1FH2-DAD, suggesting that the activity of the NH2-terminal NLS can override the masking effect of the DID-FH3 domains. As previously reported, an mDia1 deletion consisting of the FH1FH2-DAD domain also localized to the nucleus (Fig. 6D). Interestingly, the inclusion of the DID-FH3 (from mDia1) resulted in complete cytoplasmic localization of this construct, suggesting that a similar mechanism may be involved in regulating mDia1 localization.
Fig. 6.
The FH2 NLS was masked by the autoinhibited state of mDia2. A: schematic of the mDia2 and mDia1 variants used in these experiments. B: localization of the mDia2 deletion variant 256–1030 in 10T1/2 cells in the absence and presence of LMB. Scale bar = 20 μm. C: FH1FH2-DAD localization was examined in LMB-treated 10T1/2 cells coexpressing the indicated NH2-terminal (NTer) mDia2 fragments. D: localization of the indicated mDia1 variants in 10T1/2 cells.
Nuclear mDia signaling regulates nuclear actin mobility.
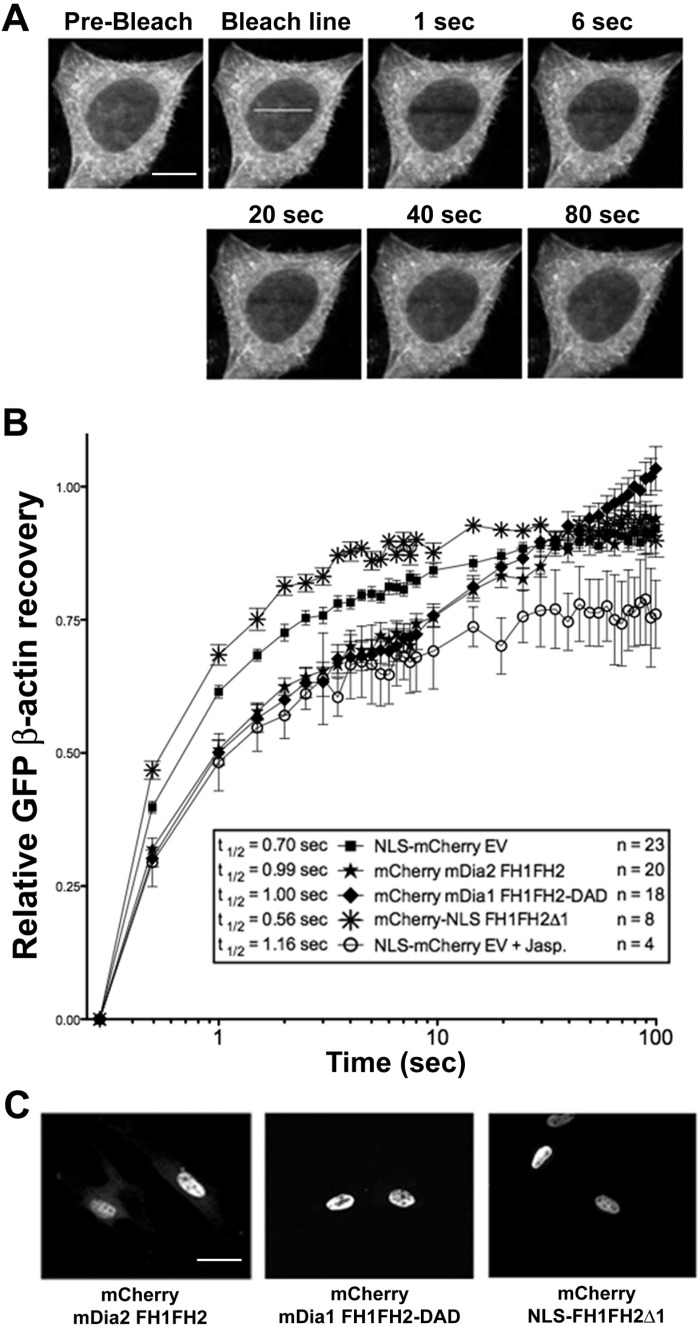
To provide support for the idea that mDia2 promoted nuclear actin polymerization, we established a FRAP-based assay in HeLa cells stably expressing GFP β-actin (Fig. 7A). In this model, changes in recovery that reflect alterations in GFP-actin diffusion are used as an indirect measure of actin polymerization. Although nuclear GFP-actin levels were relatively low, post-bleach recovery kinetics in the nucleus were similar to that seen in the cytoplasm (data not shown). Moreover, treatment of cells with jasplakinolide, a sponge toxin that facilitates actin polymerization, resulted in a slower and less complete recovery within the nuclear compartment, further validating the use of this model to measure nuclear actin dynamics (Fig. 7B). Importantly, nuclear FRAP was delayed in cells expressing mCherry-tagged constitutively active mDia variants that localize to the nucleus (mDia2FH1FH2 and mDia1FH1FH2-DAD), indicating a decrease in freely diffusible G-actin. Moreover, nuclear FRAP was enhanced in cells expressing a nuclear-targeted dominant-negative mDia variant that inhibits the activities of both mDia1 and mDia2 (NLS-FH1FH2Δ1). Interestingly, in cells expressing the constitutively active mDia variants, FRAP eventually returns to control levels between 10 and 15 s. Given the relatively slow kinetics of this recovery, we feel that it is likely due to constitutively active mDia-mediated treadmilling of polymerized GFP-β-actin molecules into the bleached region, a process that cannot occur in jasplakinolide-treated cells.
Fig. 7.
mDia signaling regulated nuclear actin dynamics. Nuclear fluorescence recovery after photobleaching (FRAP) experiments were performed on HeLa cells stably expressing GFP-β-actin using confocal microscopy (see materials and methods for more details). A: representative images illustrating nuclear GFP-β-actin FRAP in this model. Scale bar = 10 μm. B: nuclear GFP-β-actin FRAP in HeLa cells expressing mCherry-tagged constitutively active and dominant-negative mDia variants that localize predominantly to the nucleus. Data are expressed as percent recovery of the pre-bleach value set to 1 and are presented as means ± SE from the indicated number of cells from at least 3 independent transfection experiments. In some experiments, NLS-mCherry-expressing cells were treated with the actin-polymerizing reagent jasplakinolide (1 μM; Jasp) for 30 min before FRAP measurements. FRAP curves were analyzed by repeated-measures ANOVA followed by Bonferroni's post hoc test for individual significance using GraphPad Prism 5.0. For the first 10 s, all individual FRAP curves were significantly different from control (P < 0.05). C: localization of the indicated mCherry-tagged mDia variants in HeLa cells. Scale bar = 20 μm.
Nuclear localization of RhoA and LARG enhanced MRTF-dependent transcription.
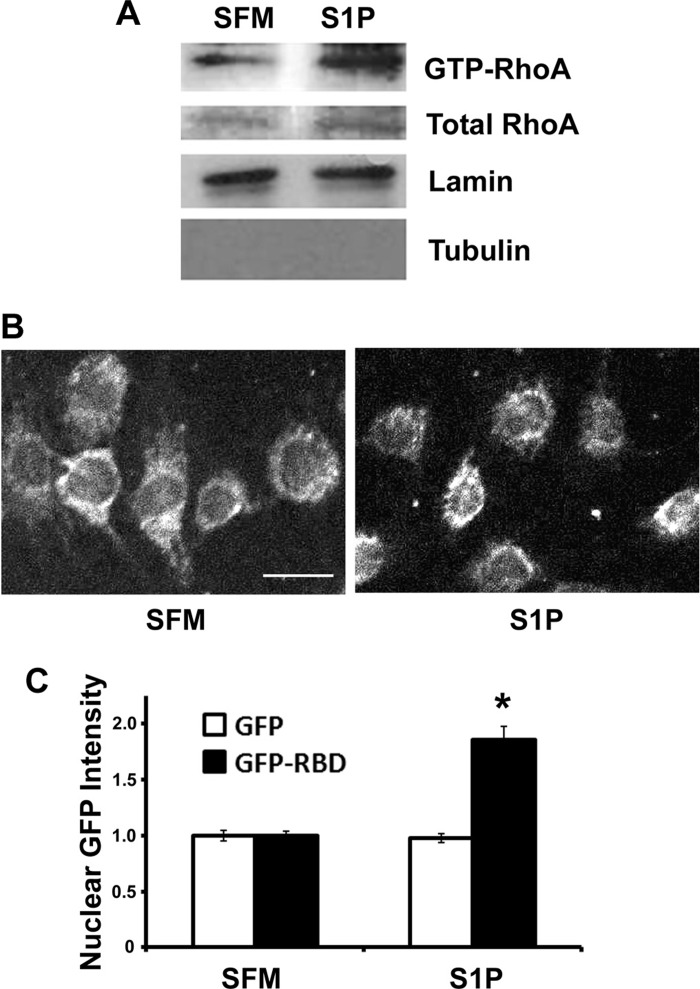
To directly measure nuclear RhoA activity in our 10T1/2 model, we used Rhotekin RBD pull-down assays to precipitate active RhoA out of nuclear lysates (15). As shown in Fig. 8A, significantly higher levels of active RhoA were detected in nuclei prepared from S1P-treated cells. To provide additional evidence for nuclear RhoA activity, we also monitored the localization of a GFP Rhotekin RBD fusion protein in our model. Because the Rhotekin RBD only binds active RhoA, its localization can be used as a measure of RhoA activity. As shown in Fig. 8, B and C, nuclear fluorescence of GFP-Rhotekin RBD was significantly increased following S1P stimulation for 7.5 min, whereas nuclear fluorescence of GFP was unaffected.
Fig. 8.
S1P activated RhoA in the nucleus. A: GST-Rhotekin Rho-binding domain (RBD) was used to precipitate active RhoA from nuclear lysates prepared from control and S1P-treated 10T1/2 cells (see materials and methods for more details). Note that lamin and tubulin were used as markers for nuclear and cytosolic fractions, respectively. B: mouse 10T1/2 cells expressing GFP or GFP-rhotekin RBD (shown) were serum starved with 0.5% FBS for 16 h and then stimulated with 10 μM S1P for 7.5 min. Cells were fixed with 4% paraformaldehyde, stained with DAPI, and then imaged by confocal microscopy. Scale bar = 20 μm. C: fluorescence intensity within an 8-μm diameter circle at the center of the nucleus was quantified by ImageJ. At least 100 GFP- and GFP-rhotekin RBD-expressing cells under each condition were quantified from 7 random fields from 2 separate experiments. *P < 0.05 vs. serum free media (SFM).
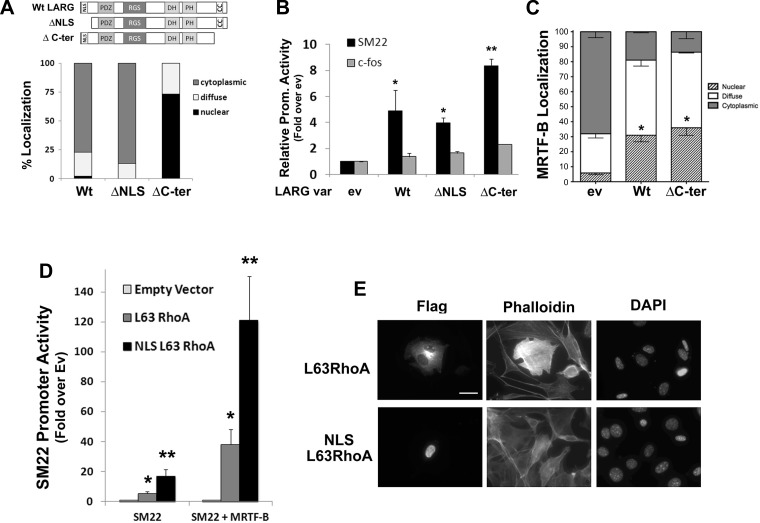
Although several RhoGEFs including Net1 and Ect2 have been shown to localize to the nucleus (4, 10, 41), and active RhoA has been measured in nuclear fractions (7), the functional significance of these findings has not been fully addressed. We have previously shown that the RhoGEF LARG is a critical regulator of RhoA activity in SMCs. Given previous studies demonstrating that monomeric LARG shuttles through the nucleus (14), we wanted to test whether this property was important for the ability of LARG to promote MRTF-dependent transcription. To this end, we generated a series of LARG nuclear localization variants and cotransfected them into 10T1/2 cells along with the SM22-luciferase reporter. Importantly, these mutations do not affect the plekstrin homology and double homology domains that mediate RhoGEF activity or the regulator of G-protein signaling domain that mediates LARG activation. As shown in Fig. 9, A and B, full-length LARG, which is mostly cytoplasmic, activated SM22 promoter activity by fivefold, whereas a COOH-terminal LARG deletion that is mostly nuclear activated SM22 promoter activity significantly more strongly. When coupled with the observation that both LARG variants significantly enhanced MRTF nuclear localization (Fig. 9C), these data suggest that activation of RhoA in either compartment can regulate MRTF-dependent transcription. Providing further support for this idea, a nuclear-targeted constitutively active variant of RhoA exhibited significantly higher transcriptional activity than its cytoplasmic counterpart (Fig. 9D) without enhancing cytoplasmic actin polymerization, as measured by phalloidin staining (Fig. 9E).
Fig. 9.
Nuclear-targeted leukemia-associated Rho guanine nucleotide exchange factor (GEF) (LARG) and RhoA-stimulated MRTF activity. A: 10T1/2 cells were transfected with the GFP-tagged LARG variants shown. After 24 h, LARG localization was scored in at least 100 cells from 3 separate experiments. C-Ter, COOH-terminal. B: 10T1/2 cells were transfected with the indicated promoter-luciferase construct along with the indicated LARG nuclear localization variant. *P < 0.05 vs. empty vector. **P < 0.05 vs. Wt. C: quantification of GFP-MRTF-B localization in 10T1/2 cells coexpressing Wt or ΔC-ter LARG. At least 100 cells were counted under each condition from 3 independent experiments. *Nuclear localization P < 0.05 vs. empty vector. D: 10T1/2 cells were transfected with SM22-luciferase, MRTF-B, and either a cytoplasmic or nuclear-targeted variant of constitutively active L63RhoA. *P < 0.05 vs. empty vector. **P < 0.05 vs. L63RhoA. E: phalloidin staining of 10T1/2 cells expressing the cytoplasmic and nuclear-targeted variants of L63RhoA.
DISCUSSION
The present study extends our understanding of the RhoA-dependent mechanisms that regulate SMC-specific gene expression. Our results support a previous study on mDia2 nuclear shuttling (29) and provide novel information on the mDia2 domains that mediate this process. More importantly, our demonstration that nuclear localization of mDia2, LARG, and RhoA is important for full activation of MRTF-dependent SMC-specific gene expression provides physiological relevance to RhoA signaling within the nuclear compartment. When coupled with our demonstration by FRAP that mDia signaling regulates nuclear actin mobility, our results support a model in which RhoA-dependent nuclear actin polymerization promotes MRTF nuclear accumulation by decreasing nuclear G-actin pools. These data add to a growing body of evidence indicating that nuclear actin polymerization has important physiological functions and highlight the need to further characterize nuclear RhoA signaling.
Given the difficulty in visualizing actin filaments in the nucleus, the precise role of actin in this compartment has remained unclear. Polymerized actin structures known as actin rods have been observed in a variety of cell types under conditions of cell stress (36), but, until recently, definitive evidence of an actin-based “nucleoskeleton” has been lacking. Importantly, during the completion of our work, results from two high-profile studies strongly support the idea that MRTF localization is regulated by nuclear actin polymerization. Baarlink et al. (2) used several nuclear-targeted approaches in combination with phalloidin staining and the actin-binding reagent, Lifeact, to demonstrate that nuclear mDia signaling promoted dynamic nuclear actin polymerization and the nuclear accumulation of MRTF-A in HeLa cells and NIH3T3 fibroblasts. In addition, Ho et al. (17) demonstrated that cardiomyocytes from lamin A/C-deficient or dystrophic mice containing a lamin mutation (N195K) have impaired MRTF-A nuclear localization (17). This effect was accompanied by defects in cytoplasmic and nuclear actin polymerization as measured by GFP-actin FRAP. When taken together with previous studies demonstrating that actin associates with the nuclear lamins (A, B, and C) and emerin (19, 39), these results suggest that nuclear actin fibers are anchored at the nuclear envelope. Because the proven existence of a RhoA-regulated actin-based nucleoskeleton provides a potential mechanism for controlling nuclear structure and compartmentalization, these findings will have profound implications on our understanding of transcriptional regulation.
It will be critical to identify the signaling pathways that regulate nuclear actin dynamics. Although we and others have detected relatively low levels of RhoA in the nucleus, the mechanisms that control RhoA levels and/or activity in this compartment nucleus are currently unclear. Because of their relatively small size (∼21 kDa), the small GTPases should be able to enter the nucleus by passive diffusion. However, many including Rac1 and RhoC contain a COOH-terminal polybasic region that facilitates nuclear import (47). The presence of profilin, ROCK2, and LIMK2 in the nucleus suggests that actin polymerization through this RhoA-dependent pathway may also be important for nuclear function (13, 43, 44). It was not surprising that the mDia2 NLS mutations only partially inhibited the transcriptional effects of mDia2 because mDia2-mediated actin polymerization in the cytoplasm is known to enhance MRTF nuclear import. However, the relationship between cytoplasmic and nuclear G-actin pools has not been well defined, and it is possible that changes in G-actin levels in the cytoplasm affect G-actin levels in the nucleus or vice versa.
Defective control of SMC differentiation has been shown to play an important role in the progression of atherosclerosis and restenosis, and our results indicate that the subcellular localization of mDia2 (or perhaps other DRFs) may be a critical mechanism for controlling SMC phenotype. For example, several studies have demonstrated that mDia2 localizes to the cell periphery, where it promotes cell migration (16, 33, 40). Thus increased localization of mDia2 to the nucleus would inhibit SMC phenotypic modulation by simultaneously inhibiting SMC migration and promoting SMC differentiation marker gene expression. Clearly, it will be important to determine whether mDia2 nuclear localization is regulated and to further identify the mechanisms involved in this process. A related formin, Formin I, has also been shown to localize to cytoplasmic and nuclear compartments. Interestingly, mice homozygous for a Formin I mutation that was predominantly cytoplasmic exhibited limb and kidney defects, providing in vivo evidence that nuclear localization can be an important determinant of formin function (5). The contributions of mDia signaling to SMC function in vivo have not been examined. mDia1 knockout mice exhibit defects in myeloid cell proliferation and lymphocyte migration, but no gross vascular abnormalities were reported (8, 34). However, given the potential overlapping and compensatory effects observed in this and other studies (35), it will be important to generate double knockout and cell-type specific knockout mice to address this question.
Another interesting question is whether the nuclear localization of mDia2 is influenced by its activation state. Given the presence of a cryptic NLS in the FH2 domain and import and export sequences at the NH2 and COOH termini, the significant conformational change that occurs following RhoA-mediated disruption of the DID-DAD interaction may lead to differential exposure of these domains. On the basis of the reported crystal structure of the FH2 domain of the yeast DRF, Bni1 (32, 49), the NLS within this region is located within an unordered, proteolytically exposed linker region that connects the NH2-terminal lasso domain with the globular knob subdomain, suggesting that this sequence would be accessible to the nuclear import machinery in the active state. In addition, a previous result from Alberts et al. (1) indicated that the leucine-rich mDia2 core DAD domain may function as an NES, and the activation-dependent exposure of this region would likely affect its NES activity. Unfortunately, most reports examining the localization of active mDia2 have used the ΔGBD version, which lacks the NH2-terminal NLS. Our preliminary examination of the localization of a full-length constitutively active mDia2 variant (A272D) demonstrated that this variant was significantly less susceptible to leptomycin B-mediated nuclear accumulation. However, additional experiments will be required to test whether mDia2 activation affects its nuclear (or subcellular) localization. Of interest, splice variants and cleavage products have been described in mDia2 and FHOD1 (11, 28), and, because the regulation of DRF subcellular localization may be modular in nature, splicing differences could have significant impacts on DRF function.
In summary, the results from the present study indicate that nuclear shuttling of mDia2 plays an important role in the control of MRTF subcellular localization and SMC-specific gene transcription and that the effects of mDia2 on nuclear actin dynamics contribute to this regulatory mechanism. Furthermore, nuclear localization of the RhoGEF LARG and RhoA enhance SMC-specific gene transcription, implying a role for nuclear RhoA signaling in MRTF-dependent gene expression. With the abundance of evidence implicating actin in a variety of nuclear processes, it will be critical to further characterize actin structure within the nucleus, to identify the molecules that regulate actin dynamics in this compartment, and to determine the contributions of actin polymerization to general and cell-type-specific gene expression.
GRANTS
This work was supported by NIH Grants HL-070953 (C. Mack), HL-102446 (J. Taylor), T32HL069768 (L. Weise-Cross), and American Heart Association Fellowship 07152340U (D. Staus).
DISCLOSURES
The authors declare that there are no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem 276: 2824–2830, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340: 864–867, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Belin BJ, Cimini BA, Blackburn EH, Mullins RD. Visualization of actin filaments and monomers in somatic cell nuclei. Mol Biol Cell 24: 982–994, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalamalasetty RB, Hummer S, Nigg EA, Sillje HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci 119: 3008–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chan DC, Leder P. Genetic evidence that formins function within the nucleus. J Biol Chem 271: 23472–23477, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Copeland SJ, Green BJ, Burchat S, Papalia GA, Banner D, Copeland JW. The diaphanous inhibitory domain/diaphanous autoregulatory domain interaction is able to mediate heterodimerization between mDia1 and mDia2. J Biol Chem 282: 30120–30130, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Dubash AD, Guilluy C, Srougi MC, Boulter E, Burridge K, Garcia-Mata R. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS One 6: e17380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem 282: 25152–25158, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Faix J, Grosse R. Staying in shape with formins. Dev Cell 10: 693–706, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Mata R, Dubash AD, Sharek L, Carr HS, Frost JA, Burridge K. The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity. Mol Cell Biol 27: 8683–8697, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat Cell Biol 5: 195–204, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gieni RS, Hendzel MJ. Actin dynamics and functions in the interphase nucleus: moving toward an understanding of nuclear polymeric actin. Biochem Cell Biol 87: 283–306, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Goyal P, Pandey D, Siess W. Phosphorylation-dependent regulation of unique nuclear and nucleolar localization signals of LIM kinase 2 in endothelial cells. J Biol Chem 281: 25223–25230, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Grabocka E, Wedegaertner PB. Disruption of oligomerization induces nucleocytoplasmic shuttling of leukemia-associated rho Guanine-nucleotide exchange factor. Mol Pharmacol 72: 993–1002, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Guilluy C, Dubash AD, Garcia-Mata R. Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus. Nat Protoc 6: 2050–2060, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Gupton SL, Eisenmann K, Alberts AS, Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J Cell Sci 120: 3475–3487, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497: 507–511, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann W, Reichart B, Ewald A, Muller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle MC. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J Cell Biol 152: 895–910, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol 2: E231, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL. Actin-protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci 117: 2481–2490, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J 24: 4176–4187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA 102: 8916–8921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol 26: 5797–5808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA 100: 9366–9370, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem 279: 42422–42430, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31: 1495–1505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ. Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol 172: 541–552, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menard I, Gervais FG, Nicholson DW, Roy S. Caspase-3 cleaves the formin-homology-domain-containing protein FHOD1 during apoptosis to generate a C-terminal fragment that is targeted to the nucleolus. Apoptosis 11: 1863–1876, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Miki T, Okawa K, Sekimoto T, Yoneda Y, Watanabe S, Ishizaki T, Narumiya S. mDia2 shuttles between the nucleus and the cytoplasm through the importin-alpha/beta- and CRM1-mediated nuclear transport mechanism. J Biol Chem 284: 5753–5762, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113: 329–342, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell 18: 273–281, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 433: 488–494, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol 15: 129–133, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Kitchen SM, West RA, Sigler R, Eisenmann KM, Alberts AS. Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res 67: 7565–7571, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol 13: 534–545, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Percipalle P, Visa N. Molecular functions of nuclear actin in transcription. J Cell Biol 172: 967–971, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 16: 588–596, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435: 513–518, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Sasseville AM, Langelier Y. In vitro interaction of the carboxy-terminal domain of lamin A with actin. FEBS Lett 425: 485–489, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol 7: 619–625, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt A, Hall A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J Biol Chem 277: 14581–14588, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol 27: 478–486, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Stuven T, Hartmann E, Gorlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin. Actin complexes. EMBO J 22: 5928–5940, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka T, Nishimura D, Wu RC, Amano M, Iso T, Kedes L, Nishida H, Kaibuchi K, Hamamori Y. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem 281: 15320–15329, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316: 1749–1752, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin- related transcription factors. Proc Natl Acad Sci USA 99: 14855–14860, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams CL. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell Signal 15: 1071–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan JL. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat Cell Biol 8: 756–763, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell 116: 711–723, 2004 [DOI] [PubMed] [Google Scholar]