Abstract
Objective
To examine changes in skinfolds in late gestation in healthy women.
Design
39 women had skinfold measures performed at 30.8 (mean) and 37.7weeks gestation. Fat mass (kg), sum of 3 skinfolds were calculated.
Results
21 women had a decrease in skinfold thickness (−3.1 ± 2.1mm) in late gestation, while 18 women had an increase (4.3 ± 3.2mm), p<0.001. The group of women who lost body fat (decrease skinfold thickness) had a trend towards greater pregravid body-mass-index (BMI, 25 vs. 22kg/m2, p=0.06), and gained less weight in late gestation (3.0 vs. 4.3 kg, p=0.042). On multiple regression, maternal age, and gestational weight gain were positively correlated with fat mass accrual, whereas pregravid BMI and dietary fibre were negative determinants of late gestational fat mass.
Conclusion
Increases in maternal fat mass in late gestation were related to maternal age and gestational weight gain while decreases were related to increased pregravid BMI and dietary fibre.
Keywords: dietary fibre, fat mass, late gestation, skinfold thickness
Introduction
Studies of changes in maternal body composition during a healthy normal pregnancy reveal that maternal body fat, as measured by skinfold thickness, accumulates through the second trimester.1,2 However in the third trimester, maternal skinfolds may decrease,1–3 indicating an increased utilization of maternal adipose tissue for maternal energy requirements and fetal growth. It is notable that these studies of maternal skinfolds in late pregnancy were performed prior to the recent increases in maternal obesity witnessed since the 1990s.4 Indeed the most recent of the above studies of maternal skinfold measures (performed in the 1980s),3 noted an increase in maternal adiposity compared to the initial study by Taggart et al.1 Studies assessing skinfold thickness in late pregnancy since the 1990s have focused on particular groups, such as adolescent mothers5 or high risk ethnic groups.6
Therefore, we reviewed our previously prospectively collected metabolic perinatal data for information which might allow us to address this question. Hence, the aim of the current study was to examine the changes in skinfolds in late gestation in healthy normal women and their relationship to various maternal metabolic parameters and fetal growth.
Materials/participants and Methods
This study was conducted in the Clinical Research Unit of the CTSC at MetroHealth Medical Center, Case Western Reserve University, Cleveland, USA from 1991 to 1994. The study protocol was approved by the hospital institutional review board, and written informed consent was obtained for each woman.
Forty-one pregnant women were recruited at 29–32 weeks’ gestation for their baseline visit through advertisements in the hospital. All women were healthy non-smokers and all except one (97%) were of white ethnic background (one woman was African American). All women had normal glucose tolerance as either a normal 50-g 1-hour glucose screening test and/or 3-hour oral glucose tolerance test for gestational diabetes according to the Carpenter and Coustan criteria.7 All pregnancies were singletons. The same 41 women returned for assessment at 34–40 weeks’ gestation, although data collection for skinfold measures was complete for n=39, thus only those participants with complete data are described here. Gestational age was determined by date of the last menstrual period confirmed by clinical assessment, and/or ultrasound before 22 weeks gestation in 31 women.
Data collected at the baseline visit in the Clinical Research Unit included: obstetric history, height, pregravid weight, paternal height and weight. Physical activity was quantified by using a validated activity questionnaire at baseline, presented as activity units/day.8 Nutritional intake was assessed at baseline using a self-reported five day food record. Subjects were instructed on how to keep and record details regarding their diet by a research nutritionist. The women were given no specific dietary instruction and did not control for total calories intake or for carbohydrates Each record was reviewed for completeness and then entered into nutrition software (Nutritionist 5, version 2.2; First DataBank Inc, San Bruno, CA). Dietary intake analyses determined estimations on calories, components, and percentage of macronutrients.
The following were collected at both study visits: maternal weight, estimated gestational age and assessment of subcutaneous adipose tissue by skinfold measurements at seven sites. Subcutaneous fat deposits were measured at seven different sites on the left side of the subject using Harpenden skinfold calipers (British Indicators, Sussex, England). The sites were triceps, biceps, subscapula, suprailiac, costal, thigh and suprapatellar. The triceps skinfold was measured in the midline of the posterior portion of the arm at the midpoint between the lateral projection of the acromion process and olecranon. The biceps skinfold was measured on the anterior aspect of the arm, midway between the acromion process and the antecubital fossa as determined by tape measure. The subscapular skinfold was measured at a 45 degree angle at a site below the inferior angle of the scapula. The suprailiac skinfold was measured at the midpoint between the anterior superior iliac spine and the lowest rib, as measured by a tape measure. The costal skinfold was measured at the midaxillary line at the level of the lowest rib. The thigh skinfold measurement was located in the midline of the anterior thigh at the midpoint between the inguinal crease and proximal border of the patella. The suprapatellar skinfold measurement was taken in the midsagittal plane on the anterior aspect of the thigh, above the patella on the proximal edge. Each measurement was obtained and recorded in triplicate, and the average was reported.9 Skinfold measurements were reproducible, with a linear correlation between repeated measures of r=0.99 (p<0.001) for triceps and r=0.98 (p<0.001) for subscapular.10 All skinfold measurements were performed by 1 examiner (NMR), trained in performing the measurements.
Fat mass (kg) was calculated using maternal weight, triceps, subscapula and suprailiac skinfold measurements according to the formula derived by Huston Presley et al10 (in healthy pregnant women at 30 weeks gestation) as follows:
Percent of body fat was calculated as fat mass divided by weight, expressed as a percentage. Sum of three skinfold measurements was calculated as triceps + subscapula + suprailiac. Change in sum of 3 skinfolds, fat mass and % body fat in late gestation were calculated as “value at second visit − value at baseline”. Groups were categorized according to loss (−FM) or gain (+FM) of sum of 3 skinfolds. These 3 skinfold measures were used as they were used to estimate fat mass (according to the above formula) and they are also the most frequently performed skinfold measures. The 7 skinfold measures were performed in order to have a broader estimate of fat distribution and change. Birth weight percentile for gestational age, gender and race was calculated using a United States national reference for the n=38 women with available birth weight data.11
Statistical Analysis
Data analysis was performed using STATA v10.0 (Stata Corporation, TX, USA). Data are presented as mean (standard deviation). Chi-square tests (categorical variables) performed to determine differences between variables; and unpaired two-sided t-tests were used to determine differences for normally distributed continuous variables. Mann-Whitney U test was used to determine differences for non normally distributed data such as physical activity and total dietary calories. Pairwise correlations were assessed using Pearson’s product-moment correlation coefficient, between variables in Table 1 and late gestational change in fat mass. Established risk factors and variables identified in univariate analyses (p value less than 0.10) were then included in backward stepwise multiple regression analysis of late gestational change in fat mass and of birth weight percentile. Statistical significance was accepted at p<0.05.
Table 1.
Participant Characteristics.
| −FM: Decrease in Sum of 3 Skinfolds, n=211 | +FM: Increase in Sum of 3 Skinfolds, n=181 | p value | |
|---|---|---|---|
| Age (years) | 30.8 ± 3.7 | 31.4 ± 3.4 | 0.586 |
| Pregravid weight (kg) | 69.1 ± 18.9 | 61.2 ± 8.3 | 0.109 |
| Pregravid BMI (kg/m2) | 24.9 ± 6.0 | 21.9 ± 3.5 | 0.064 |
| Nulliparous | 10 (48%) | 5 (28%) | 0.204 |
| Glucose Screen (mg/dL) | 113 ± 20 | 101 ± 19 | 0.059 |
| Baseline Visit: | |||
| Est Gestational Age (wk) | 30.5 ± 0.9 | 31.1 ± 0.7 | 0.057 |
| Weight (kg) | 79.6 ± 19.1 | 73.2 ± 8.9 | 0.204 |
| Fat Mass (kg) | 26.4 ± 9.1 | 22.0 ± 5.9 | 0.086 |
| Body Fat (%) | 32.6 ± 4.5 | 29.6 ± 5.2 | 0.063 |
| Sum 3 Skinfolds (mm) | 52.6 ± 18.0 | 45.1 ± 15.9 | 0.180 |
| Physical Activity2 (unit/d) | 59 (33–187) | 101 (23–185) | 0.681 |
| Total calories2 (kcal) | 1983 (1824–2386) | 2027 (1831–2453) | 0.612 |
| Dietary Fibre (gm) | 19.3 ± 6.6 | 16.0 ± 4.1 | 0.071 |
| Second Study Visit: | |||
| Est Gestational Age (wk) | 38.0 ± 1.5 | 37.5 ± 1.2 | 0.304 |
| Weight (kg) | 82.6 ± 19.5 | 77.5 ± 9.2 | 0.320 |
| Fat Mass (kg) | 26.7 ± 9.0 | 24.4 ± 6.0 | 0.362 |
| Body Fat (%) | 31.8 ± 4.8 | 31.1 ± 5.1 | 0.659 |
| Sum 3 Skinfolds (mm) | 49.5 ± 17.2 | 49.4 ± 16.8 | 0.978 |
| Change3: | |||
| Δ Est Gestation Age(wk) | 7.4 ± 2.0 | 6.4 ± 1.4 | 0.094 |
| Δ Weight (kg) | 3.0 ± 1.6 | 4.3 ± 2.2 | 0.042 |
| Δ Fat Mass (kg) | 0.3 ± 1.6 | 2.4 ± 1.6 | 0.0002 |
| Δ Body Fat (%) | −0.8 ± 1.7 | 1.5 ± 1.7 | 0.0002 |
| Δ Sum 3 Skinfolds (mm) | −3.1 ± 2.1 | 4.3 ± 3.2 | <0.0001 |
| Δ Weight (kg)4 | 13.5 ± 6.2 | 16.3 ± 5.2 | 0.135 |
| Baby Weight (kg) | 3.575 ± 0.5 | 3.579 ± 0.4 | 0.980 |
| Birth Weight Percentile | 57 ± 29 | 61 ± 27 | 0.668 |
Data are mean ± SD or n(%) unless otherwise specified.
Data are median (interquartile range).
Change from baseline to second study visit.
Change from pre-pregnancy to second study visit
Est, estimated; Δ, change in.
Results
Gestational age at baseline was 30.8 ± 0.9 weeks (range 29 – 32 weeks) and at second study visit was 37.7 ± 1.4 (range 34 – 40 weeks), the difference in gestational age between visits was not significant between the 2 groups being 6.97 ± 1.8 (range 3 – 10) weeks (for all participants, n=39). Participant characteristics are presented in Table 1, stratified by category of late gestational change in skinfold thickness (sum of the three skinfold measurements used in the calculation of body fat: triceps, subscapula, suprailiac). There was a trend towards a greater pregravid BMI in those who lost fat (−FM) compared to those who gained fat (+FM) in late gestation (25 vs. 22kg/m2, p=0.06). The −FM group gained significantly less weight in late gestation than the +FM group (3.0 vs. 4.3 kg, p=0.042). Results were similar if stratified by gain or loss in sum of 7 skinfold thickness measures (data not shown).
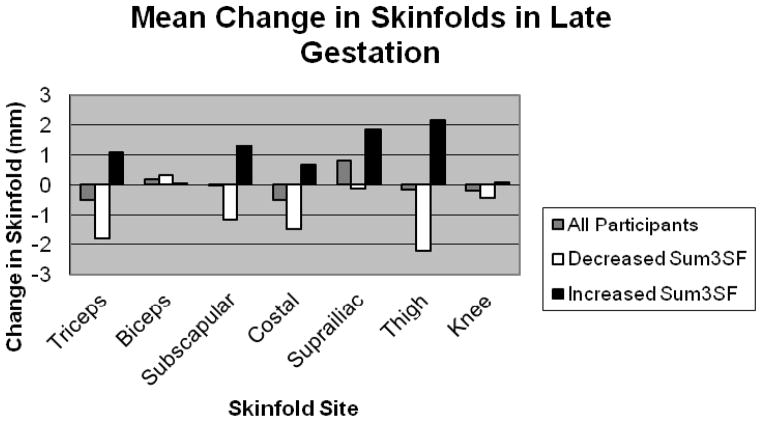
The change in skinfold thickness measurements at each of the seven sites for all participants is presented in Figure 1. For those who lost skinfold thickness in late gestation (-FM), greatest mean loss of a single skinfold was at thigh (mean, SD −2.2 ± 4.2mm), then triceps (−1.8 ± 2.5mm). For those who gained body fat (+FM), greatest gain in a single skinfold was at thigh (2.2 ± 2.9mm) then suprailiac (1.9±1.5mm). There were no differences between groups in change in the ratio of central to peripheral fat (no significant change in ratio of iliac:thigh, iliac:triceps, subscapula:thigh or subscapula:triceps ratio). The skinfold thickness measurements at each of the seven sites at baseline and visit 2 are presented in Supplementary Table S1.
Figure 1.
Change in skinfold thickness at each site in late gestation
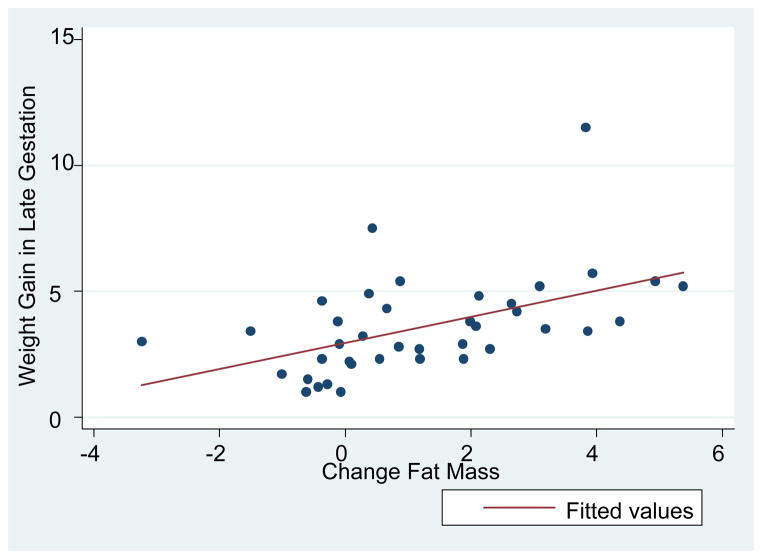
Significant pairwise correlations (Table S2) were seen with change in fat mass (calculated using the equation of Huston Presley et al)10 in late gestation and the following: pre-pregnancy weight (inverse), pregravid BMI (inverse), pregnancy weight gain, late gestational weight gain, change in sum of 7 skinfolds (in late gestation), change in percent body fat (in late gestation). There were also significant correlations for dietary fibre (inverse) with the following: pregravid weight, pregravid BMI, glucose value on gestational diabetes screen, pregnancy weight gain and late gestational weight gain. There were no significant correlations with physical activity, although a trend was seen for physical activity with both pregnancy weight gain (r=−0.321, p=0.057) and late gestational fat mass increase (r=−0.307, p=0.069). No significant correlations were seen with other dietary variables such as total calories or total fat (data not shown). Significant correlations were seen for birth weight percentile with: dietary fibre (inverse), weight gain in pregnancy, late gestational weight gain, and physical activity (inverse). The univariate correlation between change in late gestational fat mass (calculated using the equation of Huston Presley et al)10 and late gestational weight gain in all participants is depicted in Figure 2.
Figure 2.
Change in Fat Mass vs. Change in Weight in Late Gestation, r=0.50, p=0.001
On multiple regression analysis of late gestational increase in fat mass (Table 2a), maternal age and weight gain in pregnancy were independently associated with a positive increase in fat mass, while there were negative associations for pregravid BMI and dietary fibre. If late gestational weight gain was entered into the model in place of total pregnancy weight gain, results were similar, except that dietary fiber was no longer significant (β coefficient −0.091, 95%CI −0.193–0.011, p=0.071). If instead of pregravid BMI one adjusts for maternal fat mass at the time of the baseline measure the results are similar (Table 2b) with maternal age and weight gain in pregnancy independently associated with a positive increase in fat mass, while there were negative associations with baseline maternal fat mass and dietary fibre. On regression of birth weight percentile (adjusted for gestational age, gender and race), physical activity at baseline visit was the only significant predictor (beta coefficient, 95% CI: −10.98, −16.9 to −5.0, p=0.001), and explained 29.3% of the variance. Variables entered into the model (for birth weight percentile) were: age, pregravid BMI, weight gain in pregnancy, late gestational weight gain, dietary fibre and physical activity.
Table 2a.
Multiple Regression of Change in Body Fat Mass (kg) in Late Gestation
n=39, Model R2=49.5%
| Beta Coefficient (95% CI) | p | |
|---|---|---|
| Maternal Age | 0.186 (0.046, 0.326) | 0.011 |
| Pre-pregnancy BMI | −0.170 (−0.270, −0.069) | 0.002 |
| Weight Gain in Pregnancy | 0.092 (0.003, 0.181) | 0.043 |
| Dietary fibre | −0.128 (−0.226, −0.030) | 0.012 |
Variables entered into model: age, pre-pregnancy BMI, weight gain in pregnancy (from pre-pregnancy to second study visit), physical activity, dietary fibre, parity.
Table 2b.
Multiple Regression of Change in Body Fat Mass (kg) in Late Gestation
n=39, Model R2= 55.4%
| Beta Coefficient (95% CI) | p | |
|---|---|---|
| Maternal Age | 0.219 (0.089, 0.350) | 0.002 |
| Baseline Fat mass | −0.127 (−0.188, −0.066) | <0.001 |
| Weight Gain in Pregnancy | 0.159 (0.078, 0.242) | <0.001 |
| Dietary fibre | −0.126 (−0.216, −0.036) | 0.008 |
Variables entered into model: age, fat mass at baseline visit, pre-pregnancy BMI, weight gain in pregnancy (from pre-pregnancy to second study visit), physical activity, dietary fibre, parity.
Discussion
We have reported three key findings from this assessment of maternal body fat changes in late gestation. Firstly, there was a wide variation in skinfold thickness change in late gestation for these healthy pregnant women. This is in contrast to previous studies, which have reported a decrease or plateau in skinfold thickness measures at most sites in late gestation. Secondly, maternal fat accrual in late gestation was positively associated with maternal age and weight gain but negatively associated with pregravid BMI and dietary fibre. Finally, maternal fat mass change in late gestation was not associated with the baby’s birth weight percentile. Only dietary fibre, physical activity and weight gain (in pregnancy or during late gestation) were associated with birth weight percentile on univariate analysis and physical activity at 30 weeks gestation was the only variable independently associated with birth weight percentile on multiple regression analysis.
We have reported a wide variation in skinfold thickness change in late gestation. Both loss and gain of upper arm fat area were similarly seen in a multi-ethnic cohort from Camden, New Jersey.12 However earlier studies of skinfold thickness measures in late gestation observed the following pattern in skinfold changes during pregnancy: an increase to 27 weeks and then a decrease or plateau in skinfold thickness in late gestation.1,3 In their study of skinfold measures in 296 Australian women from 1981 to 1985, Ash et al3 noted that their initial skinfold thickness values (at 12 weeks gestation) were higher than those of the landmark study by Taggart (performed in the 1960s)1: 17.1, 8.4, 13.7 and 14.5mm for triceps, biceps, subscapular and suprailiac, compared to 15.7, 7.4, 11.8 and 7.9mm respectively. Skinfold thickness measures and maternal weight in the current study were higher than those of Taggart and closer to those of Ash et al. In particular, participants of the current study were not as lean as those of Taggart’s study1 of 84 Scottish women with mean weight 58.8kg at week 10, 66.5kg at week 30, consistent with the increase in obesity observed since the 1990s. Mean pregravid weight was lower in Ash et al (56.9kg) than in the current study, but weight gain in pregnancy was similar to that which we observed (14.2kg). Mean values for skinfold thicknesses at baseline in our study were very similar to those of Ash et al3 at 32 weeks for triceps and biceps (19.2, 9.3mm in Ash vs. 19.1, 9.6mm in our study), but values were lower at truncal sites in our study (subscapular, suprailiac: 18.5, 22.1mm in Ash vs. 15.9, 14.2 mm in our study).
Taggart reported variable patterns in skinfold changes at different sites from 30–38 weeks: an increase at mid-thigh, decrease at costal and triceps, with little change at the bicep, subscapular, suprailiac and knee skinfold measurements. The greatest gain at the thigh in our study is consistent with the findings of Taggart, who reported a gain in 1mm at the thigh, and a loss of 1mm at each of triceps and costal skinfolds. Villar et al6 similarly reported an increase in thigh skinfold thickness in late gestation (30–37 weeks), in a group of Guatemalan women. They also reported an increase in subscapular skinfold and decrease in both suprailiac and triceps skinfold in the same time period. Of note, the women in that study were leaner by weight and BMI criteria but had more central adiposity than those of Taggart’s study, evident by higher skinfold thicknesses at central sites (suprailiac and subscapular).
The inverse relationship between pregravid BMI and change in skinfolds (and change in fat mass) that we have described is consistent with findings of Taggart’s study. In that study, the increase in skinfolds during pregnancy was greater in underweight than in overweight women. Ehrenberg et al reported (in a different cohort from our institution) that lean women accrue more fat mass than obese women in pregnancy, regardless of glucose tolerance status,13 consistent with findings of a British study.14 Basal metabolic rate during gestation is greatly increased in obese compared to non-obese women, and maternal fat mass in late gestation is a strong predictor of basal metabolic rate.15 Thus it has been suggested that obese women appear to waste energy in pregnancy by increasing their basal metabolic rate, resulting in less fat gain than non-obese women.16 Adipokines such as leptin may play a role in the altered energy metabolism in obese pregnant women; further study of that relationship is required.16 It is possible that the decrease in maternal fat mass (in the heavier women) might also be explained by preferential shunting of fatty acids from the maternal to the fetal compartment, so that a greater proportion of birth weight of babies of the heavier women might consist of fat. Detailed study of the babies’ body composition was not available in the current study.
Our finding that fat accrual in late gestation was associated with pregnancy weight gain is consistent with the report of Hediger et al, that women who gained upper arm fat in late pregnancy had larger gestational weight gains.12 Fat mass gain throughout gestation has similarly been strongly correlated to larger gestational weight gain.17 Taggart described a greater increase in skinfolds in primiparous than multiparous women; however parity was not a significant determinant of late gestational change in fat mass in our study.
We did not find a significant relationship between change in maternal fat mass in late gestation and birth weight percentile. Loss of triceps skinfold thickness in late gestation was associated with higher birth weight in a multi-ethnic cohort from Camden, (although change in this study was assessed from 28 weeks gestation to the 4–6week postpartum visit), however when pregravid weight was low, loss of upper arm fat was associated with a lower birth weight.12 However, Villar et al6 reported that fat gain before the 30th week of gestation was an independent determinant of birth weight (with pre-pregnancy weight), rather than late gestational fat gain. The women in the later study were lean, with a lower first antenatal visit weight (50.5kg) than in the Taggart study.
Lifestyle factors (increased dietary fibre and physical activity) were important determinants of late gestational fat mass change and birth weight percentile in the current study. Dietary fibre showed significant inverse univariate associations with key variables such as glucose value at gestational screen, pregnancy weight gain and late gestational weight gain, and was a significant independent determinant of late gestational change in fat mass on regression analysis. Physical activity in late pregnancy was the only independent determinant of birth weight percentile (adjusted for gestational age, gender and race) on regression analysis. Thus these data strongly support the important role of lifestyle measures in pregnancy and the opportunity for future studies to address these potentially modifiable factors.
Limitations of the current study include small sample size, dietary content was assessed at baseline only, and that participants were volunteers. Participants were a relatively lean cohort for this US urban centre, possibly because lean women were more likely to volunteer for skinfold measures late in pregnancy. In addition, virtually all women in the current study were white and the greatest increase in maternal obesity in recent years in Cleveland was observed in African Americans.4 However the strengths of the study lie in the detailed clinical data, including seven skinfold measures at two time points in late gestation, estimates of dietary intake and physical activity, as well as standard clinical measures, collected prospectively in the era of increasing maternal obesity.
In conclusion, we have reported a varied change in skinfold measures in late gestation in healthy pregnant women from Cleveland, during the 1990s. Maternal age and weight gain in pregnancy were positively associated with maternal fat mass accrual while pregravid BMI and dietary fibre were negative independent determinants of change in fat mass in late gestation. Further study of maternal fat changes in late gestation in the current era of increasing maternal obesity may be warranted. Excessive gestational weight gain may result in weight retention post partum, with retention of adipose tissue a particular concern for future maternal metabolic risk.
Supplementary Material
Acknowledgments
The authors wish to thank participants and study staff of the Clinical Research Unit of the CTSC at MetroHealth Medical Centre, Cleveland. Financial support was provided by NICHD HD 22965-19 and Translational Science Awards Grant UL-1 RRO24989. LMB is supported by an Australian National Health and Medical Research Council Early Career Fellowship #605837.
Financial Support: NICHD HD 22965-19 and the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant number UL1TR000439. The CTSA program is led by the NIH’s National Center for Advancing Translational Sciences (NCATS).
Footnotes
Conflict of interest:
None of the authors have a conflict of interest.
References
- 1.Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21(2):439–51. doi: 10.1079/bjn19670045. [DOI] [PubMed] [Google Scholar]
- 2.Hytten FE. Weight gain in pregnancy. In: Hytten FE, Chamberlain G, editors. Clinical Physiology in Obstetrics. Blackwell Scientific Publishing; Oxford, U.K: 1980. pp. 193–233. [Google Scholar]
- 3.Ash S, Fisher CC, Truswell AS, Allen JR, Irwig L. Maternal weight gain, smoking and other factors in pregnancy as predictors of infant birth-weight in Sydney women. Aust N Z J Obstet Gynaecol. 1989;29(3 Pt 1):212–9. doi: 10.1111/j.1479-828x.1989.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Prevalence of maternal obesity in an urban center. Am J Obstet Gynecol. 2002;187(5):1189–93. doi: 10.1067/mob.2002.127125. [DOI] [PubMed] [Google Scholar]
- 5.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr. 1994;60(2):183–8. doi: 10.1093/ajcn/60.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Cogswell M, Kestler E, Castillo P, Menendez R, Repke JT. Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am J Obstet Gynecol. 1992;167(5):1344–52. doi: 10.1016/s0002-9378(11)91714-1. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31 (12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 9.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Illinois: Human Kinetics Books; 1988. [Google Scholar]
- 10.Huston Presley L, Wong WW, Roman NM, Amini SB, Catalano PM. Anthropometric estimation of maternal body composition in late gestation. Obstet Gynecol. 2000;96(1):33–7. doi: 10.1016/s0029-7844(00)00857-7. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hediger ML, Scholl TO, Schall JI, Healey MF, Fischer RL. Changes in maternal upper arm fat stores are predictors of variation in infant birth weight. J Nutr. 1994;124(1):24–30. doi: 10.1093/jn/124.1.24. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189(4):944–8. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 14.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84 (1):95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein MN, Mak RP, King JC. Unexpected relationship between fat mass and basal metabolic rate in pregnant women. Br J Nutr. 1996;75(5):659–68. doi: 10.1079/bjn19960171. [DOI] [PubMed] [Google Scholar]
- 16.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–91. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 17.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol. 1999;87(1):196–202. doi: 10.1152/jappl.1999.87.1.196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.