Abstract
Quorum sensing (QS) is a cell density-dependent signaling mechanism used by many bacteria to control gene expression. Several recent reports indicate that the signaling molecules (autoinducers) that mediate QS in Pseudomonas aeruginosa may also modulate gene expression in host cells; however, the mechanisms are largely unknown. Here we show that two P. aeruginosa autoinducers, N-3-oxododecanoyl-homoserine lactone and N-butyryl-homoserine lactone, can both enter eukaryotic cells and activate artificial chimeric transcription factors based on their cognate transcriptional activators, LasR and RhlR, respectively. The autoinducers promoted nuclear localization of chimeric proteins containing the full LasR or RhlR coding region, and the LasR-based proteins were capable of activating transcription of a LasR-dependent luciferase gene. Responsiveness to autoinducer required the N-terminal autoinducer-binding domains of LasR and RhlR. Truncated proteins consisting of only the C-terminal helix-turn-helix DNA-binding domains of both proteins attached to a nuclear localization signal efficiently translocated to the nucleus in the absence of autoinducer, and truncated LasR-based proteins functioned as constitutively active transcription factors. Chimeric LasR proteins were only activated by their cognate autoinducer ligand and not by N-butyryl-l-homoserine lactone. These data provide evidence that autoinducer molecules from human pathogens can enter mammalian cells and suggest that autoinducers may influence gene expression in host cells by interacting with and activating as-yet-unidentified endogenous proteins.
Quorum sensing (QS) is a signaling process used by many bacterial species to coordinate gene expression within a population in response to changes in cell density (12). QS systems are composed of two components, an autoinducer synthase enzyme that catalyzes the production of the signaling molecule (termed an autoinducer) and an inducible transcription factor that mediates the response to the autoinducer. Pseudomonas aeruginosa is a gram-negative pathogen that uses QS to control and coordinate the production of several factors needed to colonize and persist in a wide range of settings (20). There are at least two complete QS systems in P. aeruginosa, named the las and rhl systems, both of which use acylated homoserine lactones as signaling molecules. The LasI and RhlI proteins catalyze the synthesis of N-3-oxododecanoyl homoserine lactone, also referred to as Pseudomonas autoinducer 1 (PAI-1), and N-butyryl-homoserine lactone (PAI-2), respectively. These autoinducers bind to and activate LasR and RhlR, respectively, two members of the LuxR family of helix-turn-helix (HTH) transcription factors (24). LasR and RhlR primarily target genes encoding virulence factors and other proteins required for efficient host infection. Disruption of P. aeruginosa QS systems diminishes its virulence in plants and animals and inhibits biofilm differentiation (2, 16, 26).
Although QS has primarily been examined in bacterial communication, a growing number of reports indicate that autoinducers may also modulate gene expression in cells of a host eukaryotic organism. For example, P. aeruginosa autoinducers can influence the production of several cytokines by immune cells both in vitro and in vivo (18, 19, 21, 28). In addition, PAI-1 has been reported to increase interleukin-8 mRNA levels in epithelial cells through induction of the transcription factor NFκB (19) and to increase the expression of cyclooxygenase-2 and prostaglandin E2 in lung fibroblasts (21). Recently, PAI-1 was reported to affect apoptotic responses in macrophages and neutrophils (27). It is unclear how the effects of autoinducers on mammalian cells are mediated and specifically whether the autoinducers enter mammalian cells and bind to and activate host regulatory proteins. However, the concept that interspecies and cross-kingdom signaling may occur through diffusible small molecules such as autoinducers is supported by two additional observations. First, the marine macroalga Delisea pulchra blocks infection by the plant pathogen Serratia liquefaciens by producing a halogenated furanone that acts as a competitive inhibitor of the bacterium's QS system (17). Second, the mammalian hormone epinephrine can substitute for autoinducer in the QS system of enterohemorrhagic Escherichia coli (23). Thus, autoinducers and hormones may be interchangeable in a language common to prokaryotes and eukaryotes.
In theory, bacterial autoinducers could elicit effects in eukaryotic cells through interactions with membrane-bound receptor molecules or through intracellular proteins. On the basis of the similarity between the mechanism of action of autoinducers in bacterial cells and that of eukaryotic hormones of the nuclear hormone receptor superfamily, we hypothesize that autoinducers function as ligands for as-yet-unknown eukaryotic proteins. As an initial step in testing this hypothesis, we wished to determine whether autoinducers can indeed efficiently enter mammalian cells. To achieve this goal, we generated a set of chimeric proteins based on LasR and RhlR that could function as sensors of autoinducer action within mammalian cells. Our data demonstrate that both P. aeruginosa autoinducers can efficiently enter and function in mammalian cells.
MATERIALS AND METHODS
Construction of LasR-based expression and reporter plasmids.
LasR coding regions were amplified by PCR from the pM63.9 plasmid (4), and primers were designed on the basis of the sequence of the LasR reported in the complete sequence of the PAO1 strain of P. aeruginosa (accession number AE004572). Both the full-length coding sequence (encoding amino acids 1 to 239) and the HTH domain (encoding amino acids 174 to 239) were amplified with primers designed to introduce a BglII site at the 5′ end of the amplified region and a HindIII site immediately following the stop codon. Primers designed to generate fusion proteins containing an N-terminal nuclear localization signal (NLS) contained the sequence CCCAAGAAGAAGCGCAAGGTG, which encodes the minimal NLS from the simian virus 40 (SV40) T antigen (PKKKRKV) (8). The amplified regions were sequenced in their entirety to preclude inclusion of unintended mutations and transferred as BglII-HindIII fragments into the pMEX-FLAG expression plasmid. pMEX-FLAG is a modified version of pMEX (34) that permits the attachment of an N-terminal FLAG epitope tag to expressed proteins. The VP16 transcriptional activation domain (TAD; encoding amino acids 429 to 456) has been described previously (33) and was transferred into NcoI-BglII-digested pMEX-FLAG-based plasmids as an NcoI-BamHI fragment. Multimerized versions of the VP16 TAD were generated by directional cloning of individual TAD modules as BglII-BamHI fragments.
The full-length RhlR coding region was amplified from PAO1 genomic DNA by PCR with the primers 5′-CGGTGCTGGCATAACAGATA-3′ and 5′-CTGCGCTTCAGATGAGACC-3′. The PCR product was inserted into pGEM-T (Promega) and sequenced. The sequence matched exactly the RhlR sequence deposited in the Pseudomonas Genome Project database (www.pseudomonas.com). The full-length coding region of RhlR and the HTH domain (amino acids 178 to 241) were amplified and assembled into chimeric genes as described above for LasR. Plasmids were named on the basis of the presence or absence of a TAD (T) or NLS (N) and the identity of the LasR or RhlR sequences included. A LasR-responsive reporter plasmid was constructed by inserting either one or two copies of a palindromic LasR recognition sequence (LasBOX; Fig. 1B) upstream of the minimal promoter from the rat albumin gene and the firefly luciferase gene by a strategy described previously (33). The LasBOX sequence was based on a previously reported LasR consensus recognition sequence (32). A control plasmid containing a mutated version of the LasBOX sequence (LasBOXm; Fig. 1B) was also constructed. The LasBOXm sequence contains six nucleotide substitutions within the palindromic recognition sequence that should disrupt interactions with LasR.
FIG. 1.
Constructs and autoinducers used in this study. (A) Schematic representation of the general domain structure of LuxR homologues such as LasR and RhlR, indicating the positions of the AIBD and the HTH DNA-binding domain, and the chimeric LasR- and RhlR-based proteins constructed in this study. The chimeric proteins are named on the basis of the region of LasR or RhlR included and the identity and number of attached eukaryotic domains (VP16 TAD [T] and SV40 T-antigen NLS [N]). (B) Sequence of the LasR recognition sequence (LasBOX) inserted upstream of the albumin minimal promoter and firefly luciferase gene in (LasBOX)2-Luc. The dyad symmetry of the essentially palindromic sequence is indicated by arrows. A reporter plasmid carrying two copies of a mutated version of the LasBOX sequence containing six nucleotide substitutions (LasBOXm) was also constructed. (C) Structures of P. aeruginosa autoinducer molecules and analogs. PAI-1 (N-3-oxododecanoyl homoserine lactone) is the naturally occurring ligand for LasR. PAI-1* (oxododecanoyl homoserine lactone) is a synthetic derivative of PAI-1 that lacks the oxygen attached to carbon 3 of the acyl chain. PAI-2 (N-butyryl-l-homoserine lactone) is the naturally occurring ligand for RhlR.
Cell culture and transfections.
The monkey kidney COS-1 cell line was cultured in Dulbecco's modified Eagle's medium (BioWhittaker, Walkersville, Md.) supplemented with 10% Cosmic Calf Serum (HyClone, Logan, Utah). The cells were transfected with 1 μg (6-well dishes) or 0.6 μg (24-well dishes) of plasmid DNA at approximately 30 to 40% confluency for immunocytochemical analysis and luciferase assays, respectively. The cells destined for immunocytochemical analysis were cotransfected with 0.1 μg of pEGFP-N1 (Clontech, Palo Alto, Calif.) as a transfection control. Transfections were performed with the Effectene transfection reagents under conditions recommended by the manufacturer (Qiagen, Valencia, Calif.). Luciferase assays were performed with the Luciferase Assay Kit (Promega, Madison, Wis.). Transfections were performed in triplicate at least three times, and the results of representative experiments are presented. The natural ligands for LasR and RhlR (PAI-1 and PAI-2; Fig. 1C) were purified from bacterial cultures as described previously (15) and were a kind gift of Barbara Iglewski and Luciano Passador (University of Rochester, Rochester, N.Y.). A derivative of PAI-1 (N-dodecanoyl homoserine lactone) that lacks the oxygen attached to position 3 on the acyl side chain of PAI-1 was obtained from Sigma (St. Louis, Mo.) and will be referred to as PAI-1*. This molecule has previously been shown to be an efficient ligand for LasR (1, 14) and functioned similarly to PAI-1 in all of our assays, except that higher concentrations of PAI-1* were required. Autoinducers were prepared for addition to culture media as described previously (14). Briefly, the amount of autoinducer required to give the final concentrations indicated in each figure was first dissolved in ethyl acetate supplemented with 0.001% glacial acetic acid. The ethyl acetate was evaporated under a stream of nitrogen gas, and autoinducers were dissolved in the culture medium described above immediately prior to use. Autoinducers were added to culture medium 4 h after transfection, and control cells were cultured in the presence of the vehicle alone.
Immunocytochemical analysis.
Cells were plated on coverslips in six-well dishes and transfected as described above. The coverslips were collected 24 h after transfection, and the cells were washed three times in phosphate-buffered saline (PBS) and fixed in 3.9% formaldehyde in PBS for 30 min. The cells were washed three more times with PBS and then incubated with a 1:250 dilution of the M2 anti-FLAG monoclonal antibody (Sigma) for 1 to 3 h. After washing in PBS-0.1% Tween 20, the cells were incubated with fluorescently labeled secondary antiserum (AlexaFluor 594 donkey anti-mouse immunoglobulin G [Molecular Probes, Eugene, Oreg.]) for 1 h, followed by three washes in PBS-0.1% Tween 20. The cells were observed by epifluorescence microscopy with an Axiovert 135 TV microscope (Zeiss, Göttingen, Germany). Anti-Flag immunoreactivity and green fluorescent protein were observed with standard Texas red and fluorescein filters (Zeiss). Images were captured with a Kodak DC290 zoom camera and analyzed with MetaMorph 6.0 (Universal Imaging Corporation, Downingtown, Pa.).
RESULTS AND DISCUSSION
Construction and expression of chimeric eukaryotic-prokaryotic transcriptional activator proteins.
The LuxR family of proteins consists of more than 15 proteins that function as ligand-activated transcription factors in QS systems of multiple bacterial species (24). On the basis of sequence comparisons among family members, LuxR-type proteins possess two distinct functional domains, a carboxy-terminal HTH DNA-binding domain related to HTH domains found in a subgroup of two-component signal transduction effector proteins characterized by Escherichia coli NarL (7, 25) and an amino-terminal autoinducer-binding domain (AIBD) (Fig. 1A). These proteins recognize 18- to 20-nucleotide palindromic recognition sequences within the promoters of target genes, the prototype of which is the Lux box recognized by the founding member of the family, the LuxR protein from Vibrio fischeri (24). Functional and structural studies indicate that LuxR-type proteins function primarily as dimers (30, 35), and analysis of the LasR polypeptide identified two distinct regions of the protein that are necessary for dimerization (9). One region was located within the HTH domain, and the second was distributed throughout the N-terminal region (9).
Our initial goal was to generate expression plasmids for LasR- and RhlR-based proteins that can function as transcriptional activators in eukaryotic cells. To do this, we included functional domains from eukaryotic proteins that would permit the bacterial proteins to enter into the nuclei of eukaryotic cells and interact with the transcriptional apparatus. These chimeric proteins contained either the full-length LasR or RhlR coding sequence or the isolated C-terminal HTH domain along with the NLS from the SV40 T antigen and/or one to three copies of the potent TAD from herpes simplex virus protein VP16 (Fig. 1A).
Each chimeric protein contained an N-terminal FLAG epitope tag for immunological detection, and we first performed immunocytochemical analysis on transfected COS-1 cells to examine the subcellular location of each protein. Initial experiments were performed with the truncated proteins containing the LasR HTH domain to assess the functionality of the NLS in these chimeric proteins. As shown in Fig. 2, a chimeric protein lacking the NLS (T-LasR HTH) was diffusely localized throughout the transfected cells, while the NLS-containing protein (TN-LasR HTH) was efficiently localized to the nucleus.
FIG. 2.
The NLS is required for nuclear accumulation of LasR-based proteins. The T-LasR HTH and TN-LasR HTH chimeric proteins were expressed in COS-1 cells and detected by immunocytochemical staining with an antiserum that recognized the FLAG epitope tag on each protein. The cells were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) to permit visualization of the nucleus. T-LasR HTH-specific staining was visualized throughout the cell, and the TN-LasR HTH protein was efficiently localized to the nucleus. Individual cells are indicated by arrowheads to aid in the alignment of the two images. FITC, fluorescein isothiocyanate.
We next examined the subcellular location of fusion proteins containing the full-length LasR coding sequence in the absence and presence of autoinducer. A chimeric protein containing three copies of the VP16 TAD and the SV40 NLS (T3N-LasR) was used for this experiment. Overall, the number of cells expressing the chimeric full-length LasR protein was lower than that observed previously for the truncated protein; however, the lack of signal was not due to inefficient cell transfection, as similar numbers of cells expressed a green fluorescent protein from a second plasmid that was included as a transfection control in both experiments (data not shown). We were unable to detect any immunofluorescence in the nuclei of transfected cells in the absence of autoinducer; instead, a small number of cells exhibited significant signal in vesicular structures in the cytoplasm (a cluster of three cells is shown in Fig. 3A). To test whether the lack of nuclear localization of this chimeric protein was due to the absence of autoinducer, separate transfections were performed in which increasing concentrations of PAI-1 or its synthetic analog PAI-1* (see Materials and Methods) (Fig. 1C) were added to the cell culture 4 h after addition of DNA. PAI-1* is a commercially available derivative of PAI-1 that lacks the oxygen attached to the third carbon of the acyl side chain. Previous studies indicated that this derivative functions as a ligand for LasR, albeit with slightly lower affinity (14). As PAI-1* is more readily available, it was used in these immunofluorescence experiments. Cytoplasmic staining was observed at lower concentrations of PAI-1*; however, the fusion protein was entirely localized to the nucleus in cells exposed to a higher concentration of PAI-1* (compare the 250 and 1,000 μM parts of Fig. 3A).
FIG. 3.
Autoinducer-dependent nuclear localization of full-length LasR and RhlR proteins. (A) The T3N-LasR protein was expressed in COS-1 cells either in the absence or in the presence of different concentrations of PAI-1 (or PAI-1* in certain experiments). FLAG immunoreactivity is shown in the large images, and DAPI staining is shown in the insets to identify nuclei. T3N-LasR was detected in cytoplasmic vesicular structures when autoinducer was absent and was also predominantly localized to the cytoplasm in cells cultured in the presence of 250 μM PAI-1*, although some nuclear staining was apparent. However, the fusion protein was essentially entirely localized to the nucleus in cells cultured in 1,000 μM PAI-1*. Qualitatively similar results were obtained with both the natural LasR ligand (PAI-1) and the synthetic analog (PAI-1*) (data not shown). (B) A similar set of experiments was performed to examine the effect of PAI-2 on the subcellular location of T3N-RhlR. Similar to LasR, the recombinant RhlR protein was detected in cytoplasmic vesicles in the absence of autoinducer but became localized to the nucleus in the presence of increasing concentrations of PAI-2. (C) Immunostaining of a truncated RhlR-based protein (T3N-RhlR HTH) demonstrating nuclear localization of this protein.
A similar set of experiments was performed to examine the subcellular distribution of RhlR-based chimeric proteins in the presence or absence of its cognate autoinducer, PAI-2 (Fig. 1C). As with LasR, a chimeric protein containing only the HTH domain of RhlR (T3N-RhlR HTH) was exclusively localized to the nuclei of transfected cells (Fig. 3C). Conversely, a fusion protein containing the full-length RhlR coding region (T3N-RhlR) was excluded from the nucleus of transfected cells cultured in the absence of PAI-2 and was detected in the cytoplasm in a pattern similar to that of LasR (Fig. 3B). Nuclear accumulation of T3N-RhlR was induced upon addition of PAI-2, and the chimeric protein was entirely localized to the nuclei of cells cultured in 100 μM PAI-2. The effectiveness of smaller amounts of PAI-2 versus PAI-1* in this assay is probably a reflection of the lower hydrophobicity of PAI-2 due to its shorter acyl side chain. This feature would likely permit the more efficient transit of PAI-2 across the COS-1 cell membrane. In addition, as mentioned above, the synthetic analog of PAI-1 (PAI-1*) is a slightly less potent ligand for LasR than is the natural compound in bacterial assays (14). Taken together, these data suggest that autoinducer binding to the AIBD is required for synthesis of functional LasR and RhlR proteins in eukaryotic cells. The exclusion from the nucleus of the LasR and RhlR proteins synthesized in the absence of autoinducer may indicate that autoinducer binding is needed for proper protein folding and functionality of the attached NLS. Previous studies on the TraR protein from Agrobacterium tumefaciens indicated that autoinducer binding was required for stabilization of the nascent polypeptide, which was rapidly degraded by an unidentified protease in the absence of autoinducer (35). In addition, several LuxR-type proteins form insoluble aggregates when overexpressed in E. coli (24). Further studies are required to elucidate the reason for the cytoplasmic localization of LasR in the absence of autoinducer.
Transcriptional activity of LasR-based fusion proteins.
To assess the functionality of LasR-based fusion proteins, we constructed a luciferase reporter plasmid containing two copies of a consensus palindromic LasR recognition sequence (Fig. 1B) upstream of the proximal promoter region from the serum albumin gene [(LasBOX)2-Luc]. As mentioned above, LuxR-type proteins bind DNA as dimers (or possibly higher-order multimers) and the DNA-binding activity and at least one dimerization interface have been localized to the C-terminal HTH domain of LasR (9). Therefore, we first performed transcription assays with fusion proteins containing only the LasR HTH, hypothesizing that these proteins should be capable of functioning as autoinducer-independent, constitutive DNA-binding proteins. These experiments addressed the functional requirements for the attached eukaryotic domains (NLS and TAD). Proteins containing the LasR HTH, either alone or combined with the NLS or the TAD, were relatively inactive in these transcription assays, with luciferase activities in cells expressing these proteins only slightly above the background level (Fig. 4). However, a protein containing both the NLS and the TAD (TN-LasR HTH) exhibited significantly elevated activity, and the inclusion of additional copies of the TAD resulted in increased transcriptional activity proportional to the number of TAD modules. To examine the specificity of this response, additional transfections were performed with a reporter plasmid carrying two copies of a mutated version of the LasBOX [(LasBOXm)2-Luc]. Cotransfection with the T3N-LasR HTH resulted in approximately twofold higher luciferase activity from the mutant reporter plasmid, while this protein was capable of increasing luciferase activity 20-fold on the reporter containing the wild-type LasBOX sequences (Fig. 4). Thus, the LasR HTH was capable of functionally interacting with its cognate DNA recognition sequence in eukaryotic cells. Although it is not conclusively demonstrated by this experiment, these data suggest that the protein-protein interface identified in the HTH domain of LasR is sufficient for the formation of functional LasR homodimers.
FIG. 4.
Transcriptional activity of LasR HTH-based chimeric proteins. Expression plasmids encoding the indicated chimeric proteins were cotransfected with (LasBOX)2-Luc (shaded bars) or (LasBOXm)2-Luc (black bars) into COS-1 cells, and luciferase activities were measured. Each experiment was performed in triplicate at least three times, and the results of a representative experiment are shown. The activity of each protein is shown relative to the activity of the reporter plasmid in the absence of any activator protein (Control). The presence of both an NLS and at least one TAD was necessary to observe transcriptional activity significantly above the background.
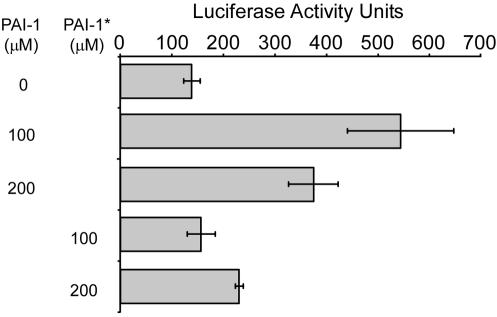
Next, we examined the transcriptional activity of fusion proteins containing the full-length LasR coding region (Fig. 5). For this experiment, we used a fusion protein (T3N-LasR) containing the SV40 NLS and three copies of the VP16 TAD to maximize its transcriptional potency and tested its activity in the absence or presence of either its cognate autoinducer (PAI-1*) or the RhlR autoinducer (PAI-2) to assess the specificity of ligand-dependent activation. The full-length protein was essentially inactive in the absence of either autoinducer. However, the inclusion of increasing amounts of PAI-1* resulted in a dose-dependent increase (above 250 μM) in transcriptional activity. The ligand-dependent activation of T3N-LasR was not observed when similar concentrations of PAI-2 were added to the medium (Fig. 5) or in the presence of the vehicle alone (data not shown). Luciferase activities from the wild-type reporter plasmid [(LasBOX)2-Luc] were unaffected by autoinducer in cells transfected with either the reporter plasmid alone or LasR HTH-based plasmids (data not shown). Furthermore, T3N-LasR was essentially inactive on the (LasBOXm)2-Luc reporter either in the absence or in the presence of PAI-1*. Thus, the specific response of T3N-LasR to PAI-1* is likely to be due to direct ligand binding leading to LasR activation.
FIG. 5.
Autoinducer-specific activation of LasR-based chimeric proteins. An expression plasmid encoding T3N-LasR was cotransfected with (LasBOX)2-Luc (shaded bars) or (LasBOXm)2-Luc (black bars) into COS-1 cells. The cells were incubated in the absence or presence of increasing concentrations of either PAI-1* or PAI-2, and luciferase activities were measured. The data are presented as fold activation relative to the activity of T3N-LasR in the absence of autoinducer. The activity of T3N-LasR increased significantly in a dose-dependent manner in cells cultured in the presence of PAI-1* but was unaffected by PAI-2. Each experiment was performed in triplicate at least three times, and the results of a representative experiment are shown.
Previous studies indicated that the effects of PAI-1 on gene expression in mammalian cells were apparent at an autoinducer concentration of 100 μM (19, 21). Therefore, we hypothesized that the requirement for higher concentrations of the synthetic PAI-1* compound was due to the lower potency of this molecule compared to that of the natural autoinducer (14). To test this idea, additional transfections were performed to directly compare the abilities of PAI-1 and PAI-1* to activate T3N-LasR at lower concentrations. As shown in Fig. 6, addition of 100 μM PAI-1 to the medium increased luciferase activities approximately four- to fivefold over the activities observed in the absence of autoinducer. Consistent with our earlier data, PAI-1* had no significant effect at this lower concentration.
FIG. 6.
Comparison of natural and synthetic ligands for LasR. The effect of lower concentrations of either PAI-1 or PAI-1* on the activity of T3N-LasR was examined in transient transfection assays with COS-1 cells. The activity of the recombinant protein was increased approximately fourfold in cells incubated in the presence of 100 μM PAI-1 (the concentration at which PAI-1 has been reported to influence gene expression in mammalian cells), whereas PAI-1* was ineffective at this concentration.
In summary, these data show that the two major autoinducer molecules from the human pathogen P. aeruginosa can enter and function in mammalian cells. The concentration of PAI-1 required for efficient activation of the chimeric sensor proteins described above was similar to that previously shown to activate cellular genes such as that for cyclooxygenase 2 in human fibroblasts and epithelial cells (19, 21). These data therefore support the hypothesis that the effects of autoinducers on the transcription of genes in host cells may result from specific interactions of these bacterial proteins with host proteins, a phenomenon we refer to as global sensing. In addition, we showed that the autoinducers retained their inherent specificity for their cognate receptor proteins in mammalian cells. Thus, the selective entry of autoinducers of different chemical structures may represent an additional level of complexity in the putative interactions between bacteria and their eukaryotic hosts.
Although specific mammalian receptor proteins for bacterial autoinducers remain to be identified, some clues as to their possible structure have emerged from the recent elucidation of the crystal structure of the TraR protein (30). These studies revealed that the N-terminal region of TraR that encompasses the AIBD resembled a GAF or PAS domain, which is a small-molecule-sensing domain found in a large number of mammalian signaling proteins and transcription factors (6). It is therefore possible that GAF or PAS domain-containing proteins, such as the aryl hydrocarbon receptor and hypoxia-inducible factor transcription factors (6) or mammalian phosphodiesterases (22), could serve as intracellular receptors for autoinducers. Further studies to identify additional target genes for autoinducers in mammalian cells are under way to test the feasibility of this concept.
Several recent studies have begun to elucidate the molecular mechanisms that control activation of LuxR-type proteins by autoinducer binding (see, for example, references 9 and 30). By testing the activity and regulation of LasR and RhlR in mammalian cells, we were able to examine the contributions of individual domains to their function in the absence of potential confounding interactions with other bacterial proteins. First, our data suggest that the C-terminal HTH domain of LasR is sufficient to permit efficient dimerization of LasR-derived chimeric proteins, consistent with earlier studies with bacteria (9). Second, we showed that the AIBD of either LasR or RhlR functions as a potent inhibitory domain in mammalian cells, and inclusion of these domains resulted in the localization of chimeric proteins to cytoplasmic vesicular structures. The identity of these structures is not clear, but they may be lysosomes or their precursors. Previous studies with bacteria suggested that efficient and correct folding of TraR requires the coordinated binding of autoinducer to the AIBD during polypeptide synthesis and that this protein was improperly folded and unstable when synthesized in the absence of autoinducer (35). Although this mechanism has not been demonstrated for all LuxR-type proteins, we speculate that the cytoplasmic staining of LasR- and RhlR-based proteins in the absence of autoinducer represents unfolded protein or protein aggregates that accumulate in the cell. Eukaryotic cells possess several quality control systems that recognize and dispose of improperly folded proteins, often culminating in ubiquitylation and degradation in the proteasome (3). By extension, therefore, we propose that proteins synthesized in cells exposed to autoinducer fold correctly and can be transported to the nucleus, where they function as transcription factors. This mammalian expression system should permit the rapid fine mapping of critical residues within specific domains of these proteins that mediate functions such as dimerization and ligand binding and to elucidate the mechanisms by which these domains control the functions of these proteins. In addition, this system will allow direct comparisons between different LuxR-type proteins to identify common and/or unique properties of each protein. For example, the RhlR-based proteins described here did not function as ligand-inducible transcription factors in cells treated with PAI-2 (data not shown). Previous data indicate that RhlR forms both homodimers and heterodimers with other proteins such as QscR (also known as PhzR), another LuxR protein from P. aeruginosa (10, 11, 31). Furthermore, RhlR homodimers bind to DNA in the absence of autoinducer and function as transcriptional repressors (11); however, these homodimers dissociate in the presence of PAI-2, perhaps permitting the released monomers to form heterodimers that can function as transcriptional activators. Therefore, it is likely that further examination of the intrinsic activity of RhlR in mammalian cells in the absence and presence of autoinducer and of potential heterodimerization partners should reveal important insights into the unique functions of this LuxR homologue.
During the performance of the experiments described here, Neddermann and colleagues (13) reported that a modified version of the TraR protein from Agrobacterium tumefaciens can also function as an autoinducer-regulated transcription factor in mammalian cells. These findings therefore provide further confirmation that bacterial autoinducers can enter mammalian cells. These authors also suggested that this system could potentially be used as an alternative or complement to the widely used tetracycline-inducible system (5) to control protein expression in mammalian cells. In addition, we have found that attachment of the AIBD from LasR to heterologous proteins can confer ligand inducibility on the resulting chimeric proteins (EKP, KPR, and SCW; unpublished observations). Thus, this strategy, which is similar to one previously developed with the ligand-binding domain of the estrogen receptor alpha protein (29), may also be used to provide tight control of protein activity in mammalian cells. Regulatory proteins from QS systems of different bacteria may therefore have important and powerful biotechnological applications, which will undoubtedly be enhanced by detailed analysis of the functions of these proteins in mammalian cells.
Acknowledgments
We thank Brandt Schneider and Curt Pfarr for comments on the manuscript and Dan Webster for assistance with microscopic techniques. We gratefully acknowledge the gift of purified PAI-1 and PAI-2 from Barbara Iglewski and Luciano Passador (University of Rochester Medical Center).
REFERENCES
- 1.Chhabra, S. R., C. Harty, D. S. Hooi, M. Daykin, P. Williams, G. Telford, D. I. Pritchard, and B. W. Bycroft. 2003. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J. Med. Chem. 46:97-104. [DOI] [PubMed] [Google Scholar]
- 2.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 3.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell. Biol. 4:181-191. [DOI] [PubMed] [Google Scholar]
- 4.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu, Y.-Z., J. B. Hogenesch, and C. A. Bradfield. 2000. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40:519-561. [DOI] [PubMed] [Google Scholar]
- 7.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 8.Kalderon, D., B. L. Roberts, W. D. Richardson, and A. E. Smith. 1984. A short amino acid sequence able to specify nuclear location. Cell 39:499-509. [DOI] [PubMed] [Google Scholar]
- 9.Kiratisin, P., K. D. Tucker, and L. Passador. 2002. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J. Bacteriol. 184:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledgham, F., I. Ventre, C. Soscia, M. Foglino, J. N. Sturgis, and A. Lazdunski. 2003. Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol. Microbiol. 48:199-210. [DOI] [PubMed] [Google Scholar]
- 11.Medina, G., K. Juarez, B. Valderrama, and G. Soberon-Chavez. 2003. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol. 185:5976-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 13.Neddermann, P., C. Gargioli, E. Muraglia, S. Sambucini, F. Bonelli, R. De Francesco, and R. Cortese. 2003. A novel, inducible, eukaryotic gene expression system based on the quorum-sensing transcription factor TraR. EMBO Rep. 4:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passador, L., K. D. Tucker, K. R. Guertin, M. P. Journet, A. S. Kende, and B. H. Iglewski. 1996. Functional analysis of the Pseudomonas aeruginosa autoinducer PAI. J. Bacteriol. 178:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson, J., K. Gray, L. Passador, K. Tucker, A. Eberhard, B. Iglewski, and E. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen, T. B., M. Manefield, J. B. Andersen, L. Eberl, U. Anthoni, C. Christophersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2000. How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology 146 Pt. 12:3237-3244. [DOI] [PubMed] [Google Scholar]
- 18.Saleh, A., C. Figarella, W. Kammouni, S. Marchand-Pinatel, A. Lazdunski, A. Tubul, P. Brun, and M. D. Merten. 1999. Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect. Immun. 67:5076-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J. Immunology 167:366-374. [DOI] [PubMed] [Google Scholar]
- 20.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 21.Smith, R. S., R. Kelly, B. H. Iglewski, and R. P. Phipps. 2002. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169:2636-2642. [DOI] [PubMed] [Google Scholar]
- 22.Soderling, S. H., and J. A. Beavo. 2000. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 12:174-179. [DOI] [PubMed] [Google Scholar]
- 23.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens, A. M., and E. P. Greenberg. 1999. Transcriptional activation by LuxR, p. 231-242. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 25.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 26.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateda, K., Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro, and K. Yamaguchi. 2003. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umek, R. M., A. D. Friedman, and S. L. McKnight. 1991. CCAAT-enhancer binding protein: a component of a differentiation switch. Science 251:288-292. [DOI] [PubMed] [Google Scholar]
- 30.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. D. Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventre, I., F. Ledgham, V. Prima, A. Lazdunski, M. Foglino, and J. N. Sturgis. 2003. Dimerization of the quorum sensing regulator RhlR: development of a method using EGFP fluorescence anisotropy. Mol. Microbiol. 48:187-198. [DOI] [PubMed] [Google Scholar]
- 32.Whiteley, M., and E. P. Greenberg. 2001. Promoter specificity elements in Pseudomonas aeruginosa quorum-sensing-controlled genes. J. Bacteriol. 183:5529-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams, S. C., M. Baer, A. J. Dillner, and P. F. Johnson. 1995. CRP2 (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA-binding activity and cell specificity. EMBO J. 14:3170-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, S. C., C. A. Cantwell, and P. F. Johnson. 1991. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 5:1553-1567. [DOI] [PubMed] [Google Scholar]
- 35.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 98:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]