Abstract
Purpose
To investigate relationships between contrast sensitivity (CS), color vision, and retinal nerve fiber layer (RNFL) among people with human immunodeficiency virus (HIV) infection; to evaluate the effect of time since diagnosis of HIV infection on RNFL thickness.
Design
Noninterventional cross-sectional study.
Methods
We evaluated 102 eyes of 57 HIV-infected individuals without ocular opportunistic infections. Peripapillary RNFL thickness was determined with spectraldomain optical coherence tomography in 4 quadrants. CS was measured with the Pelli-Robson technique (expressed as logCS); color vision was measured with the Lanthony desaturated 15-hue technique (expressed as color confusion index [C-index], with higher scores indicating worse color vision). Correlations between values were assessed using Spearman correlation coefficients.
Results
Median RNFL thickness (average of 4 quadrants) was 102.9 μm (range, 75.0–134.7 μm). Median logCS was 1.90 (range, 1.25–1.95). Median C-index was 1.58 (range, 0.96–4.07). Temporal RNFL thickness was correlated with logCS (r = 0.295, P = .003) and C-index (r = −0.338, P = .0005). Time since diagnosis of HIV infection was shorter for those with thick average RNFL than for those with thin average RNFL (P = .18).
Conclusions
Both worse CS and worse color vision are correlated with thinning of the temporal RNFL, with possible threshold effects. Increased prevalences of abnormal CS and abnormal color vision in this population are therefore likely attributable to neuroretinal compromise. This pattern of structural and functional losses may reflect preferential damage to small-caliber axons in the maculopapillary bundle, possibly associated with mitochondrial dysfunction, providing a potential disease mechanism for HIV-associated “neuroretinal disorder.”
Subtle vision abnormalities (reduced contrast sensitivity [CS],1–4 altered color vision,2,3,5 visual field loss6–13), in the absence of ocular opportunistic infections, are more common in people with human immunodeficiency virus (HIV) disease than in the general population, even among those whose immune function has improved because of antiretroviral therapy.3,4 Changes in vision are thought to be caused by HIV-associated “neuroretinal disorder,” which is characterized by changes in the retinal nerve fiber layer (RNFL).14 Autopsy studies have revealed a 40% reduction of axons in the optic nerves of people with AIDS, with severe degeneration of the axons that remain, when compared to normal controls.15 Previous studies have suggested that host genetic factors and certain mitochondrial haplogroups may be associated with an increased risk of developing neuroretinal disorder.16,17 Proposed mechanisms for neuroretinal disorder include direct damage of neural tissue by HIV, collateral damage from the body's immunologic response to HIV infection, and cumulative damage to the retina and optic nerve from a long-standing microvasculopathy and associated hemorheologic abnormalities.3,4,17
Visual field loss has been associated with RNFL thinning in HIV-infected individuals, as measured by optical coherence tomography (OCT),12 but similar direct relationships have not yet been shown for other measures of visual function. In this study, we compared RNFL thickness to CS and color vision among HIV-infected individuals without clinically apparent retinal infections. We hypothesize that the increased prevalences of psychophysical losses seen in this population are attributable to a retinopathy that manifests as changes in the RNFL of susceptible individuals.
Methods
We recruited volunteer study participants from the Los Angeles County – University of Southern California (USC) Medical Center and from among individuals enrolled in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA)18 at the Jules Stein Eye Institute, University of California, Los Angeles (UCLA). Inclusion criteria were the following: HIV infection; at least 1 eye with best-corrected visual acuity (BCVA) of 20/25 or better; clear media; and no retinal, choroidal, or optic nerve lesions that could affect visual function, such as cytomegalovirus retinitis, other ocular opportunistic infections, neoplasia, glaucoma, or other maculopathies. All participants were evaluated during the period October 2009 through April 2011. Data relevant to the study for those participants enrolled in LSOCA were extracted from the LSOCA database. Data were collected from participants not involved in LSOCA by identical techniques.
The following demographic and medical data were collected from all subjects: age; sex; race/ethnicity; HIV risk factor (men who have sex with men [MSM], intravenous drug use [IDU], other); weight; interval since HIV diagnosis; interval since AIDS diagnosis; CD4+ T-lymphocyte count (nadir and most recent); CD8+ T-lymphocyte count (nadir and most recent); HIV RNA blood levels (maximum ever and most recent); and antiretroviral drug use. Participants were asked during the study visit to self-report presence or absence of the following comorbidities: hypertension; diabetes mellitus; cardiovascular disease (myocardial infarction, peripheral vascular disease, coronary artery disease); stroke; renal disease; and smoking history (current smoker, past smoker, never smoked). These comorbidities were confirmed by review of medical records and laboratory reports, when available.
The following ophthalmic data were collected for each eye of all study participants: BCVA, CS, color vision measurement, and intraocular pressure. All examinations for an individual study participant were performed on the same day. Data from both eyes of each participant were used for the analyses, unless there were ocular abnormalities that could affect visual function in 1 eye.
BCVA was measured by the Snellen method. CS was determined using the Pelli-Robson chart and technique.19 The log of the CS measurement (logCS) was calculated and used in analyses. It has been determined that different Pelli-Robson charts can result in different CS measurements (M.L. Van Natta, SOCA Coordinating Center, Johns Hopkins Bloomberg School of Public Health, personal communication, October 2009). To standardize CS scores between the 2 study sites, which used 2 different Pelli-Robson charts, a statistical correction factor was determined by external justification. Based on the results of 13 individuals who were tested with both charts, a correction factor of 3 (mean difference between charts for the external group) was added to the CS score for each study participant who was examined at USC. Individuals in the external group consistently saw more letters on the UCLA chart than on the USC chart, under identical lighting conditions. A logCS value of 1.5 or below was considered abnormal; the rationale for using this method and threshold is described in a previous publication.3
Color vision was determined using the Lanthony desaturated 15-hue color vision test.20 This test is more sensitive to subtle color discrimination deficiencies and is easier to administer and score than the Farnsworth-Munsell 100-hue test (FM-100).21 Feitosa-Santana and associates confirmed the validity of the technique as a reliable measure of subtle color vision losses, when compared to the Cambridge Colour Test, in a study of participants with type 2 diabetes mellitus who did not have clinically apparent retinopathy.22 Testing was performed in ambient light with test materials viewed under standard illuminant conditions. Participants used near vision correction, if needed, and were allowed 5 minutes to complete the test. Color confusion index (C-index), as described by Vingrys and King-Smith,23 was determined for each eye by inputting participant responses into a web-based application available at http://www.univie.ac.at/Vergl-Physiologie/colortest/colortestF-en.html. Higher values indicate worse color vision. The minimum possible score for C-index is 0.96. A C-index value of 1.78 or higher was considered abnormal, as described by Vingrys and King-Smith.23
RNFL thickness was obtained using the RTVue spectral-domain optical coherence tomographer (Optovue Inc, Fremont, California, USA). RTVue uses a scanning laser diode with a wavelength of 840 ± 10 nm to provide images of ocular microstructures. A peripapillary RNFL protocol was used to determine RNFL thickness in this study; this technique and normal values for average and quadrant-specific RNFL thickness (superior, temporal, inferior, nasal) are described in detail by Rao and associates.24
We compared logCS and C-index with average and quadrant-specific RNFL thickness for each eligible eye of each study participant. To evaluate for cumulative effects of chronic HIV infection on the RNFL, we compared time since diagnosis of HIV infection to average and temporal RNFL thickness. We separated study participants into 2 groups: those diagnosed with HIV infection less than vs greater than 180 months previously (the midpoint for the longest duration of any study participant). Both subgroups were evaluated for correlations between time since diagnosis of HIV infection and RNFL thickness. We also sought a relationship between temporal RNFL thickness and use of the following nucleoside reverse transcriptase antiviral drugs, which are known to have mitochondrial toxicity: zidovudine, stavudine, didanosine, zalcitabine.25
Statistical Analysis
Statistical analyses were performed using software SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). Spearman correlation coefficients were used to assess relationships between time since diagnosis of HIV infection, logCS, C-index, and RNFL thickness. The following assessments were repeated in multivariate analyses: 1) comparisons of logCS and of C-index between those with thin vs those with normal temporal RNFL thickness; and 2) correlations of logCS and of C-index with temporal RNFL thickness. Adjustment was performed using repeated-measures linear regression models with a compound symmetry covariance structure to account for correlations between the 2 eyes of same participants and with the inclusion of the following covariates to account for potential confounding effects: study sites; age; race/ethnicity (white vs nonwhite); duration of HIV infection; nadir CD4+ T-lymphocyte count; hypertension; and history of smoking (ever smoked vs never smoked). A P value less than .05 was considered to be statistically significant.
Results
We enrolled 57 study participants; 102 eyes met inclusion criteria. Eyes were excluded because of visually significant cataract (4 eyes), primary open-angle glaucoma (2 eyes), macular hole (2 eyes), amblyopia (1 eye), epiretinal membrane (1 eye), traumatic optic neuropathy (1 eye), and unexplained vision loss worse than 20/25 (1 eye).
Table 1 shows demographic, medical, and laboratory characteristics of all study participants. Median intraocular pressure was 14 mm Hg (range, 9–21 mm Hg; mean ± standard deviation [SD], 14.3 ± 2.93 mm Hg). No study participants included in the analysis had undergone previous cataract extraction. Table 2 shows visual function results for the 102 study eyes. There was substantial variation in measured values for both parameters, but the majority of individuals had values considered to be normal. Only 2.9% of eyes (3/102) had abnormal CS; 40.2% of eyes (41/102) had abnormal color vision, based on study definitions.
Table 1. Demographic, Medical, and Laboratory Factors for 57 HIV-Infected Study Participants.
| Characteristics | Summary Statistics |
|---|---|
| Study site (n [percentage])a | |
| USC | 21 (37%) |
| UCLA | 36 (63%) |
| Age (years) | |
| Mean ± SD | 51.9 ± 9.9 |
| Median (range) | 53 (27 to 72) |
| Male sex (n [percentage])a | 56 (98%) |
| Race/ethnicity (n [percentage])a | |
| White | 31 (54%) |
| Black | 8 (14%) |
| Hispanic | 17 (30%) |
| Asian | 1 (2%) |
| HIV risk factor, (n [percentage])a,b | |
| MSM only | 41 (75%) |
| IDU only | 4 (7%) |
| Both MSM and IDU | 1 (2%) |
| Heterosexual | 7 (12%) |
| IDU and heterosexual | 1 (2%) |
| Other | 1 (2%) |
| Time since diagnosis of HIV infection (months) | |
| Mean ± SD | 187.1 ± 91.7 |
| Median (range) | 194 (3 to 359) |
| Diagnosis of AIDS (n [percentage])a | 51 (89%) |
| Time since diagnosis of AIDS (months) | |
| Mean ± SD | 134.4 ± 67.7 |
| Median (range) | 149 (0 to 247) |
| Current CD4+ T-lymphocyte count (cells/μL) | |
| Mean ± SD | 473.9 ± 226.7 |
| Median (range) | 420 (80 to 1037) |
| Nadir CD4+ T-lymphocyte count (cells/μL) | |
| Mean ± SD | 118.2 ± 139.8 |
| Median (range) | 66 (0 to 602) |
| Current HIV blood level (RNA copies/μL) | |
| Mean ± SD | 6011 ± 27816 |
| Median (range) | 0 (0 to 188393) |
| Smoking history (n [percentage])a | |
| Current smoker | 11 (19%) |
| Former smoker | 1 (2%) |
| Never smoked | 45 (79%) |
| Comorbidities (n [percentage])a | |
| Hypertension | 23 (40%) |
| Diabetes mellitus | 7 (12%) |
| Cardiovascular disease | 2 (4%) |
| Cerebrovascular accident | 0 |
| Renal disease | 3 (5%) |
IDU = intravenous drug use; HIV = human immunodeficiency virus; MSM = men who have sex with men; SD = standard deviation.
Number of individuals with characteristic among 57 study participants evaluated, unless otherwise stated.
HIV risk factor known for 55 of 57 participants.
Table 2. Visual Function Among 102 Eyes of 57 HIV-Infected Study Participants.
| Visual Function | Summary Statistics |
|---|---|
| Visual acuitya (median [range]) | 20/20 (20/20 to 20/25) |
| Contrast sensitivityb (logCS) | |
| Mean ± SD | 1.85 ± 0.14 |
| Median (range) | 1.90 (1.25 to 1.95) |
| <1.5 (abnormal;c n [percentage] d) | 3 (2.9%) |
| Color visione(C-index) | |
| Mean ± SD | 1.74 ± 0.74 |
| Median (range) | 1.58 (0.96 to 4.07) |
| ≥1.78 (abnormal;f n [percentage] d) | 41 (40.2%) |
C-index = color confusion index; SD = standard deviation.
Snellen technique.
Determined using the Pelli-Robson technique.19
Based on criteria described by Shah and associates.3
Number of eyes (percentage of 102 eyes).
Determined using the Lanthony D-15 technique.20
Based on a cohort of HIV-negative individuals, as described by Vingrys and King-Smith.23
Mean RNFL thickness values reported by Rao and associates for a population of HIV-negative individuals were considered to be normal values.24 For purposes of analysis, study participants were considered to have normal RNFL thickness if their measurement values fell within the range mean ± 1 SD for the corresponding control population measurements (104.68 ± 12.61 μm for average peripapillary RNFL; 79.78 ± 14.62 μm for temporal RNFL). There were participants whose average and temporal RNFL thickness values were greater than normal and others whose thickness values were less than normal, based on these defined ranges.
Table 3 shows relationships of average RNFL thickness values (grouped as thick, normal, and thin) with visual function results and with time since diagnosis of HIV infection. There was a statistically significant association between thin average RNFL and worse logCS when compared to the normal average RNFL thickness group.
Table 3. Comparison Of LogCS, Color Confusion Index, and Time Since Diagnosis of HIV Infection vs Average Retinal Nerve Fiber Layer Thickness Grouped by Thickness for 102 Eyes of 57 Study Participants.
| Normal RNFLa (n = 74) | Thick RNFLa (n = 10) | P Valueb | Thin RNFLa (n = 18) | P Valuec | |
|---|---|---|---|---|---|
| RNFL thickness (μm) | |||||
| Mean ± SD | 104.4 ± 6.4 | 121.4 ± 5.1 | 82.5 ± 4.6 | ||
| Median (range) | 104.0 (93.3 to 116.5) | 119.7 (117.3 to 134.7) | 83.1 (75.0 to 90.0) | ||
| LogCS | .43 | .009 | |||
| Mean ± SD | 1.87 ± 0.12 | 1.84 ± 0.14 | 1.78 ± 0.19 | ||
| Median (range) | 1.90 (1.45 to 1.95) | 1.90 (1.50 to 1.95) | 1.85 (1.25 to 1.95) | ||
| C-index | .47 | .42 | |||
| Mean ± SD | 1.72 ± 0.71 | 1.53 ± 0.60 | 1.95 ± 0.88 | ||
| Median (range) | 1.58 (0.96 to 4.07) | 1.31 (0.96 to 2.45) | 1.72 (0.96 to 3.50) | ||
| Time since diagnosis of HIV infection (months) | |||||
| Mean ± SD | 187.3 ± 94.7 | 159.5 ± 76.6d | 216.4 ± 81.2d | ||
| Median (range) | 202 (3 to 359) | 167 (29 to 243) | 209.5 (75 to 332) | ||
C-index = color confusion index; CS = contrast sensitivity; HIV = human immunodeficiency virus; RNFL = retinal nerve fiber layer; SD = standard deviation.
Study participants were included in the normal subgroup if their measurements fell within 1 standard deviation of the mean value (104.68 ± 12.61 μm) of parapapillary RNFL measurements in 4 quadrants (superior, temporal, inferior, nasal) for a population of HIV-negative individuals described by Rao and associates.24 Participants with values above or below that range were included in the thick and thin subgroups respectively.
Based on Kruskal-Wallis test for the comparison of the thick RNFL subgroup to normal RNFL subgroup.
Based on Kruskal-Wallis test for the comparison of the thin RNFL subgroup to normal RNFL subgroup.
P value = .18 for the comparison of time since diagnosis of HIV infection between eyes with thin and thick RNFL.
Time since diagnosis of HIV infection was shorter for those with thick average RNFL than for those with thin average RNFL, but the difference did not reach statistical significance (P = .18). Figure 1 shows a comparison of average RNFL thickness to time since diagnosis of HIV infection. Review of the scatterplot suggests increasing thickness vs time among those with shorter durations of disease, but decreasing thickness vs time among those with longer durations of disease. When lines of best fit are plotted as a linear function for the intervals ≤ 180 months and > 180 months (Figure 1, Bottom), there is a slightly positive slope for the shorter interval (slope ± standard error [SE] = 0.014 ± 0.04 μm per month) and a slightly negative slope for the longer interval (-0.026 ± 0.03 μm per month), although the values were not statistically significant (P = .76 and P = .40, respectively).
Figure 1.
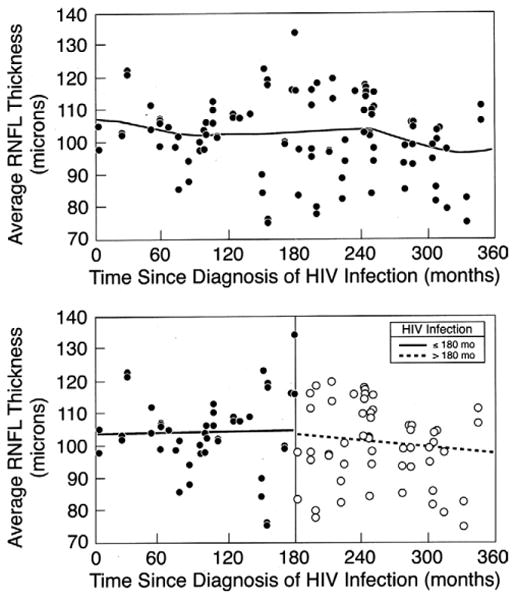
Scatterplot showing retinal nerve fiber layer (RNFL) thickness (average of 4 quadrants) vs time since HIV diagnosis for 102 eyes of 57 HIV-infected individuals without ocular opportunistic infections. The distribution of points suggests greater thickness during the mid portion of the interval range. The top graph shows the nonlinear line of best fit, while the bottom graph shows the linear line of best fit for data points grouped according to time since HIV infection (≤ 180 months and > 180 months). The slope ± standard error is 0.014 ± 0.044 μm/month (P = .76) for the interval 0 to 180 months and −0.026 ± 0.030 μm/month (P = .40) for the interval 181 to 360 months.
Table 4 shows relationships of temporal RNFL thickness (grouped as thick, normal, and thin) with visual function results. There were statistically significant associations between thin temporal RNFL and both worse logCS and worse C-index when compared to the normal temporal RNFL thickness group. After adjusting for possible correlation between eyes and for other potential confounders, the relationship between reduced CS and thin temporal RNFL remained statistically significant (P = .027), while the relationship between the abnormal color vision and thin temporal RNFL group did not (P = .12). Time since diagnosis of HIV infection was not found to be related to temporal RNFL thickness. We could not identify a relationship between the use of specific nucleoside reverse transcriptase inhibitors and temporal RNFL thickness (data not shown).
Table 4. Comparison of LogCS, Color Confusion Index, and Time Since Diagnosis of HIV Infection vs Temporal Retinal Nerve Fiber Layer Thickness Grouped by Thickness for 102 Eyes of 57 Study Participants.
| Normal RNFLa (n = 76) | Thick RNFLa (n = 9) | P Valueb | Thin RNFLa (n = 17) | P Valuec | |
|---|---|---|---|---|---|
| RNFL thickness (μm) | |||||
| Mean ± SD | 77.9 ± 7.7 | 103.1 ± 8.6 | 57.6 ± 7.0 | ||
| Median (range) | 78.5 (65.7 to 93.0) | 100.7 (94.7 to 119.0) | 58.0 (37.7 to 65.0) | ||
| LogCS | .99 | .030 | |||
| Mean ± SD | 1.86 ± 0.12 | 1.88 ± 0.07 | 1.76 ± 0.21 | ||
| Median (range) | 1.90 (1.45 to 1.95) | 1.90 (1.75 to 1.95) | 1.80 (1.25 to 1.95) | ||
| C-index | .24 | .018 | |||
| Mean ± SD | 1.69 ± 0.70 | 1.37 ± 0.64 | 2.16 ± 0.83 | ||
| Median (range) | 1.58 (0.96 to 4.07) | 1.10 (0.96 to 3.04) | 2.02 (1.10 to 3.53) | ||
| Time since diagnosis of HIV infection (months) | |||||
| Mean ± SD | 188.0 ± 96.3 | 208.9 ± 58.0d | 187.2 ± 85.4d | ||
| Median (range) | 198.5 (3 to 359) | 213 (110 to 314) | 194 (29 to 332) | ||
C-index = color confusion index; CS = contrast sensitivity; HIV = human immunodeficiency virus; RNFL = retinal nerve fiber layer thickness; SD = standard deviation.
Study participants were included in the normal subgroup if their measurements fell within 1 standard deviation of the mean value (79.78 ± 14.62 μm) of the temporal parapapillary RNFL for a population of HIV-negative individuals described by Rao and associates.24 Participants with values above or below that range were included in the thick and thin subgroups respectively.
Based on Kruskal-Wallis test for the comparison of the thick RNFL subgroup to normal RNFL subgroup.
Based on Kruskal-Wallis test for the comparison of the thin RNFL subgroup to normal RNFL subgroup.
P value = .50 for the comparison of time since diagnosis of HIV infection between eyes with thin and thick RNFL.
Table 5 shows the correlation coefficients for the comparison of visual function results and RNFL thickness for each quadrant. Temporal RNFL thickness was positively correlated with logCS (r = 0.29, P = .003) and inversely correlated with C-index (r = −0.34, P = .0005). After adjusting for possible correlation between eyes of the same individual and for other potential confounders, the correlations between visual function measures and temporal RFNL thickness remained statistically significant: for logCS, slope ± SE was 0.0041 ± 0.0010 per μm (P = .0001); for D15, slope ± SE was −0.013 ± 0.005 per μm (P = .008). Average RNFL thickness was not correlated with CS or color vision.
Table 5. Spearman Correlation Coefficients for LogCS and Color Confusion Index vs Retinal Nerve Fiber Layer Thickness by Quadrant for 102 Eyes of 57 HIV-Infected Study Participants.
| Visual Function | Nerve Fiber Layer Thickness By Quadrant | ||||
|---|---|---|---|---|---|
|
| |||||
| All (Average) | Temporal | Superior | Nasal | Inferior | |
|
|
|||||
| LogCS | R = 0.145 | R = 0.295 | R = 0.017 | R = 0.061 | R = 0.183 |
| P = .14 | P = .003 | P = .87 | P = .54 | P = .07 | |
| C-index | R = −0.083 | R = −0.338 | R = 0.066 | R = −0.065 | R = −0.028 |
| P = .41 | P = .0005 | P = .51 | P = .52 | P = .78 | |
C-index = color confusion index; CS = contrast sensitivity; HIV = human immunodeficiency virus.
Figure 2 shows the relationship between logCS and RNFL thickness. There is an apparent threshold effect for the positive relationship between temporal RNFL thickness and logCS, at the lower RNFL values. Figure 3 shows the relationship between color vision and RNFL thickness. There is an apparent threshold effect for the negative relationship between temporal RNFL thickness and C-index, at the lower RNFL values.
Figure 2.
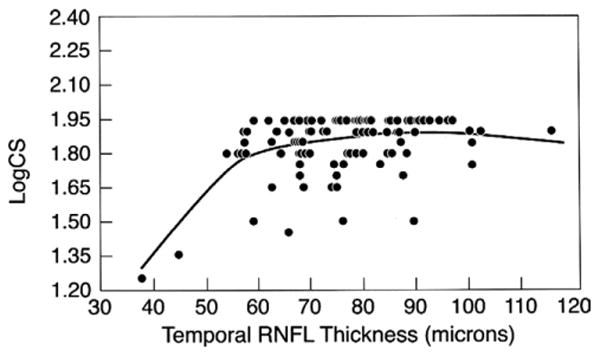
Scatterplot showing logCS vs temporal retinal nerve fiber layer thickness for 102 eyes of 57 HIV-infected individuals without ocular opportunistic infections. Smaller logCS values indicate worse contrast sensitivity.
Figure 3.
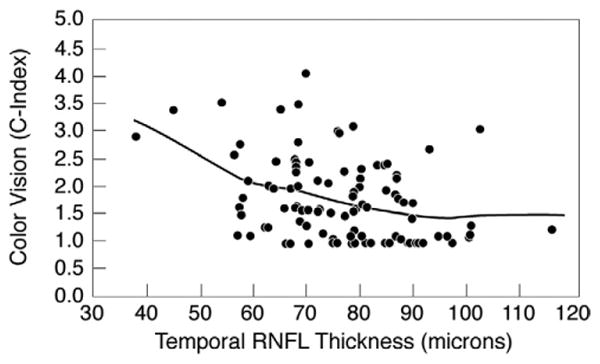
Scatterplot showing color confusion index (C-index) vs temporal retinal nerve fiber layer thickness for 102 eyes of 57 HIV-infected individuals without ocular opportunistic infections. Larger C-index values indicate worse color vision.
Discussion
In our cross-sectional study, worse CS and worse color vision were associated with thinner peripapillary RNFL, most apparent in the temporal quadrant. These results are biologically plausible; both CS and color vision are macular functions, and it is not surprising that correlations were found primarily in the temporal RNFL, which reflects the maculopapillary bundle. Our results provide direct evidence of a link between abnormal vision and neuroretinal disorder.
Other investigators have compared RNFL thickness of HIV-infected individuals without opportunistic ocular infections to HIV-negative controls. Kozak and associates found that HIV-infected individuals with histories of CD4+ T-lymphocyte counts <100/μL for at least 6 months had significantly thinner average, temporal, superior, and inferior RNFL thicknesses than HIV-negative controls, using time-domain OCT.14 Moschos and associates, assessing RNFL thickness in HIV-infected children, found significantly thinner RNFL in all quadrants when compared to HIV-negative children.26 Plummer and associates, using confocal scanning laser tomography, found significantly thinner RNFL when compared to HIV-negative healthy controls.27 None of these studies compared RNFL thickness directly to psychophysical measures of visual function. Cheng and associates have shown a relationship between RNFL thickness and driving ability among HIV-infected individuals.28 Although such a relationship might be indirect, reflecting a variety of associated neurologic factors, it is reasonable to assume that it is mediated at least in part by visual changes, pointing out the importance of the current research.
Visual disturbances similar to those found in our study have been described in asymptomatic carriers of Leber hereditary optic neuropathy (LHON),29–31 a maternally inherited genetic disorder associated with mitochondrial DNA mutations that cause severe mitochondrial dysfunction, leading to decreased ATP production and increased reactive oxygen species. Histopathologic findings associated with LHON suggest a low-grade active degenerative process involving the optic nerve, with evidence of axonal loss and impaired axoplasmic transport.32 LHON provides a potential model for understanding HIV-associated neuroretinal disorder, as both disorders can have similar patterns of vision abnormalities and histologic changes.33,34 The RNFL and the unmyelinated prelaminar optic nerve have high concentrations of mitochondria, required for the energy-intensive maintenance of axonal membrane potentials and axonal transport.35 There is evidence suggesting that HIV infection itself or its treatment (specifically, treatment with certain nucleoside reverse transcriptase inhibitors) is toxic to mitochondrial function, resulting in skeletal myopathies, peripheral neuropathies, insulin resistance, and life-threatening lactic acidosis.25 Studies have shown that certain mitochondrial genotypes are related to HIV-associated neuroretinal disorder,17 providing additional support for our hypothesis. We could not, however, identify a specific association between use of nucleoside or nucleotide reverse transcriptase inhibitors and temporal RNFL thinning.
The relative RNFL thinning seen in study participants with worse visual function could be a result of preferential axonal damage, especially to smaller-caliber fibers in the papillomacular bundle; such changes have been shown by light and electron microscopy in patients with LHON who have dyschromotopsia and central visual loss.36 These axons seem to be particularly sensitive because of their small volume-to-surface-area ratio, making them most susceptible to deficits in energy expenditure, leading to disruption in axonal transport and eventual retinal ganglion cell death.36 In a small cohort study, Barboni and associates followed 4 patients with LHON longitudinally during 4 distinct time points: the presymptomatic stage, time of visual loss, and 3 and 9 months later. A significant increase in the RNFL thickness was seen between the presymptomatic stage and the onset of the disease, followed by a significant reduction of the RNFL thickness by the 9-month follow-up.37
Among our study participants, there was a subgroup with average RNFL thickness greater than normal. Duration of HIV disease was shorter for this subgroup than for those whose RNFL thickness was less than normal. Although the difference was not significant, it suggests the possibility that, as the axons are compromised, the RNFL goes through an initial phase of swelling before becoming atrophic. In contrast to changes associated with LHON, retinal damage and vision loss associated with HIV-related neuroretinal disorder probably occur over years, rather than over weeks to months, as with LHON. The course of this process may proceed at variable rates in different individuals, making it difficult to confirm the sequence of events in a cross-sectional study, and it is possible that not all individuals are susceptible to the observed changes.
Our results suggest a possible threshold effect, with visual changes occurring only in cases of more severe RNFL thinning. This impression is based on 1) the fact that we did not find evidence of a dose effect when those with thicker RNFL were analyzed, and 2) the shapes of best-fit lines shown in Figures 2 and 3. These lines are influenced by small numbers of cases at both extremes of RNFL thickness. Additional study will be needed to confirm that these cases are representative of the entire population of HIV-infected individuals.
Although RNFL changes can occur as a result of primary optic neuropathy, we hypothesize that it reflects retinal disease, possibly at the retinal ganglion cell layer, in HIV-infected individuals. Reduced CS is believed to be related, at least in part, to retinal microvasculopathy,38 and Tenhula and associates found evidence that axonal loss in the optic nerves of people with AIDS was attributable to retinal disease in addition to primary optic neuropathy.15
Abnormal CS has been used as a surrogate for neuroretinal disorder in previous studies.16,17 Although the correlation between CS and RNFL thickness was highly significant, there were too few participants in our study whose vision met definitions of abnormal CS (2.9%) to determine the predictive values of this measure for the presence of RNFL thinning; however, the fact that the correlation coefficient itself was relatively weak suggests that abnormal CS is not a sensitive marker of mild changes of RNFL thickness in people infected with HIV. The proportion of participants with abnormal color vision was higher (40.2%), but there are no generally accepted definitions of abnormal color vision, and results are test-dependent; in a previous study at our institution, only 9.9% of HIV-infected individuals had abnormal color vision, when tested with the FM-100.3 Additional study will be necessary to determine the utility of color vision measurement as a marker of neuroretinal disorder.
Visual field abnormalities are more common among HIV-infected individuals than either CS or color vision abnormalities.4 In contrast to our study, Faria E Arantes and associates found relationships between visual field parameters and average RNFL thickness among HIV-infected individuals whose median duration of disease was shorter than that of our cohort.12 Different RNFL relationships in that study may be attributable to the fact that most visual field abnormalities involve axons from the peripheral retina, while CS and color vision abnormalities reflect changes in the maculopapillary bundle.
There are a number of limitations to this study. As with any cross-sectional analysis, we could not establish cause-and-effect relationships between RNFL thickness and vision loss. Although our results suggest a temporal relationship between duration of HIV infection and RNFL thinning, this issue will be better clarified in a longitudinal study. Sample sizes were small, which may have affected our ability to identify some potential associations, such as the influence of drug therapy on RNFL thickness. Data were collected from different sites, using different equipment, but relationships between reduced CS and temporal RNFL thickness remained significant after adjustment for study site. We also included both eyes of each participant; results remained significant, however, after adjustment for the potential correlation between eyes. Other limitations include the fact that visual function testing techniques involve an element of subjectivity; we did, however, use standardized protocols (ie, testing distance, lighting conditions) to minimize this problem. We did not recruit an HIV-negative control group to be tested under the same conditions specific to this project, and much of the study participants' medical history was self-reported, as medical records were not available. The Lanthony D-15 test can be associated with intra-subject variability of test results.21 Other methods of color vision assessment, such as the FM-100 or Cambridge Colour Test, might be used to supplement Lanthony D-15 results in future studies. With regard to RNFL thickness measurements, an animal study of glaucoma has shown that substantial axonal loss can occur before changes in parapapillary RNFL thickness are identified by OCT (Cull G, Reynaud J, Wang L, et al, unpublished data, presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, May 2011, Abstract no. 179).
In summary, temporal RNFL thinning is correlated with worse CS and worse color vision among people infected with HIV who have no history of ocular opportunistic infections. This study provides additional confirmation of the suspected association between vision dysfunction and neuroretinal disorder. Further investigation of the relationships described in our study may provide some insight into the mechanism of vision dysfunction among people with HIV disease. Small-caliber axons of the papillomacular bundle may be preferentially susceptible to mitochondrial dysfunction as a potential disease mechanism. A longitudinal study will be necessary to understand more fully the effects of chronic HIV infection and its treatment on visual function.
Acknowledgments
Publication of this article was supported by Longitudinal Study of the Ocular Complications of AIDS (LSOCA) grant support from the National Eye Institute (Bethesda, Maryland). Additional support provided by National Center for Research Resources through General Clinical Research Center Grant 5M01 RR 00865 and cooperative agreement U01 AI 27660 (University of California, Los Angeles [UCLA]). Additional support was provided by the Elizabeth Taylor AIDS Foundation (Los Angeles, California) through a gift to the UCLA Herb Ritts, Jr Memorial Vision Fund (Dr Holland); the Jack H. Skirball Endowed Professorship (Dr Holland); and the Vernon O. Underwood Family Endowed Fellowship (Dr Kalyani). Involved in study design (P.S.K., G.N.H., A.A.F., A.A.S.); data collection (P.S.K., T.E.F.A.); data management and analysis (P.S.K., G.N.H., A.A.F., T.E.F.A., F.Y., A.A.S.); data interpretation (P.S.K., G.N.H., A.A.F., T.E.F.A., F.Y., A.A.S.); preparation of initial draft of manuscript (P.S.K., G.N.H., A.A.S.); review and approval of manuscript (all authors, as well as Study Officers of LSOCA, representing the SOCA Research Group, reviewed and approved the manuscript).
Biography

Partho S. Kalyani, MD, undertook this project during research and clinical uveitis fellowships at the Jules Stein Eye Institute, David Geffen School of Medicine at UCLA (2009–2011). He has been involved in a variety of research projects dealing with HIV-related eye disease, and has a clinical interest in AIDS-related CMV retinitis in the developing world. Dr Kalyani is currently a vitreoretinal fellow at the University of Michigan in Ann Arbor.
Footnotes
All Authors have completed and submitted the ICMJE form for Disclosure of Potential Conflicts of Interest and none were reported.
Informed consent was obtained from all subjects, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations. This study was approved by the University of Southern California, UCLA, and Johns Hopkins University Institutional Review Boards prior to the collection of data.
Contributor Information
Partho S. Kalyani, Ocular Inflammatory Disease Center, Jules Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, California; Doheny Eye Institute and Department of Ophthalmology, University of Southern California Keck School of Medicine, Los Angeles, California
Gary N. Holland, Ocular Inflammatory Disease Center, Jules Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, California
Amani A. Fawzi, Doheny Eye Institute and Department of Ophthalmology, University of Southern California Keck School of Medicine, Los Angeles, California
Tiago E.F. Arantes, Ocular Inflammatory Disease Center, Jules Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, California
Fei Yu, Ocular Inflammatory Disease Center, Jules Stein Eye Institute and the Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, California.
Alfredo A. Sadun, Doheny Eye Institute and Department of Ophthalmology, University of Southern California Keck School of Medicine, Los Angeles, California
References
- 1.Mutlukan E, Dhillon B, Aspinall P, Cullen JF. Low contrast visual acuity changes in human immuno-deficiency virus (HIV) infection. Eye (Lond) 1992;6(Pt 1):39–42. doi: 10.1038/eye.1992.6. [DOI] [PubMed] [Google Scholar]
- 2.Quiceno JI, Capparelli E, Sadun AA, et al. Visual dysfunction without retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113(1):8–13. doi: 10.1016/s0002-9394(14)75745-9. [DOI] [PubMed] [Google Scholar]
- 3.Shah KH, Holland GN, Yu F, Van Natta M, Nusinowitz S. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol. 2006;142(2):284–292. doi: 10.1016/j.ajo.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Freeman WR, Van Natta ML, Jabs D, et al. Vision function in HIV-infected individuals without retinitis: report of the Studies of Ocular Complications of AIDS Research Group. Am J Ophthalmol. 2008;145(3):453–462. doi: 10.1016/j.ajo.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommerhalder J, Baglivo E, Barbey C, et al. Colour vision in AIDS patients without HIV retinopathy. Vision Res. 1998;38(21):3441–3446. doi: 10.1016/s0042-6989(98)00011-x. [DOI] [PubMed] [Google Scholar]
- 6.Brodie SE, Friedman AH. Retinal dysfunction as an initial ophthalmic sign in AIDS. Br J Ophthalmol. 1990;74(1):49–51. doi: 10.1136/bjo.74.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geier SA, Nohmeier C, Lachenmayr BJ, Klauss V, Goebel FD. Deficits in perimetric performance in patients with symptomatic human immunodeficiency virus infection or acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119(3):335–344. doi: 10.1016/s0002-9394(14)71177-8. [DOI] [PubMed] [Google Scholar]
- 8.Mueller AJ, Plummer DJ, Dua R, et al. Analysis of visual dysfunctions in HIV-positive patients without retinitis. Am J Ophthalmol. 1997;124(2):158–167. doi: 10.1016/s0002-9394(14)70780-9. [DOI] [PubMed] [Google Scholar]
- 9.Plummer DJ, Marcotte TD, Sample PA, et al. Neuropsychological impairment-associated visual field deficits in HIV infection. HNRC Group. HIV Neurobehavioral Research Center. Invest Ophthalmol Vis Sci. 1999;40(2):435–442. [PubMed] [Google Scholar]
- 10.Falkenstein I, Kozak I, Kayikcioglu O, et al. Assessment of retinal function in patients with HIV without infectious retinitis by multifocal electroretinogram and automated perimetry. Retina. 2006;26(8):928–934. doi: 10.1097/01.iae.0000250009.60908.35. [DOI] [PubMed] [Google Scholar]
- 11.Kozak I, Sample PA, Hao J, et al. Machine learning classifiers detect subtle field defects in eyes of HIV individuals. Trans Am Ophthalmol Soc. 2007;105:111–118. discussion 119–120. [PMC free article] [PubMed] [Google Scholar]
- 12.Faria E, Arantes TE, Garcia CR, Mello PA, Muccioli C. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol. 2010;149(4):571–576. e572. doi: 10.1016/j.ajo.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Goldbaum MH, Kozak I, Hao J, et al. Pattern recognition can detect subtle field defects in eyes of HIV individuals without retinitis under HAART. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):491–498. doi: 10.1007/s00417-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak I, Bartsch DU, Cheng L, Kosobucki BR, Freeman WR. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. 2005;139(2):295–301. doi: 10.1016/j.ajo.2004.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenhula WN, Xu SZ, Madigan MC, et al. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113(1):14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- 16.Sezgin E, Hendrickson SL, Jabs DA, et al. Effect of host genetics on incidence of HIV neuroretinal disorder in patients with AIDS. J Acquir Immune Defic Syndr. 2010;54(4):343–351. doi: 10.1097/QAI.0b013e3181deaf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrickson SL, Jabs DA, Van Natta M, et al. Mitochondrial haplogroups are associated with risk of neuroretinal disorder in HIV-positive patients. J Acquir Immune Defic Syndr. 2010;53(4):451–455. doi: 10.1097/QAI.0b013e3181cb8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vision Sci. 1991;6:471–475. [Google Scholar]
- 20.Lanthony P. The desaturated panel D-15. Doc Ophthalmol. 1978;46(1):185–189. doi: 10.1007/BF00174107. [DOI] [PubMed] [Google Scholar]
- 21.Good GW, Schepler A, Nichols JJ. The reliability of the Lanthony Desaturated D-15 test. Optom Vis Sci. 2005;82(12):1054–1059. doi: 10.1097/01.opx.0000192351.63069.4a. [DOI] [PubMed] [Google Scholar]
- 22.Feitosa-Santana C, Paramei GV, Nishi M, et al. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge Colour Test. Ophthalmic Physiol Opt. 2010;30(5):717–723. doi: 10.1111/j.1475-1313.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Vingrys AJ, King-Smith PE. A quantitative scoring technique for panel tests of color vision. Invest Ophthalmol Vis Sci. 1988;29(1):50–63. [PubMed] [Google Scholar]
- 24.Rao HL, Zangwill LM, Weinreb RN, et al. Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology. 2010;117(9):1692–1699. 1699, e1691. doi: 10.1016/j.ophtha.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J Antimicrob Chemother. 2009;64(5):901–909. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]
- 26.Moschos MM, Mostrou G, Psimenidou E, Spoulou V, Theodoridou M. Objective analysis of retinal function in HIV-positive children without retinitis using optical coherence tomography. Ocul Immunol Inflamm. 2007;15(4):319–323. doi: 10.1080/09273940701375154. [DOI] [PubMed] [Google Scholar]
- 27.Plummer DJ, Bartsch DU, Azen SP, et al. Retinal nerve fiber layer evaluation in human immunodeficiency virus-positive patients. Am J Ophthalmol. 2001;131(2):216–222. doi: 10.1016/s0002-9394(00)00787-x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S, Klein H, Bartsch DU, et al. Relationship between retinal nerve fiber layer thickness and driving ability in patients with human immunodeficiency virus infection. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1643–1647. doi: 10.1007/s00417-011-1735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura DF, Quiros P, Carelli V, et al. Chromatic and luminance contrast sensitivities in asymptomatic carriers from a large Brazilian pedigree of 11778 Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2005;46(12):4809–4814. doi: 10.1167/iovs.05-0455. [DOI] [PubMed] [Google Scholar]
- 30.Sadun AA, Salomao SR, Berezovsky A, et al. Subclinical carriers and conversions in Leber hereditary optic neuropathy: a prospective psychophysical study. Trans Am Ophthalmol Soc. 2006;104:51–61. [PMC free article] [PubMed] [Google Scholar]
- 31.Quiros PA, Torres RJ, Salomao S, et al. Colour vision defects in asymptomatic carriers of the Leber's hereditary optic neuropathy (LHON) mtDNA 11778 mutation from a large Brazilian LHON pedigree: a case-control study. Br J Ophthalmol. 2006;90(2):150–153. doi: 10.1136/bjo.2005.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002;40(6):573–584. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 33.Sadun AA, Pepose JS, Madigan MC, et al. AIDS-related optic neuropathy: a histological, virological and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233(7):387–398. doi: 10.1007/BF00180941. [DOI] [PubMed] [Google Scholar]
- 34.Saadati HG, Hsu HY, Heller KB, Sadun AA. A histopathologic and morphometric differentiation of nerves in optic nerve hypoplasia and Leber hereditary optic neuropathy. Arch Ophthalmol. 1998;116(7):911–916. doi: 10.1001/archopht.116.7.911. [DOI] [PubMed] [Google Scholar]
- 35.Hollander H, Makarov F, Stefani FH, Stone J. Evidence of constriction of optic nerve axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 1995;27(5):296–309. doi: 10.1159/000267739. [DOI] [PubMed] [Google Scholar]
- 36.Sadun AA, Win PH, Ross-Cisneros FN, Walker SO, Carelli V. Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc. 2000;98:223–232. discussion 232–225. [PMC free article] [PubMed] [Google Scholar]
- 37.Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117(3):623–627. doi: 10.1016/j.ophtha.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Holland GN, Kappel PJ, Van Natta ML, et al. Association between abnormal contrast sensitivity and mortality among people with acquired immunodeficiency syndrome. Am J Ophthalmol. 2010;149(5):807–816. doi: 10.1016/j.ajo.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


