Abstract
Background
R-etomidate possesses unique desirable properties, but potently suppresses adrenocortical function. Consequently, efforts are being made to define structure-activity relationships with the goal of designing analogues with reduced adrenocortical toxicity. We explored the pharmacological impact of modifying etomidate’s chiral center utilizing R-etomidate, S-etomidate and two achiral etomidate analogues (cyclopropyl etomidate and dihydrogen etomidate).
Methods
The γ-aminobutyric acid type A (GABAA) receptor modulatory potencies of drugs were assessed in oocyte-expressed α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors (for each drug, n=6 oocytes per subtype). In rats, hypnotic potencies and durations of action were measured using a righting reflex assay (n = 26 – 30 doses per drug) and adrenocortical potencies were quantified using an adrenocorticotropic hormone stimulation test (n = 20 experiments per drug).
Results
All four drugs activated both GABAA receptor subtypes in vitro and produced hypnosis and suppressed adrenocortical function in rats. However, drug potencies in each model ranged by 1–2 orders of magnitude. R-etomidate had the highest GABAA receptor moulatory, hypnotic, and adrenocortical inhibitory potencies. Respectively, R-etomidate, S-etomidate and cyclopropyl etomidate were 27.4-fold, 18.9-fold, and 23.5-fold more potent activators of receptors containing β3 subunits than β1 subunits; however, dihydrogen etomidate’s subunit selectivity was only 2.48-fold and similar to that of propofol (2.08-fold). S-etomidate was 1/23rd as potent an adrenocortical inhibitor as R-etomidate.
Conclusion
The linkage between the structure of etomidate’s chiral center and its pharmacology suggests that altering etomidate’s chiral center may be used as part of a strategy to design analogues with more desirable adrenocortical activities and/or subunit selectivities.
Introduction
Etomidate is a potent and rapidly acting imidazole-based anesthetic agent that is highly valued for its minimal effects on breathing and blood pressure and consequent high therapeutic index. 1–5 It produces sedation and hypnosis by enhancing the function of γ-aminobutyric acid type A (GABAA) receptors in the brain, an action that is highly dependent upon the receptor’s subunit composition 6; GABAA receptor’s containing β2 or β3 subunits are substantially more sensitive to the actions of etomidate than those containing β1 subunits. 7 Among clinical anesthetics, such high selectivity is unusual and may account for distinguishing aspects of etomidate’s pharmacology, potentially including its tendency to produce myoclonus and lower seizure thresholds or maintain cardiovascular stability. 7,8
In addition to producing sedation and hypnosis, etomidate also potently inhibits the function of 11β-hydroxylase, suppressing the biosynthesis of adrenocortical steroids (i.e. cortisol, corticosterone, and aldosterone). 9–12 This deleterious side effect has great clinical significance because it can increase morbidity and mortality, particularly in the critically ill. 13,14 Therefore, etomidate is not administered as a prolonged continuous infusion to maintain anesthesia or sedation, and the use of even a single bolus dose to induce anesthesia at the start of surgery is highly controversial. 15–19
Etomidate contains a single chiral center and thus exists as R and S enantiomers. Its ability to enhance GABAA receptor function and produce sedation/hypnosis is known to be enantiomerically-selective as the R enantiomer – which is the one used clinically – is significantly more potent than the S enantiomer. 20 It is not known whether etomidate’s effects on adrenocortical function are similarly enantioselective. However, enantiomers of the structural analogue metomidate inhibit in vitro cortisol secretion by adrenocortical carcinoma cells with different potencies, suggesting that etomidate’s two enantiomers may differ in their abilities to suppress in vivo adrenocortical function. 21
Because etomidate possesses important desirable pharmacological properties found in no other clinical anesthetic agent, efforts are being made to define etomidate structure-activity relationships with the long-term goal of rationally designing analogues with reduced adrenocortical toxicity. 22 To date, such studies have identified etomidate’s imidazole ring and ester moiety as potential structural elements in the molecule that may be modified to reduce the magnitude or duration of adrenocortical suppression. 23–26 Conversely in the endocrinology field, the focus has been on designing etomidate analogues to treat hypercortisolemia that retain potent adrenocortical activity but are non-sedating, or developing new radiotracers for the diagnostic imaging and ablation of adrenocortical tumors. 21 The purpose of the present study was to explore the pharmacological impact of modifying etomidate’s chiral center. Our hypothesis was that etomidate’s pharmacological properties could be altered (potentially in desirable ways) by modifying the structure of its chiral center. To test this hypothesis, we utilized the two etomidate enantiomers (i.e. R-etomidate and S-etomidate) and two achiral etomidate analogues (figure 1). We defined their potencies for enhancing GABAA receptor function using two receptor subtypes known to have differing etomidate sensitivities and contains a gating mutation that facilitates quantitation of hypnotic sensitivity. We also measured their potencies for producing hypnosis and suppressing adrenocortical function in rats following single intravenous bolus administration. Our studies show that the structure of the chiral center is a highly sensitive determinant of GABAA receptor modulatory potency and subunit selectivity, and hypnotic and adrenocortical potencies.
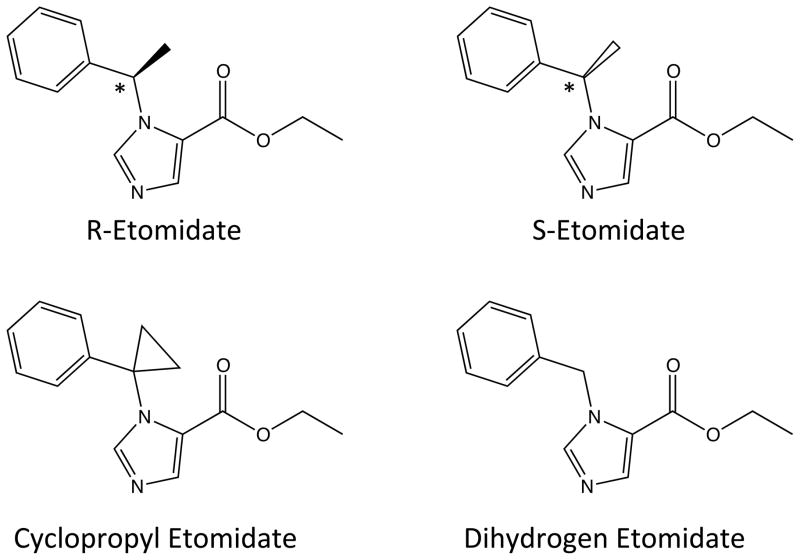
Figure 1.
Structures of the R-etomidate (R-ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate), S-etomidate (S-ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate), cyclopropyl etomidate (ethyl 1-(1-phenylcyclopropyl)-1H-imidazole-5-carboxylate) and dihydrogen etomidate (ethyl 1-benzyl-1H-imidazole-5-carboxylate). The chiral center in the two etomidate enantiomers are indicated by the asterisk.
Materials and Methods
Animals
All studies were conducted with the approval of and in accordance with rules and regulations of the Institutional Animal Care and Use Committee at the Massachusetts General Hospital, Boston, Massachusetts. Xenopus laevis adult female frogs were purchased from Xenopus One (Ann Arbor, MI). Adult male Sprague-Dawley rats (300–450 gm) were purchased from Charles River Laboratories (Wilmington, MA).
Sources of Drugs and Chemicals
Dexamethasone was obtained from American Regent (Shirley, NY), and adrenocorticotropic hormone1–24 (ACTH1–24) and GABA was from Sigma-Aldrich Chemical Company (St. Louis, MO). R-etomidate was purchased from Bachem (Torrance, CA) and S-etomidate was synthesized by Aberjona Laboratories (Beverly, MA). Dihydrogen etomidate and cyclopropyl etomidate were synthesized in our laboratory as described below.
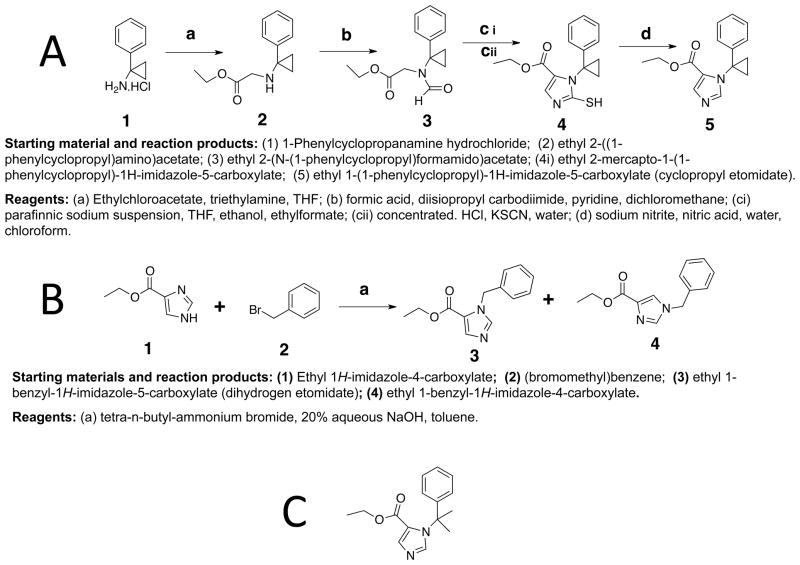
Synthesis of Cyclopropyl Etomidate
Preparation of ethyl 2-(1-(phenylcyclopropyl)-amino)acetate (2)
A stirred solution of 1-phenyl cyclopropamine hydrochloride (2.55 g, 15 mmol) in anhydrous dimethylformamide (15 ml) under Argon was cooled in an ice bath and treated drop-wise with triethylamine (4.2 ml, 30 mmol; figure 2A). Ethylchloroacetate (1.6 ml, 15 mmol) was slowly added, the ice bath was removed and the solution stirred at room temp for 48 hrs. The reaction mixture was diluted with ether (50 ml), filtered and the precipitate repeatedly washed with ether. The combined ethereal layer was extracted three times with 50 ml portions of water and once with brine (25 ml) and the ethereal layer dried over Na2SO4. The crude product was purified on a silica gel column, equilibrated with dichloromethane/ethyl acetate 8.5:1.5 V/V to yield the colorless, liquid product 2 (1.94 g, 59 %). 1H nuclear magnetic resonance spectrum: (CDCl3) d 0.92 (m, 2H), 1.04 (m, 2H), 1.21 (t, 3H), 3.36 (s, 2H), 4.08 (q, 2H), 7.22 (m, 1H) 7.30 (m, 3H)
Figure 2.
Scheme for the synthesis of cyclopropyl etomidate (A) and dihydrogen etomidate (B). Structure of dimethyl etomidate (ethyl 1-(2-phenylpropan-2-yl)-1H-imidazole-5-carboxylate).
Preparation of ethyl 2-(N-(1-phenylcyclopropyl)formamidoacetate (3)
Formylation of the secondary amine 2 was performed with formic anhydride by the procedure of Waki & Meienhofer. 27 A solution of of 2M formic acid in dichloromethane (30 ml) was added drop-wise to a stirred solution of diisopropylcarbodiimide (4.87 g, 31.4 mmol) in anhydrous dichloromethane (30 ml), cooled in an ice bath. After stirring for 5 min, the mixture was added over a period of 30 min to an ice-cooled solution of the amino compound 2 (3.22 g, 14.7 mmol) in anhydrous pyridine (30 ml). The solution was stirred at ice-bath temperature for 3 hrs and then overnight at 4 °C. The mixture was filtered and the pyridine removed by rotary evaporation. The residue was suspended in ethyl acetate (60 ml) and extracted twice with 60 ml portions of water and once with brine (60 ml). The ethyl acetate layer was dried over sodium sulfate. The crude product obtained after rotary evaporation was purified by chromatography on a silica gel column, equilibrated with ethyl acetate/hexane 40:60 V/V. to yield pale colored, viscous formyl derivative 3 (3 g, 82.5 %). 1H nuclear magnetic resonance spectrum: (CDCl3) d 1.239 (t, 3H), 1.30 (m, 2H), 1.58 (m, 2H), 4.07 (s, 2H), 4.15 (q, 2H), 7.10 (m, 2H), 7.26 (m, 1 H masked by the solvent signal), 7.33, (m, 2H), 8.545 (s, 1H).
Preparation of ethyl 2-mercapto-1-(1-phenylcyclopropyl)-1H-imidazole-5-carboxylate (4)
Ring closure of the formyl compound 3 to mercapto imidazole derivative was performed by the procedure of Jones et al., as modified by Godefroi et al.. 28,29 Sodium ethoxide was freshly prepared by slowly adding anhydrous ethanol (785 μl, 13.44 mmol) to 34% paraffinic suspension of sodium (912 mg suspension, containing 310 mg, 13.44 mmol, sodium) in anhydrous tetrahydrofuran (10 ml) under argon. To this suspension was added at 10 °C, ethyl formate (2.93 ml, 36.4 mmol), followed by the formyl derivative 3 (3 g, 12.14 mmol). The reaction mixture was stirred overnight at room temperature. The suspension was rotary evaporated, the residue was vortexed with a mixture of xylene (13 ml) and water (13 ml), the aqueous layer was separated and acidified with 12.1 M HCl (2.4 ml, 29.7 mmol). Potassium thiocyanate (1.3 g, 113 mmol) was added, and the suspension stirred at room temp for 24 hrs. The mixture was extracted twice with 17 ml portions of chloroform and the organic layer dried by rotary evaporation to yield brownish, crude mercapto derivative 4 that was purified by flash chromatography on silica gel column with dichloromethane/ether 9:1 V/V to yield pale-white solid mercapto compound 4 (2.07 g).
Preparation of ethyl -1-(1-phenylcyclopropyl)-1H-imidazole-5-carboxylate (cyclopropyl-etomidate, 5)
A solution of the mercapto compound 4 (2.07 g, 7.2 mmol) in chloroform (7 ml) was slowly added to a stirred solution of sodium nitrite (14.4 mg), concentrated nitric acid (1.44 ml, 20.4 mmol) and water (6 ml) at 10 °C. The solution was stirred at room temp for 1.5 hrs. The reaction mixture was neutralized with sodium carbonate. The mixture was diluted with choloroform (50 ml) and extracted with two 25 ml portions of brine. The organic layer was dried over Na2SO4. Rotary evaporation of the solvent yielded brownish colored crude, oily product which was purified by flash chromatography on silica gel column with ethyl acetate/hexane 7:3 to give colorless crystalline product 5 (0.85 g). 1H nuclear magnetic resonance spectrum: (CDCl3) δ 1.22 (t, 3H), 1.64 (m, 4H), 4.20 (q, 2H), 6.84 (m, 2H), 7.26 (m, 3 H), 7.79 (d, 1H), 7.86 (d, 1H).
Synthesis of Dihydrogen Etomidate
The dihydrogen derivative (3) was synthesized by the procedure described by Sonegawa et al. for phase transfer-catalyzed N-alkylation of imidazole esters with phenyl halides (figure 2B).30 Tetra-n-butyl ammonium bromide (645 mg, 2.0 mmol) was added to a mixture of 4-ethyl imidazole carboxylate (700 mg, 5 mmol), benzyl bromide (961 mg, 5.5 mmol), toluene (21 ml) and 20% NaOH (6.5 ml) and the suspension vigorously stirred for 2 hrs at room temperature. The reaction mixture was treated with a saturated solution of ammonium chloride (7 ml) and extracted with three 50 ml portions of toluene. The combined extract was washed with brine (50 ml) and the organic layer dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation to yield a viscous oily product that was shown to be a mixture of two major components, Rf 0.41 and 0.21 on a silica gel thin layer chromatography plate with ethyl acetate/ether (9:1 V/V) solvent. The crude product was purified by preparative flash chromatography on silica gel column, equilibrated with ethyl acetate/ether 9:1 V/V. Evaporation of the faster moving fractions yielded colorless, crystalline ethyl-1-phenylmethyl-1H-imidazole-5-carboxylate (560 mg). 1H NMR (CDCl3) d 1.31 (t, 3H), 4.28 (q, 2H), 6.52 (s, 2H), 7.16 (m, 2H), 7.31 (m, 3H), 7.61 (s, 1H), 7.78 (d, 1H). The slower moving fractions yielded colorless, viscous oily residue (275 mg) of ethyl 1-phenylmethyl)-1H-imidazole-4-carboxylate. 1H nuclear magnetic resonance spectrum: (CDCl3) δ 1.37 (t, 3H), 4.35 (q, 2H), 5.14 (s, 2H), 7.18 (m, 2H), 7.37 (m, 3H), 7.56 (d, 1H), 7.59 (d, 1H).
Attempted Synthesis of Dimethyl Etomidate
An attempt to synthesize dimethyl-etomidate (figure 2C), following the synthetic route used to prepare cyclopropyl etomidate was unsuccessful, probably due the instability of the product or the precursor under the reaction condition.
Alternate synthetic routes, using nucleophilic substitution of ethyl 1H-imidazole-4-carboxylate with the mesylate of 2-phenylpropan-2-ol or with the chloro compound 2-(chloropropan-2-yl)benzene did not yield the desired compound.
Reaction of the chloro compound with the sodium salt of ethyl 1H-imidazole-4-carboxylate produced the desired N-substituted derivatives in very small quantities, just enough to perform nuclear magnetic resonance spectroscopy analyses. However, attempts to obtain better yield of the product by prolonging the reaction time or by increasing the temperature were not successful.
Determination of Octanol:Water Partition Coefficients
One mg of each hypnotic was added to 10 ml of water buffered with 10 mM Tris (pH 7.4) and 1 ml of octanol. The mixture was stirred overnight and then centrifuged to more fully separate the organic and aqueous phases. The relative hypnotic concentration in each phase (i.e. the partition coefficient) was determined by high performance liquid chromatography.
GABAA Receptor Direct Activation Assay
Oocytes were harvested from frogs as previously described and injected with messenger RNA encoding the α1(L264T), γ2, and either the β1 or β3 subunits of the human GABAA receptor (5 ng of messenger RNA total at a subunit ratio of 1:3:1). As in previous studies, we chose to study GABAA receptors harboring a mutation that significantly enhances channel-gating efficacy because it increases anesthetic sensitivity. 25 This allows us to generate more complete concentration-response curves for direct activation by hydrophobic drugs using concentrations that are below the aqueous solubility limit and without the potentially confounding influence of a co-administered agonist. After RNA injection, oocytes were incubated for at least 18 hours at 18°C in ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 0.8 mM MgCl2, 10 mM HEPES, pH=7.4) containing 0.1 mg/mL of ciprofloxicin, 0.1 mg/mL of amikacin, and 0.05 mg/mL of gentamicin before electrophysiological study.
Electrophysiological recordings were performed using the whole cell two-electrode voltage-clamp technique. Oocytes were voltage clamped at −50 mV using an Oocyte Clamp OC-725C amplifier (Warner Instruments, Hamden, CT) and perfused with ND-96 buffer with 1 mM ethylene glycol tetraacetic acid (EGTA) at a rate of 4–6 ml/min. Buffer perfusion was controlled using an eight-channel valve controller (Warner Instruments) interfaced with a Digidata 1322A data acquisition system (Molecular Devices, Sunnyvale, CA) and driven by a Dell personal computer (Round Rock, TX). Clampex 9.2 and Clampfit software (Molecular Devices) were used to record and analyze electrophysiological data. Peak current amplitudes elicited by a 30 second application of drug were normalized to control currents elicited by 100 μM GABA in the same oocyte. EC50s for direct activation were calculated by fitting the concentration-mean response data to a Hill equation with minima and maxima constrained to 0% and 100%, respectively. Because of the limited aqueous solubility of these drugs, the maximum concentration studied was 1000 μM. Pilot studies showing that preexposure of oocytes to the GABAA receptor inhibitor picrotoxin (2 mM) reduced the peak current amplitudes elicited by sedative-hypnotic drugs (100 μM) by > 98% in both receptor subtypes confirmed that the observed currents were mediated by GABAA receptors (data not shown).
Measurement of In Vivo Hypnotic Potency and Duration of Action
The hypnotic potencies of drugs were assessed in rats using a loss of righting reflexes (LORR) assay. 23,24,26 Briefly, the desired dose of drug in dimethyl sulfoxide vehicle (0.1–0.3 ml) was rapidly injected through either a femoral venous catheter preimplanted by the vendor or a 24 gauge intravenous catheter placed in a tail vein. This was followed by a 1-ml normal saline flush. Immediately after injection, rats were turned supine. A rat was judged to have LORR if it failed to right (i.e. turn itself back onto all four paws) after drug administration. The duration of LORR, which was defined as the time from drug injection until the animal spontaneously righted itself, was determined using a stopwatch. For each drug, the median effective dose (ED50) for LORR was determined from a data set of at least 24 separate doses using the method of Waud. 31 Rats that failed to recover (i.e. died) after bolus injection were not included in the ED50 calculation, but were used to estimate the median lethal dose (LD50).
Measurement of In Vivo Adrenocortical Toxicity
The in vivo adrenocortical inhibitory potencies of drugs were assessed in rats using an adrenocorticotropic hormone (ACTH)-stimulation test as previously described. 24 Each rat was given dexamethasone (0.2 mg/kg IV) to suppress baseline corticosterone production. Two hours later, dexamethasone was readministered and the desired dose of drug in dimethyl sulfoxide vehicle was rapidly injected through either a femoral venous catheter preimplanted by the vendor or a 24 gauge intravenous catheter placed in a tail vein. This was followed by a 1-ml normal saline flush. Immediately after the saline flush, ACTH1–24 (25 μg/kg) was injected through the catheter followed by another normal saline flush. Fifteen minutes after ACTH1–24 administration, a blood sample was removed from the catheter (~ 0.4 ml) for measurement of the corticosterone concentration to determine the adrenocortical response to the ACTH1–24. The blood sample was allowed to clot at room temperature before centrifugation at 16000 g for 15 min. The corticosterone concentration in the resulting serum was determined using an Enzyme-Linked ImmunoSorbent Assay (ELISA) (Diagnostic Systems Laboratories, Webster, TX) and a 96-well plate reader (Molecular Devices). For each drug, the median adrenocortical inhibitory dose (ID50) was determined from the dose-corticosterone response relationship using a Hill equation.
Statistical Analysis
All data are reported as mean +/− SD. For quantities (i.e. subunit selectivity ratio and adrenocortical toxicity index) defined by the ratio of two experimental values, the reported SDs were determined by error propagation. Linear and nonlinear errors are from fits in Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR). Statistical comparisons among all four drugs (i.e. to test for differences among octanol:buffer partition coefficients and surge current amplitudes in each receptor subtype) were made using a one-way analysis of variance with Tukey’s multiple comparisons test in Prism v6 for the Macintosh (GraphPad Software, Inc., LaJolla, CA). Comparisons between two drugs (i.e. ID50 value of an etomidate analogue versus that of R-etomidate) or two receptor subtypes (i.e. direct activation EC50s or surge current amplitudes mediated by α1(L264T)β3γ2L versus α1(L264T)β1γ2L GABAA receptors) were made using an extra-sum-of-squares F test or t-test with Walsh’s correction, respectively, in Prism v6 for the Macintosh. We tested the null hypothesis that there were no differences among drugs or between receptor subtypes with no prediction regarding which group would have the larger value prior to collecting data (i.e. two-tailed test). Statistical significance was defined by a p value <0.05. The sample sizes are indicated in the figure legends. We did not exclude any data in this study.
Results
Octanol:Buffer Partition Coefficients
Table 1 shows the measured octanol:buffer partition coefficients of the four drugs. As expected for enantiomeric pairs, R- and S-etomidate had partition coefficients that were not significantly different from one another. The partition coefficients of dihydrogen etomidate and cyclopropyl etomidate were also not significantly different from one another, but were significantly lower than those of R- and S-etomidate.
Table 1.
Octanol:Buffer Partition Coefficients and Subunit-Dependent EC50s for Direct Activation of γ-aminobutyric acid type A (GABAA) receptors
| Sedative-Hypnotic | Octanol:Buffer Partition Coefficient | GABAA Receptor EC50# β3 Subunit (μM) |
GABAA Receptor EC50# β1 Subunit (μM) |
Subunit Selectivity Ratio* |
|---|---|---|---|---|
| R-Etomidate | 731 ± 72 | 1.83 ± 0.28 | 50.17 ± 0.83 | 27.4 ± 4.2 |
| S-Etomidate | 711 ± 90 | 57.0 ± 5.1 | 1080 ± 230 | 18.9 ± 4.4 |
| Cyclopropyl Etomidate | 458 ± 45 | 67.6 ± 8.1 | 1590 ± 140 | 23.5 ± 3.5 |
| Dihydrogen Etomidate | 388 ± 32 | 161 ± 13 | 400 ± 28 | 2.48 ± 0.27 |
EC50 = Drug concentration that produces the half-maximal effect.
Subunit Selectivity Ratio = EC50 β1 subunit/EC50 β3 subunit.
Hill coefficients for GABAA receptor EC50s ranged from 0.64 to 0.92.
For every drug, the EC50 for direct activation was significantly lower in receptors containing β3 subunits (i.e. α1(L264T)β3γ2L GABAA receptors) than β1 subunits (i.e α1(L264T)β1γ2L GABAA receptors).
All reported errors are Standard Deviations.
Subunit-Dependent Direct Activation of GABAA Receptors
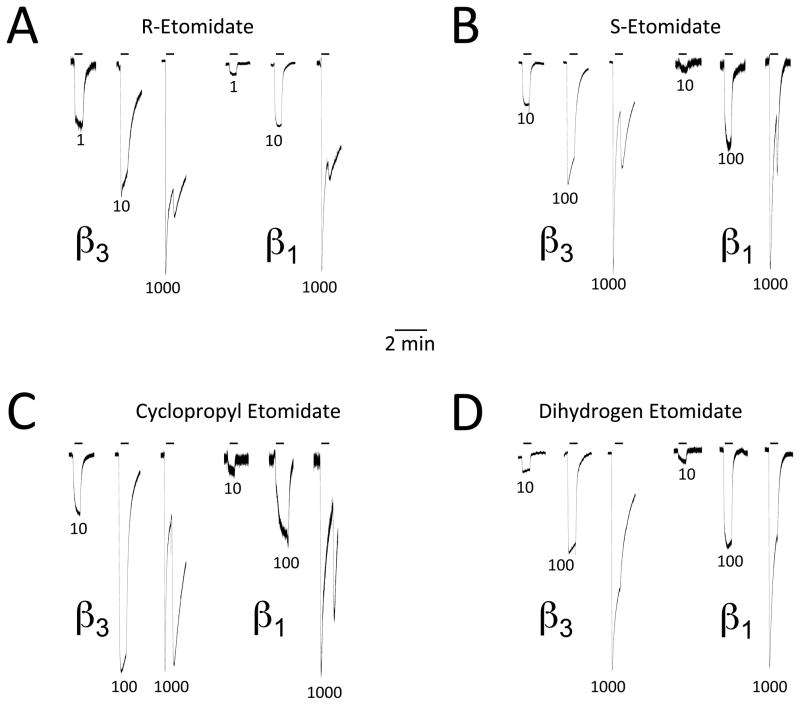
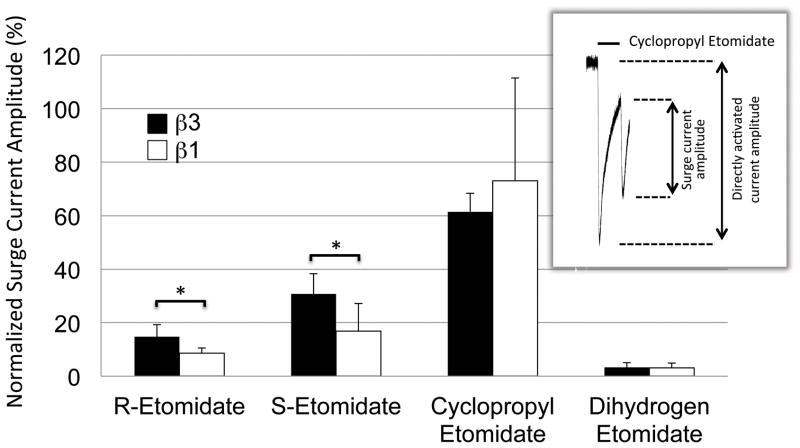
We quantified the GABAA receptor modulatory potencies and β subunit selectivities of the four drugs by assessing their abilities to directly activate α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors expressed in Xenopus oocytes. Figure 3 shows representative electrophysiological traces recorded upon 30-sec drug application and demonstrates that all four drugs directly activated α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors in a concentration-dependent manner. Inspection of the electrophysiological traces in this figure also reveals the presence of “surge currents” upon washout of a high (i.e. 1000 μM) drug concentration. Such currents, which have been attributed to low affinity anesthetic blockade of the ion channel that reverses upon anesthetic removal, varied in amplitude for the four drugs. 7 In both receptor subtypes, the amplitude of the surge currents were significantly larger for cyclopropyl etomidate than for any other drug, with values (at 1000 μM) in α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors that were 61.5 ± 6.9% and 73.0 ± 38.5%, respectively, of the directly activated peak current (figure 4). In contrast, the same concentration of dihydrogen etomidate produced surge currents that were barely perceptible (3 ± 2% of the initial directly activated peak current) in both receptor subtypes. R- and S-etomidate (also at 1000 μM) produced surge currents having amplitudes that were between these two extremes and for both etomidate enantiomers, the surge currents were significantly larger in the β3-containing subtype than the β1-containing one (figure 4).
Figure 3.
Representative two-microelectrode electrophysiological traces recorded upon applying R-etomidate (A), S-etomidate (B), cyclopropyl etomidate (C), or dihydrogen etomidate (D) at the indicated concentrations (in μM) for 30 sec. Traces obtained from oocytes expressing α1(L264T)β3γ2L and α1(L264T)β1γ2L γ-aminobutyric acid type A receptors are labeled as β3 and β1, respectively. Each panel shows the effect of three different drug concentrations on currents mediated by the two receptor subtypes. In each set of three traces, the peak current amplitudes have been normalized to that produced by 100 μM γ-aminobutyric acid in the same oocyte.
Figure 4.
Normalized surge current amplitude produced by R-etomidate, S-etomidate, cyclopropyl etomidate, or dihydrogen etomidate (all at 1000 μM). Each bar represents the average normalized amplitude (± SD) obtained from 6 separate oocytes. The inset shows a representative current trace recorded upon 30-sec application of 1000 μM cyclopropyl etomidate to α1(L264T)β1γ2L γ-aminobutyric acid type A receptors with the surge and directly activated current amplitudes indicated by the arrows. The normalized surge current amplitude is defined as the surge current amplitude divided by the directly activated current amplitude. In the inset example, this value was 58%. For each receptor subtype, statistical differences among the four drugs was tested using a one-way analysis of variance with Tukey’s multiple comparisons test. This analysis showed that cyclopropyl etomidate produced significantly larger normalized surge current amplitudes than the other drugs (for clarity, this comparison is not indicated in the figure). Comparisons among the other three drugs were not statistically significant. For each drug, a statistical difference between receptor subtypes was tested using a t-test with Walsh’s correction. Both etomidate enantiomers produced larger surge current amplitudes in α1(L264T)β3γ2L versus α1(L264T)β1γ2L γ-aminobutyric acid type A receptors. *, p < 0.05.
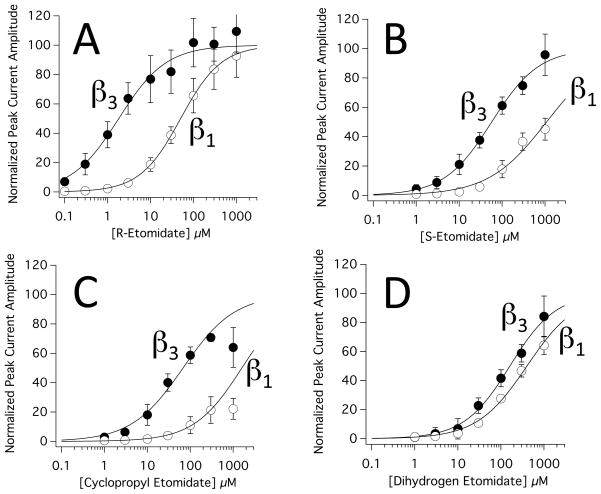
Figure 5 shows the concentration-response relationships for peak current activation of α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors by R-etomidate (panel A), S-etomidate (panel B), cyclopropyl etomidate (panel C), and dihydrogen etomidate (panel D). A fit of each data set to a Hill equation revealed that the receptor modulatory potencies of these four drugs and their subunit selectivities ranged by 1–2 orders of magnitude.
Figure 5.
Concentration-response relationships for direct activation of α1(L264T)β3γ2L and α1(L264T)β1γ2L γ-aminobutyric acid type A receptors by R-etomidate (A), S-etomidate (B), cyclopropyl etomidate (C), and dihydrogen etomidate (D). Data obtained from oocytes expressing α1(L264T)β3γ2L and α1(L264T)β1γ2L γ-aminobutyric acid type A receptors are labeled as β3 and β1, respectively. Each directly activated peak current amplitude was normalized to the peak current elicited by 100 μM γ-aminobutyric acid in the same oocyte. Each data point represents the average normalized amplitude (± SD) obtained from 6 separate oocytes. The curves are fits of the data sets to a Hill equation in the form: Y=100/(1+[EC50/X]Hill coefficient), where EC50 is a drug’s half-maximal direct activating concentration. In the case of cyclopropyl etomidate, the 1000 μM data points were not included in the fits. For all drugs, the EC50 for direct activation was significantly lower in α1(L264T)β3γ2L than α1(L264T)β1γ2L γ-aminobutyric acid type A receptors.
In both receptor subtypes, R-etomidate was the most potent of the four drugs with EC50s for direct activation of 1.83 ± 0.28 μM and 50.17 ± 0.83 μM in α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors, respectively (figure 5A and table 1). The subunit selectivity ratio for this action, which was calculated as the ratio of these two potency values, was 27.4 ± 4.2. Thus, the β3-containing subtype is ~27-fold more sensitive to direct activation by R-etomidate than the β1-containing subtype.
With respective EC50s of 57.0 ± 5.1 μM and 1080 ± 230 μM in α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors, S-etomidate was 1/20th – 1/30th as potent as R-etomidate as a direct activator of both GABAA receptor subtypes (figure 5B and table 1) but had a subunit selectivity ratio (18.9 ± 4.4) that was similar to that of R-etomidate.
The achiral etomidate analogues cyclopropyl etomidate and dihydrogen etomidate were also significantly less potent than R-etomidate as direct activators of both GABAA receptor subtypes (figures 5C and 5D and table 1). However whereas the subunit selectivity ratio of cyclopropyl etomidate (23.5 ± 3.5) was similar to those of the two etomidate enantiomers, the subunit selectivity ratio of dihydrogen etomidate was an order of magnitude lower (2.48 ± 0.27).
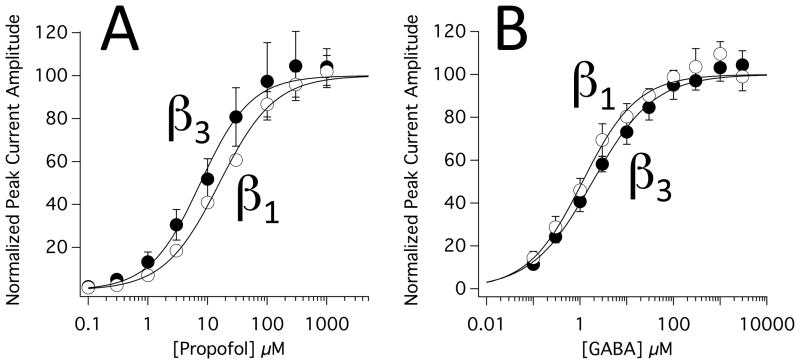
To provide additional context for the above electrophysiological studies, we also assessed the subunit selectivities of propofol and GABA in these two receptor constructs. Figure 6 shows the concentration-response relationships for activation of α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors by propofol (A) and GABA (B). Propofol’s EC50 for activation was 7.58 ± 0.83 μM and 15.8 ± 1.0 in α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors, respectively. These values were significantly different from one another and define propofol’s subunit selectivity ratio as 2.08 ± 0.26. GABA’s EC50 for activation was 1.85 ± 0.17 μM and 1.13 ± 0.16 μM in α1(L264T)β3γ2L and α1(L264T)β1γ2L GABAA receptors, respectively. These values were also significantly different from one another and define GABA’s subunit selectivity ratio as 0.61 ± 0.10.
Figure 6.
Concentration-response relationships for direct activation of α1(L264T)β3γ2L and α1(L264T)β1γ2L γ-aminobutyric acid type A receptors by propofol (A) or γ-aminobutyric acid type A (B). Data obtained from oocytes expressing α1(L264T)β3γ2L and α1(L264T)β1γ2L γ-aminobutyric acid type receptors are labeled as β3 and β1, respectively. Each directly activated peak current amplitude was normalized to the peak current elicited by 100 μM γ-aminobutyric acid in the same oocyte. Each data point represents the average normalized amplitude (± SD) obtained from 6 separate oocytes. The curves are fits of the data sets to a Hill equation in the form: Y=100/(1+[EC50/X]Hill coefficient) where EC50 is a drug’s half-maximal direct activating concentration.
Hypnotic Potency in Rats
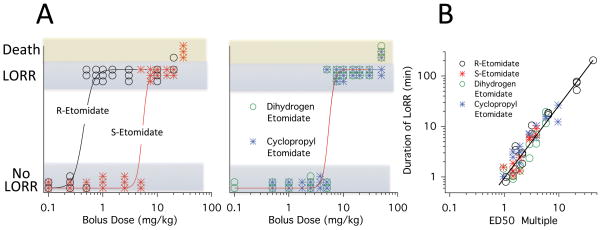
To quantify the hypnotic potencies of the four etomidate-like drugs, we administered a range of intravenous boluses doses and assessed their abilities to produce LORR. As shown in figure 7A, all four drugs produced LORR in rats in a dose-dependent manner and at high doses all drugs produced LORR. Paralleling our potency studies with GABAA receptors, R-etomidate was the most potent drug with a hypnotic ED50 of 0.47 ± 0.17 mg/kg (table 2). The hypnotic potencies of the remaining three drugs were identical with ED50s of 5.2 mg/kg (table 2).
Figure 7.
Intravenous bolus dose-response relationships for loss of righting reflexes and death in rats. The curves are fits of each data set using the quantal method of Waud. Panel A (left) shows the dose-response relationships for the two etomidate enantiomers. The loss of righting reflexes (LORR) median effective doses (ED50s) for R-etomidate and S-etomidate were 0.47 ± 0.17 mg/kg and 5.2 ± 0.9 mg/kg, respectively. Panel A (right) shows the dose-response relationships for cyclopropyl etomidate and dihydrogen etomidate. The LORR ED50 for cyclopropyl etomidate and dihydrogen etomidate were 5.2 ± 1.0 mg/kg and 5.2 ± 1.1 mg/kg, respectively. Panel B plots the duration of LORR as a function of the ED50 multiple. The line is a linear fit of the logarithm transformed data which had a slope of 1.42 ± 0.06 and an intercept of 0.01 ± 0.05. In both panels, each data point is the response from a single rat.
Table 2.
In Vivo Pharmacology in Rats
| Sedative-Hypnotic | Hypnotic ED50* (mg/kg) | LD50# (mg/kg) | Therapeutic Index+ | Adrenocortical ID50§ (mg/kg) | Adrenocortical Toxicity Index¶ |
|---|---|---|---|---|---|
| R-Etomidate | 0.47 ± 0.17 | 15 | 40 | 0.46 ± 0.05 | 0.98 ± 0.37 |
| S-Etomidate | 5.2 ± 0.9 | 25 | 5 | 10.7 ± 1.2 | 2.1 ± 0.4 |
| Cyclopropyl Etomidate | 5.2 ± 1.0 | 50 | 10 | 2.0 ± 0.2 | 0.38 ± 0.08 |
| Dihydrogen Etomidate | 5.2 ± 1.1 | 40 | 8 | 2.7 ± 0.3 | 0.52 ± 0.12 |
Hypnotic ED50 = Dose that produces hypnosis in 50% of rats.
LD50 = Dose that produces death in 50% of rats.
Therapeutic Index = LD50/Hypnotic ED50
Adrenocortical ID50 = Dose that reduces the plasma corticosterone concentration by 50%.
Adrenocortical Toxicity Index = Adrenocortical ID50/Hypnotic ED50
Hill coefficients for Hypnotic ID50s ranged from 12 to 16.
Hill coefficients for Adrenocortical ID50s ranged from −1.3 to −1.6.
All reported errors are Standard Deviations.
The duration of hypnosis (i.e. LORR) produced by the four drugs increased with the dose. Figure 7B reveals that when a drug’s dose is normalized to its hypnotic ED50 (i.e. the ED50 multiple), the duration of hypnotic action increased comparably for all four drugs with each doubling of the dose lengthening the duration of action by 142 ± 6%.
Although it was not our intent to examine the lethality of these drugs, we note that some of our rats died when administered high drug doses (figure 7A). In the case of R-etomidate, 1/2 rats died after receiving a 20 mg/kg dose. For S-etomidate and dihydrogen etomidate, 3/3 rats died after receiving doses of 30 mg/kg and 50 mg/kg, respectively. For cyclopropyl etomidate, 2/4 rats died after receiving a dose of 50 mg/kg. These data allowed us to estimate an LD50 for each drug from our data as either (1) the dose that caused death in half of the rats (R-etomidate and cyclopropyl etomidate); or (2) the average of the highest dose that produced no deaths and the lowest dose that produced death in all rats (S-etomidate and dihydrogen etomidate). For R-etomidate, S-etomidate, cyclopropyl etomidate, and dihydrogen etomidate, these LD50 values were 20 mg/kg, 25 mg/kg, 50 mg/kg, and 40 mg/kg, respectively (table 2). Although in the case of R-etomidate this estimate (20 mg/kg) is based on the death of a single rat, it is essentially identical to a previously reported value (20.4 mg/kg) using a larger number of Sprague Dawley rats. 32 Therefore, we elected not to subject additional rats to potentially lethal etomidate doses to obtain a more secure LD50 value. From our LD50 estimates and calculated hypnotic ED50s, we estimate the therapeutic indices of R-etomidate, S-etomidate, cyclopropyl etomidate, and dihydrogen etomidate to be 40, 5, 10, and 8, respectively.
Adrenocortical Inhibitory Potency in Rats
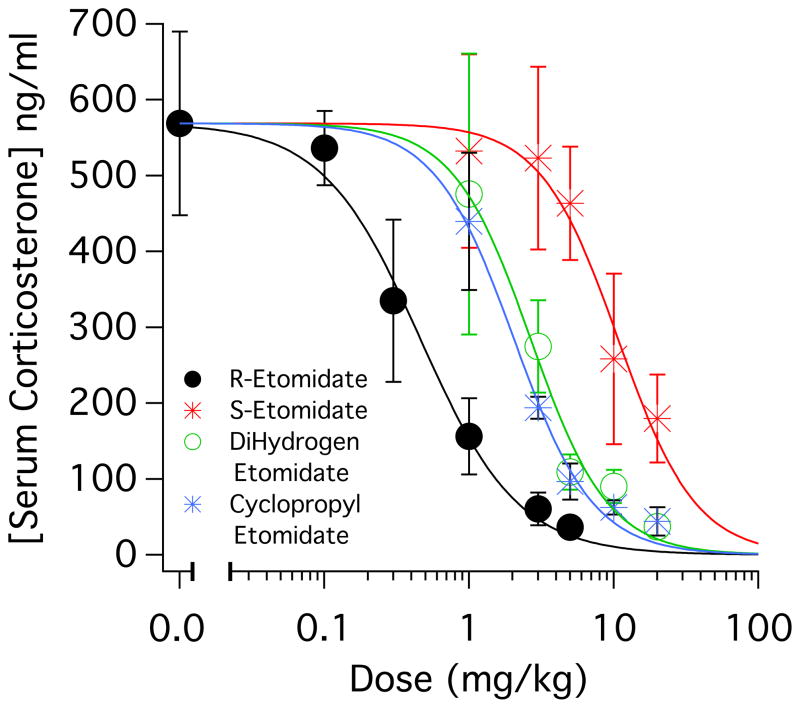
To quantify the in vivo adrenocortical inhibitory potencies of the four drugs, we administered the desired drug as a bolus (in doses ranging from 0.1 mg/kg to either 5 mg/kg or 20 mg/kg) and then immediately assessed adrenocortical function using an ACTH1–24 stimulation test. We found that all four drugs produced a dose-dependent decrease in the serum corticosterone concentration in blood serum sampled 15 minutes after administering ACTH1–24 along with the test drug (figure 8). Table 2 shows that the adrenocortical inhibitory potencies (as defined by their ID50s) ranged by 23-fold from 0.46 ± 0.05 mg/kg (R-etomidate) to 10.7 ± 1.2 mg/kg (S-etomidate). We used this data to define an “adrenocortical toxicity index” as measure of each drug’s adrenocortical inhibitory potency that is normalized to its hypnotic potency (table 2); a higher value indicates less adrenocortical suppression upon administering a hypnotic dose. The value of this toxicity index ranged 4-fold from 0.52 ± 0.12 (dihydrogen etomidate) to 2.12 ± 0.4 (S-etomidate).
Figure 8.
Intravenous bolus dose-response relationships for serum corticosterone concentrations determined in rats. Each data point represents the average concentration (± SD) obtained from 4 rats. In the absence of drug, the mean (± SD) adrenocorticotropic hormone-stimulated corticosterone concentration was 569 ± 121 ng/ml. The curves are fits of the data sets to a Hill equation in the form: Y=569/(1+[IC50/X]Hill coefficient), where IC50 is the drug’s half-inhibitory concentration. The half-inhibitory dose (ID50) for cyclopropyl etomidate and dihydrogen etomidate were not significantly different (extra-sum-of-squares F test). All other ID50 pair-wise comparisons were significantly different.
Discussion
The aim of this study was to better define the relationship between an etomidate analogue’s structure and its in vitro and in vivo pharmacological activities, with a specific focus on the chiral center. In previous work, we demonstrated that R-etomidate’s adrenocortical inhibitory potency or duration of action could be markedly reduced by modifying its imidazole ring or adding a metabolically-labile ester moiety, respectively. 23,24,26,33 In the present study, we show that etomidate’s in vitro GABAA receptor modulatory potency and subunit selectivity, and in vivo hypnotic and adrenocortical inhibitory potencies can be significantly altered by modifying the structure of its chiral center.
Although the site of anesthetic action of many anesthetics (particularly volatile inhaled agents) remains a debated question, there is overwhelming evidence that R-etomidate produces hypnosis by enhancing the function of GABAA receptors. 34,35 The binding site for this sedative-hypnotic is found at the interface between the receptor’s α and β subunits. 36,37 This is the same interface that forms the receptor’s GABA binding site; however, R-etomidate’s binding site is located within the receptor’s hydrophobic transmembrane domain rather than in the extracellular domain. Propofol is, by comparison, a more promiscuous ligand as it also binds to other GABAA receptor subunit interfaces. 37,38
Previous studies have shown that R-etomidate is a much more potent modulator of GABAA receptors containing β2 or β3 subunits than those containing β1 subunits. 7 Our results with R-etomidate are consistent with those studies as we determined its β subunit selectivity ratio in our constructs to be 27.4. Our studies go further to show that this subunit selectivity is largely maintained even when the chiral center is inverted to form S-etomidate (selectivity ratio: 18.9) or when chirality is eliminated completely by replacing the methyl group at the chiral center with a cyclopropyl group (selectivity ratio: 23.5). Surprisingly, this maintenance of subunit selectivity occurs even as GABAA receptor potency (in both receptor subtypes) is reduced by more than an order of magnitude. In contrast, the β subunit selectivity of dihydrogen etomidate (2.48) is ~1/10th that of the other etomidate-like drugs in this study and similar to that of propofol (2.08).
In addition to activating GABAA receptors, the four drugs also inhibited them at a high concentration (1000 μM) as evidenced by the presence of surge currents in the electrophysiological traces. The largest surge currents were obtained with cyclopropyl etomidate in both receptor subtypes, implying that this drug inhibits GABAA receptors with greatest potency (and/or efficacy). Such inhibition likely explains why the cyclopropyl etomidate concentration-response curves for direct activation shown figure 5C flatten or decrease upon reaching a concentration of 1000 μM. It has been suggested that such inhibition, which is also produced by high concentrations of other anesthetics, results from interactions with receptor sites that are distinct from those that produce activation. 39,40 Our data generally support this concept as the apparent potencies for direct activation (as indicated by EC50 values) and inhibition (as reflected by the amplitudes of surge currents) have different rank orders for the four drugs with R-etomidate being the most potent direct activator and cyclopropyl etomidate the most potent inhibitor.
In addition to having the highest potency for activating GABAA receptors, R-etomidate also had the highest hypnotic potency in rats. This parallel between GABAA receptor and hypnotic potencies is consistent with a cause and effect relationship between these two actions. Our finding that S-etomidate both enhances GABAA receptor function and produces hypnosis in rats (albeit with potencies that are 1–2 orders of magnitude lower than R-etomidate) contrasts with a previous study that reported that S-etomidate is “pharmacologically inactive”. 41 We cannot explain this discrepancy as the data for their conclusion was reported only as “unpublished results”. However, our results are consistent with subsequent studies by Tomlin et al. showing that S-etomidate weakly modulates GABAA receptors and produces hypnosis in tadpoles at high concentrations. 20
All of the etomidate-like drugs produced deaths in rats at the highest doses studied. Our estimated LD50s ranged by only 2.5-fold (from 20 mg/kg to 50 mg/kg). This may be contrasted with the respective 31-fold and 22-fold range in their potencies for activating our β3 subunit-containing and β1 subunit-containing GABAA receptors, and 11-fold range for producing hypnosis in rats. Our findings that these four drugs have rather similar lethal doses while differing by 1–1.5 orders of magnitude in their GABAA receptor and hypnotic potencies suggest that their lethal effects (1) involve targets other than GABAA receptors that are not highly sensitive to the structure of the chiral center and (2) is not simply a manifestation of excessive hypnotic depth.
We utilized an in vivo functional assay for evaluating the adrenocortical inhibitory potencies of the four drugs because it allows us to directly compare their adrenocortical and hypnotic potencies in the same animal model. Once again, R-etomidate was the most potent drug with an adrenocortical ID50 of 0.46 ± 0.05 mg/kg. As this is essentially identical to its hypnotic ED50 (0.47 ± 0.17 mg/kg), the calculated adrenocortical toxicity ratio is ~ 1 (0.98 ± 0.37, table 2). Although to our knowledge analogous adrenocortical inhibitory potency studies have not been performed in humans, this toxicity ratio is likely lower in humans than rats as even subhypnotic doses of R-etomidate can be highly effective in treating hypercortisolemia in humans. 42–44 With an ID50 of 10.7 ± 1.2 mg/kg, S-etomidate was 1/23rd as potent as R-etomidate making it the least potent inhibitor of adrenocortical function (table 2). This stereoselectivity in in vivo adrenocortical activity may offer an alternative strategy to producing etomidate analogues with higher adrenocortical toxicity indices. Specifically, one might seek to design S-etomidate analogues with higher anesthetic potencies rather than R-etomidate analogues with lower adrenocortical inhibitory potencies. Further studies to define the relationship between the structure of an etomidate analogue and its ability to produce hypnosis and suppress adrenocortical function are necessary to identify how this can be achieved.
In summary, we hypothesized that R-etomidate’s pharmacological properties could be altered by modifying the structure of its chiral center. We tested this by defining the in vitro and in vivo pharmacological properties of R-etomidate and three structural analogues. We found that modifying R-etomidate’s chiral center significantly altered its in vitro GABAA receptor modulatory potency and subunit selectivity, and in vivo hypnotic and adrenocortical inhibitory potencies. Such tight linkage between the structure of the chiral center and the pharmacological activity of etomidate analogues suggests the possibility of modifying etomidate’s chiral center as part of a strategy to produce analogues with more desirable adrenocortical activities and/or subunit selectivities.
Final Boxed Summary Statement.
What we already know about this topic
Etomidate not only produces sedation and hypnosis but also suppresses adrenocortical steroid biosynthesis.
The R-enantiomer of etomidate is a more potent sedative and hypnotic than the S-enantiomer.
What this article tells us that is new
The R-enantiomer of etomidate is a more potent suppressor of adrenocortical steroid biosynthesis than the S-enantiomer.
Two achiral etomidate analogs had lower hypnotic and adrenocortical suppression potencies than the R-enantiomer of etomidate.
Modification of the chiral center of etomidate may be part of a strategy to produce analogs that cause less adrenocortical suppression.
Acknowledgments
The authors would like to thank Dr. Stuart A. Forman, M.D., Associate Professor, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA for his helpful comments during the writing of this manuscript.
Supported by grant R01-GM087316 from the National Institutes of Health, Bethesda, MD and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Conflicts of Interest: The authors declare no competing interests
References
- 1.Janssen PA, Niemegeers CJ, Marsboom RP. Etomidate, a potent non-barbiturate hypnotic. Intravenous etomidate in mice, rats, guinea-pigs, rabbits and dogs. Arch Int Pharmacodyn Ther. 1975;214:92–132. [PubMed] [Google Scholar]
- 2.Gooding JM, Corssen G. Effect of etomidate on the cardiovascular system. Anesth Analg. 1977;56:717–9. doi: 10.1213/00000539-197709000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Morgan M, Lumley J, Whitwam JG. Etomidate, a new water-soluble non-barbiturate intravenous induction agent. Lancet. 1975;1:955–6. doi: 10.1016/s0140-6736(75)92011-5. [DOI] [PubMed] [Google Scholar]
- 4.Lamalle D. Cardiovascular effects of various anesthetics in man. Four short-acting intravenous anesthetics: Althesin, etomidate, methohexital and propanidid. Acta Anaesthesiol Belg. 1976;27 (suppl):208–24. [PubMed] [Google Scholar]
- 5.Colvin MP, Savege TM, Newland PE, Weaver EJ, Waters AF, Brookes JM, Inniss R. Cardiorespiratory changes following induction of anaesthesia with etomidate in patients with cardiac disease. Br J Anaesth. 1979;51:551–6. doi: 10.1093/bja/51.6.551. [DOI] [PubMed] [Google Scholar]
- 6.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. Faseb J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 7.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br J Pharmacol. 1997;120:749–56. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: Seizures and general anesthetic drugs. Anesth Analg. 2008;107:1689–703. doi: 10.1213/ane.0b013e3181852595. [DOI] [PubMed] [Google Scholar]
- 9.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–21. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 10.de Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab. 1984;59:1143–7. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 11.Wagner RL, White PF. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology. 1984;61:647–51. doi: 10.1097/00000542-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57:156–9. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 13.Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet. 1983;1:1270. doi: 10.1016/s0140-6736(83)92712-5. [DOI] [PubMed] [Google Scholar]
- 14.Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973–81. doi: 10.1111/j.1365-2044.1984.tb08885.x. [DOI] [PubMed] [Google Scholar]
- 15.den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, Joosten KF. One single dose of etomidate negatively influences adrenocortical performance for at least 24h in children with meningococcal sepsis. Intensive Care Med. 2008;34:163–8. doi: 10.1007/s00134-007-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson WL., Jr Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: A critical appraisal. Chest. 2005;127:1031–8. doi: 10.1378/chest.127.3.1031. [DOI] [PubMed] [Google Scholar]
- 17.Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med. 2011;37:901–10. doi: 10.1007/s00134-011-2160-1. [DOI] [PubMed] [Google Scholar]
- 18.Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: A meta-analysis*. Crit Care Med. 2012;40:2945–53. doi: 10.1097/CCM.0b013e31825fec26. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu R, You J, Mascha EJ, Sessler DI, Kasuya Y, Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117:1329–37. doi: 10.1213/ANE.0b013e318299a516. [DOI] [PubMed] [Google Scholar]
- 20.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–17. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Zolle IM, Berger ML, Hammerschmidt F, Hahner S, Schirbel A, Peric-Simov B. New selective inhibitors of steroid 11beta-hydroxylation in the adrenal cortex. Synthesis and structure-activity relationship of potent etomidate analogues. J Med Chem. 2008;51:2244–53. doi: 10.1021/jm800012w. [DOI] [PubMed] [Google Scholar]
- 22.Sneyd JR. Novel etomidate derivatives. Curr Pharm Des. 2012;18:6253–6. doi: 10.2174/138161212803832362. [DOI] [PubMed] [Google Scholar]
- 23.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: A novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–9. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotten JF, Forman SA, Laha JK, Cuny GD, Husain SS, Miller KW, Nguyen HH, Kelly EW, Stewart D, Liu A, Raines DE. Carboetomidate: A pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology. 2010;112:637–44. doi: 10.1097/ALN.0b013e3181cf40ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge RL, Pejo E, Haburcak M, Husain SS, Forman SA, Raines DE. Pharmacological Studies of Methoxycarbonyl Etomidate’s Carboxylic Acid Metabolite. Anesth Analg. 2012;115:305–8. doi: 10.1213/ANE.0b013e318239c6ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husain SS, Pejo E, Ge R, Raines DE. Modifying methoxycarbonyl etomidate inter-ester spacer optimizes in vitro metabolic stability and in vivo hypnotic potency and duration of action. Anesthesiology. 2012;117:1027–36. doi: 10.1097/ALN.0b013e31826d3bef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waki M, Meienhofer J. Efficient preparation of N alpha-formylamino acid tert-butyl esters. J Org Chem. 1977;42:2019–20. doi: 10.1021/jo00431a046. [DOI] [PubMed] [Google Scholar]
- 28.Jones RG. The synthesis of 5-imidazolecarboxylates from glycine and substituted glycine esters. J Am Chem Soc. 1949;71:644–7. doi: 10.1021/ja01170a072. [DOI] [PubMed] [Google Scholar]
- 29.Godefroi EF, Janssen PA, Vandereycken CA, Vanheertum AH, Niemegeers CJ. Dl-1-(1-arylalkyl)imidazole-5-carboxylate esters. A novel type of hypnotic agents. J Med Chem. 1965;8:220–3. doi: 10.1021/jm00326a017. [DOI] [PubMed] [Google Scholar]
- 30.Sonegawa M, Yokota M, Tomiyama H, Tomiyama T. Regioselective alkylation of 2-alkyl-5,6,7,8-tetrahydro-3h-cycloheptimidazol-4-ones and 2-alkyl-3h-cycloheptimidazol-4-ones. Chem Pharm Bull (Tokyo) 2006;54:706–10. doi: 10.1248/cpb.54.706. [DOI] [PubMed] [Google Scholar]
- 31.Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- 32.Kissin I, McGee T, Smith LR. The indices of potency for intravenous anaesthetics. Can Anaesth Soc J. 1981;28:585–90. doi: 10.1007/BF03007157. [DOI] [PubMed] [Google Scholar]
- 33.Ge R, Pejo E, Husain SS, Cotten JF, Raines DE. Electroencephalographic and hypnotic recoveries after brief and prolonged infusions of etomidate and optimized soft etomidate analogs. Anesthesiology. 2012;117:1037–43. doi: 10.1097/ALN.0b013e31826d3de2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Rampil IJ, Trudell J, Vissel B, Eger EI., 2nd Inhaled anesthetics and immobility: Mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–40. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 35.Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114:695–707. doi: 10.1097/ALN.0b013e3181ff72b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human alpha1beta3gamma2 gamma-aminobutyric acid type A (GABAA) receptor. J Biol Chem. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, Townsend RR, Fuchs K, Sieghart W, Evers AS, Franks NP. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol. 2013;9:715–20. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall AC, Lieb WR, Franks NP. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br J Pharmacol. 1994;112:906–10. doi: 10.1111/j.1476-5381.1994.tb13166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heykants JJ, Meuldermans WE, Michiels LJ, Lewi PJ, Janssen PA. Distribution, metabolism and excretion of etomidate, a short-acting hypnotic drug, in the rat. Comparative study of (R)-(+)-(--)-Etomidate. Arch Int Pharmacodyn Ther. 1975;216:113–29. [PubMed] [Google Scholar]
- 42.Allolio B, Schulte HM, Kaulen D, Reincke M, Jaursch-Hancke C, Winkelmann W. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in Cushing’s syndrome. Klin Wochenschr. 1988;66:361–4. doi: 10.1007/BF01735795. [DOI] [PubMed] [Google Scholar]
- 43.Schulte HM, Benker G, Reinwein D, Sippell WG, Allolio B. Infusion of low dose etomidate: Correction of hypercortisolemia in patients with Cushing’s syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab. 1990;70:1426–30. doi: 10.1210/jcem-70-5-1426. [DOI] [PubMed] [Google Scholar]
- 44.Soh LM, Gunganah K, Akker SA, Jones P, Khachi H, Dodzo K, Drake WM. Etomidate in the emergency management of hypercortisolemia. Eur J Endocrinol. 2012;167:727–8. doi: 10.1530/EJE-12-0698. [DOI] [PubMed] [Google Scholar]