Abstract
PURPOSE
To investigate the relationship between contrast sensitivity (CS) and mortality among people with acquired immunodeficiency syndrome (AIDS); and to explore the hypothesis that abnormal CS is a marker of systemic, life-threatening microvascular disease.
DESIGN
Longitudinal, observational cohort study.
METHODS
We evaluated 3395 eyes of 1706 individuals enrolled in the Longitudinal Study of the Complications of AIDS (1998–2008). CS was evaluated as a risk factor for death, and was compared to the presence of systemic diseases characterized by microvasculopathy (diabetes, cardiovascular disease, stroke, renal disease) and to laboratory markers of those diseases. Abnormal CS was defined as logCS <1.5 (lower 2.5th percentile for a normal control population).
RESULTS
CS was abnormal in 284 of 1691 (16.8%) study participants at enrollment. There was a positive relationship between the presence of abnormal CS at study entry and mortality (relative risk 2.0, 95% confidence interval 1.7-2.3, P < .0001). Abnormal CS was related to the presence of cardiovascular disease, stroke, and renal disease (all P values < .01), but abnormal CS remained associated with death even after adjustment for these diseases and for other known predictors of death among people with AIDS. Diseases characterized by microvasculopathy were more often identified as causes of death among individuals with abnormal CS than among those with normal CS, although the strength of the association was moderate (P = .06).
CONCLUSIONS
Abnormal CS among people with AIDS is associated with increased mortality, and is independent of other risk factors for death that are monitored routinely. The relationship may indicate life-threatening microvascular disease in other organs.
Abnormalities of vision are common among people infected with human immunodeficiency virus (HIV), even in the absence of cytomegalo-virus (CMV) retinitis or other intraocular opportunistic infections. It has been shown that the prevalence of reduced contrast sensitivity (CS) and abnormal color vision among HIV-infected individuals without clinically apparent retinal disease is 2- to 3-fold greater than in the general population.1–4 It is believed that these vision abnormalities are related to retinal damage, characterized by loss of ganglion cell axons, which is common among HIV-infected individuals. This condition, which has been termed neuroretinal disorder (NRD), may be caused, in part, by the retinal microvasculopathy of HIV disease.3
Studies of CS in the general population and among specific groups, such as diabetics, have shown that reduced CS can have functional consequences, and may be a marker for systemic disorders. Among diabetics, for example, reduced CS is associated with retinal vascular disease,5,6 which in turn is associated with increased mortality.7–10
In this study, we sought to ascertain similar relationships among HIV-infected individuals enrolled in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), and explored whether those who demonstrated reduced CS at baseline examination had an increased risk for death. We also explored the hypothesis that the association between CS and mortality reflects the presence of potentially life-threatening systemic diseases that are characterized by microvasculopathies, such as cardiovascular disease, stroke, and renal disease.
METHODS
lsoca is an ongoing, prospective, observational cohort study designed to collect data on ophthalmic conditions associated with acquired immunodeficiency syndrome (AIDS) that have been seen since the introduction of highly active antiretroviral therapies (HAART). Enrollment began in September 1998. Study participants must have a diagnosis of AIDS, as defined by 1993 Centers for Disease Control and Prevention criteria.1,2 A description of LSOCA design, methods, and baseline data has been published elsewhere.1,2 The current study includes data collected through December 31, 2008. Excluded from this investigation were study participants who had ocular opportunistic infections, which are associated with mortality and may cause abnormal CS.
DATA COLLECTION
By design, LSOCA participants without ocular opportunistic infections were seen every 6 months. At baseline (study enrollment) and at each follow-up visit, participants were interviewed about medical and treatment histories, and each underwent an ophthalmic examination that included determination of best-corrected visual acuity, determination of CS, Goldmann visual field testing, slit-lamp biomicroscopy, and dilated indirect ophthalmoscopy. Laboratory tests obtained at each visit included complete blood cell count, including leukocyte differential; platelet count; lymphocyte subset analyses; serum creatinine; serum lipid determinations; plasma HIV RNA level; and urine protein level. The method by which proteinuria was determined was not requested from investigators at the time of data collection. Beginning in 2005, blood pressure was measured and smoking status was determined at each visit. A Framingham mortality risk score was calculated for each participant; this score takes into account the following factors: gender, age, systolic blood pressure, treatment for hypertension, total cholesterol, high-density lipoprotein, and smoking status.11 Continuous covariates were categorized using clinically meaningful cut points, where applicable.
CS was determined using the method of Pelli-Robson.12,13 Briefly, participants sat or stood 1 m from the Pelli-Robson contrast sensitivity chart, using a +0.75-diopter lens in addition to their refractive corrections. Participants read the chart until at least 2 letters in a triple were missed. The logCS score was calculated as the total number of letters read correctly minus 3, then multiplied by 0.05.14 Because CS is linear on a log10 scale, analyses used log10CS. The protocol used in LSOCA does not permit confusion between “C” and “O,” which is consistent with the technique described by Myers and associates14 but is unlike the protocol used in some other reported studies; this convention will potentially lower the distribution of CS values in LSOCA when compared to established norms in those other studies. This issue has been addressed previously by the SOCA Research Group.4
We categorized study participants who died during follow-up on the basis of immediate and contributing causes of death, using information from available death certificates; specifically, we identified whether death was associated with diseases characterized by microvasculopathy (diabetes, cardiovascular disease, renal disease, stroke), liver disease (a major cause of morbidity and mortality among people with AIDS), AIDS-related opportunistic infections and malignancies, other AIDS-related disorders, or trauma.
DEFINITIONS
Abnormal CS was defined as logCS <1.5, which corresponds to the lower 2.5th percentile of a normal control population described by Myers and associates.14 The rationale for use of this threshold in studies of the LSOCA population has been discussed elsewhere.3 For purposes of this study, HAART was defined as the concurrent use of 3 or more antiretroviral drugs.
A participant was considered to have diabetes mellitus if the condition was self-reported at baseline or during follow-up, or if he or she was on any therapy for diabetes mellitus.
A participant was considered to have hypertension if the condition was self-reported at baseline; if he or she received treatment with antihypertensive medications; or if he or she had a systolic blood pressure >140 mm Hg or a diastolic blood pressure >85 mm Hg on at least 1 LSOCA visit.
A participant was considered to have cardiovascular disease if either coronary heart disease or peripheral vascular disease was self-reported or mentioned in medical records at baseline or if he or she was reported to have a cardiovascular event during follow-up.
A participant was considered to have a history of stroke if the condition was self-reported at baseline or if he or she was reported to have had a cerebrovascular event during follow-up.
A participant was considered to have renal disease if the condition was self-reported at baseline; if he or she had proteinurea of 2+ or more; or if his or her calculated glomerular filtration rate (GFR) was <60 mL/min, using the Modification of Diet in Renal Disease (MDRD) Study equation: 186 × (serum creatinine [mg/dL])−1.154 × (age [y])−0.203 × (0.742, if female) × (1.212, if black).15
DATA ANALYSIS
Unless otherwise noted, the unit of analysis was the eye. Demographic, medical, and laboratory data were compared between study participants with abnormal CS in at least 1 eye versus those with normal CS in both eyes. CS at baseline was compared to the occurrence of death, both as a categorical variable (abnormal CS vs normal CS) and as a continuous variable; relationships were investigated for all participants and for subgroups based on best-corrected visual acuity (20/20 or better vs worse than 20/20), with and without adjustment for the following factors: CD4+ T-lymphocyte count, plasma HIV RNA level, HAART status (current use vs no current use), and date of CS measurement. We adjusted for date of examination in all multivariate analyses because we observed a secular trend in which there was a small, but significant, increase in mean logCS that may have been related to replacement of old contrast sensitivity charts in some clinics during the latter years of the study.
In a secondary, person-specific (as opposed to eyespecific) analysis, we compared abnormal CS to death after categorizing study participants into the following groups: abnormal CS in both eyes at baseline; abnormal CS in 1 eye at baseline; and abnormal CS in neither eye at baseline.
CS (abnormal vs normal) at each visit was compared to the following factors at the same visit: history of stroke; the presence or absence of diabetes, hypertension, cardiovascular disease, or renal disease; laboratory markers of renal disease (proteinuria, GFR); and Framingham 10-year mortality risk score.
Abnormal CS at baseline and abnormal CS as a time-dependent variable were assessed as risk factors for death in crude comparisons, and time-dependent abnormal CS was assessed as a risk factor for death in several models that adjusted for various combinations of the following potential confounders: HAART status; current and nadir CD4+ T-lymphocyte counts; plasma HIV RNA level; visual acuity; history of stroke; the presence or absence of diabetes, hypertension, cardiovascular disease, or renal disease; Framingham 10-year mortality risk score; and date of CS measurement.
For study participants who died and for whom death certificates were available, causes of, or factors contributing to, death were compared between those with and those without abnormal CS.
STATISTICAL TECHNIQUES
Differences were assessed using the Wilcoxon rank sum test for continuous data and the χ2 test for categorical data.16 Assessment of time-independent and time-varying risk factors for death used Cox regression with staggered entry; time since diagnosis of AIDS was the time metric, and analysis accounted for the correlation between eyes of a given participant.17 P values were 2-sided and were not adjusted for multiple outcomes or multiple looks. Analyses were performed with both SAS (SAS/STAT User’s Guide, Version 9.2, 2008; SAS Institute, Cary, North Carolina, USA) and Stata (Stata Statistical Software: Release 10, 2007; StataCorp LP, College Station, Texas, USA) statistical packages.
RESULTS
a total of 2221 individuals had enrolled in lsoca as of December 31, 2008. There were no ocular opportunistic infections in 1712 individuals. CS measurements were available for 3363 eyes of 1691 study participants without ocular opportunistic infections at baseline; CS data were available for at least 1 visit (baseline or follow-up) for 3395 eyes (1706 participants without ocular opportunistic infections). Data were available from 14 250 patient visits, consisting of 28 319 eye visits. The percentage of eye visits for which data were missing was 83% for Framingham risk score; 60% for presence or absence of renal disease; 21% for cardiovascular disease; 22% for stroke; 7% for plasma HIV RNA level; 2% for CD4+ T-lymphocyte count; and ≤1% each for nadir CD4+ T-lymphocyte count, diabetes mellitus, hypertension, and use of HAART.
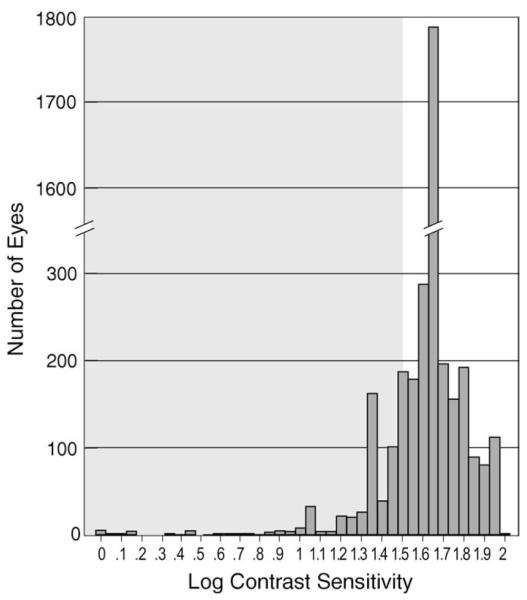
At the baseline visit, CS values ranged from logCS 0.00 to logCS 2.00 (median and mode logCS = 1.65). The distribution of CS values is shown in Figure 1. CS was considered to be abnormal in 284 individuals. Demographic and medical data at baseline are described in Table 1; there were numerous relationships between abnormal CS and factors that reflect worse health status.
FIGURE 1.
Histogram showing the distribution of contrast sensitivity (CS) values for 3363 eyes of 1691 participants without ocular opportunistic infections at study enrollment (baseline examination) in the Longitudinal Study of the Ocular Manifestations of AIDS. Abnormal CS (shaded area) was defined as logCS <1.5 (the lower 2.5th percentile for a normal control population described by Myers and associates.14 The mode (interrupted bar) was logCS 1.65 (n = 1778 eyes).
TABLE 1.
Demographic, Medical, and Laboratory Factors at Baseline for 1691 Study Participants Without Ocular Opportunistic Infections in the Longitudinal Study of the Ocular Complications of AIDS
| Abnormal CS (<1.5 logCS)a in Either Eye (n=284) |
Normal CS (≥1.5 logCS) in Both Eyes (n = 1407) |
||||
|---|---|---|---|---|---|
| Factor | Nc | Valued | Nc | Valued | P Valueb |
| Demographics | |||||
| Age | 283 | 43 years | 1402 | 43 years | .16 |
| Female | 283 | 26% | 1402 | 18% | .001 |
| Black | 283 | 46% | 1402 | 35% | .0005 |
| HIV infection risk group | 275 | 1385 | <.0001 | ||
| MSM only | — | 38% | — | 58% | |
| IDU only | — | 14% | — | 9% | |
| Both MSM and IDU | — | 6% | — | 4% | |
| Heterosexual contact | — | 36% | — | 25% | |
| Other | — | 6% | — | 4% | |
| AIDS history | |||||
| Interval since AIDS diagnosis (median) | 273 | 4.5 years | 1371 | 3.7 years | .006 |
| Lymphocytopenia as AIDS-defining illness | 283 | 61% | 1402 | 66% | .11 |
| Weight | 261 | 72 kg | 1283 | 75 kg | .0007 |
| Immunology/virology | |||||
| CD4+ T-lymphocyte count | 282 | 166 cells/μL | 1393 | 194 cells/μL | .02 |
| Nadir CD4+ T-lymphocyte count | 273 | 38 cells/μL | 1387 | 44 cells/μL | .28 |
| CD8+ T-lymphocyte count | 280 | 731 cells/μL | 1385 | 776 cells/μL | .15 |
| HIV RNA blood level | 258 | 3.0 log copies/mL | 1348 | 2.8 log copies/mL | .28 |
| Peak HIV RNA blood level | 264 | 5.3 log copies/mL | 1348 | 5.3 log copies/mL | .12 |
| Hematology | |||||
| Hemoglobin concentration | 283 | 13.3 g/dL | 1397 | 13.8 g/dL | .0004 |
| Median hematocrit | 282 | 39.2% | 1399 | 40.4% | .0008 |
| Mean corpuscular volume | 282 | 97.2 fL | 1400 | 98.0 fL | .45 |
| Platelet count | 281 | 217 × 103 cells/μL | 1390 | 212 × 103 cells/μL | .44 |
| Leukocyte count | 283 | 4.5 × 103 cells/μL | 1400 | 4.6 × 103 cells/μL | .51 |
| Absolute neutrophil count | 282 | 2.4 × 103 cells/μL | 1394 | 2.3 × 103 cells/μL | .90 |
| Comorbidities | |||||
| Hypertension | 282 | 26% | 1402 | 20% | .03 |
| Diabetes mellitus | 283 | 10% | 1402 | 9% | .71 |
| Renal disease | 283 | 5% | 1402 | 7% | .43 |
| Cardiovascular diseasee | 199 | 9% | 1103 | 7% | .33 |
| Peripheral vascular disease | 198 | 7% | 1097 | 4% | .11 |
| Stroke | 198 | 8% | 1093 | 4% | .02 |
| Antiretroviral treatment | |||||
| Ever on HAART | 225 | 88% | 1172 | 90% | .20 |
| Currently on HAART | 282 | 79% | 1402 | 85% | .006 |
| Mean Karnofsky score | 283 | 82 | 1401 | 85 | .0002 |
| Visual function (better eye) | |||||
| Visual acuity (standardized letters) | 283 | 83 | 1402 | 90 | <.0001 |
| Goldmann visual field (degrees) | 279 | 730 | 1398 | 756 | <.0001 |
| Presence of cotton-wool spots | 283 | 5% | 1401 | 3% | .02 |
AIDS = acquired immunodeficiency syndrome; CS = contrast sensitivity; HAART = highly active antiretroviral therapy; HIV = human immunodeficiency virus; IDU = intravenous drug user; MSM = men who have sex with men.
Corresponds to the lower 2.5th percentile for a normal control population described by Myers and associates.14
Wilcoxon rank sum test for continuous variables; χ2 test for categorical variables.
The number of study participants for whom data were available.
Values other than percentages represent medians unless otherwise stated.
Includes coronary heart disease and peripheral vascular disease.
Abnormal CS and reduced CS (when considered as a continuous variable) at baseline were associated with increased risk of death (Table 2, Figure 2). Despite the known relationship between abnormal CS and low CD4+ T-lymphocyte count,4 the relationship between abnormal CS and mortality remained significant after adjustment for CD4+ T-lymphocyte count, indicating that CS is not simply a marker for the immunodeficiency reflected by a low CD4+ T-lymphocyte count.
TABLE 2.
Abnormal Contrast Sensitivity at Baseline as a Risk Factor for Death Among 1691 Study Participants (3363 Eyes) Without Ocular Opportunistic Infections in the Longitudinal Study of the Ocular Complications of AIDS
| Risk Factor | Eye Deaths | Eye Visits | RRa | 95% CI | P Valueb |
|---|---|---|---|---|---|
| CS threshold (logCS ≤1.5 vs logCS >1.5) | |||||
| All eyes | |||||
| Crude | 801 | 3363 | 2.0 | 1.6–2.5 | <.0001 |
| Adjustedc | 748 | 3178 | 1.9 | 1.5–2.4 | <.0001 |
| Eyes <20/20 at baseline | |||||
| Crude | 200 | 692 | 1.5 | 1.1–2.1 | .01 |
| Adjustedc | 181 | 637 | 1.6 | 1.1–2.3 | .006 |
| Eyes ≥20/20 at baseline | |||||
| Crude | 601 | 2671 | 2.1 | 1.6–2.8 | <.0001 |
| Adjustedc | 567 | 2541 | 1.8 | 1.3–2.6 | .0002 |
| Interaction of visual acuity threshold and CS | |||||
| Crude | — | — | — | — | .14 |
| Adjustedc | — | — | — | — | .72 |
| CS as continuous variable (per 0.05 logCS) | |||||
| All eyes | |||||
| Crude | 801 | 3363 | 0.96 | 0.94–0.97 | <.0001 |
| Adjustedc | 748 | 3178 | 0.95 | 0.94–0.97 | <.0001 |
| Eyes ≥20/20 at baseline | |||||
| Crude | 200 | 692 | 0.98 | 0.96–1.00 | .06 |
| Adjustedc | 181 | 637 | 0.97 | 0.95–1.00 | .02 |
| Eyes ≥20/20 at baseline | |||||
| Crude | 601 | 2671 | 0.92 | 0.88–0.95 | <.0001 |
| Adjustedc | 567 | 2541 | 0.94 | 0.90–0.98 | .002 |
| Interaction of visual acuity threshold and CS | |||||
| Crude | — | — | — | — | .003 |
| Adjustedc | — | — | — | — | .17 |
CI = confidence interval; CS = contrast sensitivity; RR = relative risk for death.
Calculated from Cox regression using method of staggered entry for interval since AIDS diagnosis as time metric and accounting for correlation of eyes within a given study participant.
Based on Cox proportional hazards model, accounting for correlation between eyes.
Adjusted for baseline CD4+ T-lymphocyte count, HIV RNA blood level, highly active antiretroviral therapy (current use vs no current use), and date of CS measurement.
FIGURE 2.
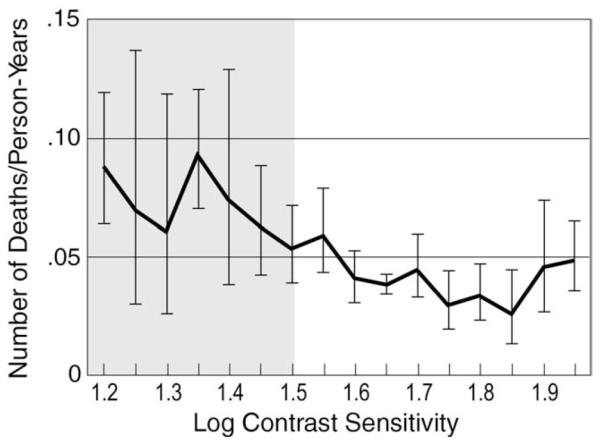
Deaths per person-year of follow-up versus contrast sensitivity (CS) at study enrollment (baseline examination) in the Longitudinal Study of the Ocular Manifestations of AIDS. Vertical bars represent 95% confidence intervals. The shaded area corresponds to abnormal CS (corresponds to the lower 2.5th percentile for a normal control population described by Myers and associates).14
Because CS is associated with visual acuity, we analyzed the relationship between CS and death both for eyes with visual acuity of 20/20 or better and for eyes with visual acuity worse than 20/20; CS was strongly related to death for both subgroups. Lack of significant interaction between the visual acuity threshold and CS after adjustment for CD4+ T-lymphocyte count, plasma HIV RNA level, and HAART status provided further evidence that the relationship between CS and death was independent of visual acuity. All subsequent analyses considered all eyes, regardless of visual acuity.
In the secondary, person-specific analysis that compared abnormal CS to death after categorizing the 1691 study participants on the basis of abnormal CS in 2 versus 1 versus 0 eyes, 131 participants (8%) had abnormal CS in both eyes at baseline; 153 (9%) had abnormal CS in 1 eye at baseline; and 1407 (85%) had abnormal CS in neither eye at baseline. The relative risk of death comparing participants with abnormal CS in 2 versus 0 eyes was 2.3 (95% CI, 1.7-3.0; P < .0001), while the relative risk of death comparing participants with abnormal CS in 1 versus 0 eyes was 1.6 (95% CI, 1.1-2.1; P = .006), consistent with a dose effect. Despite the discrepant CS values between eyes in some participants, there was a strong relationship between CS values when right and left eyes of the same participant were compared (rank correlation coefficient, 0.69; P < .0001), consistent with the hypothesis that abnormal CS is related to a generalized disease process.
Table 3 shows relationships between abnormal CS and diseases that are associated with microvasculopathy, laboratory measures that are associated with renal disease, and Framingham 10-year risk scores. There were significant positive associations with cardiovascular disease, stroke, renal disease, laboratory abnormalities that reflect renal disease, and Framingham 10-year risk score.
TABLE 3.
Relationship Between Abnormal Contrast Sensitivity and Disease Factors Associated With Microvasculopathy or Death Among 1706 Study Participants Without Ocular Opportunistic Infections in the Longitudinal Study of the Ocular Complications of AIDS
| Disease Factora | Abnormal CSa,b in Either Eye vs Normal CS in Both Eyes |
Number of Person-Visits |
||
|---|---|---|---|---|
| ORc | 95% CI | P Value | ||
| Diabetes (yes vs no) | 1.3 | 0.9–1.8 | .18 | 14215 |
| Hypertension (yes vs no) | 1.0 | 0.8–1.2 | .83 | 14214 |
| Cardiovascular diseased (yes vs no) | 1.6 | 1.1–2.4 | .01 | 12 669 |
| Stroke (yes vs no) | 2.6 | 1.5–4.5 | .001 | 12 590 |
| Renal disease (yes vs no) | 1.7 | 1.2–2.4 | .006 | 6393 |
| Proteinurea (≥2+ vs < 2+) | 1.7 | 1.0–2.8 | .04 | 6399 |
| Glomerular filtration ratee (<60 vs ≥60 mL/min) | 1.6 | 1.1–2.4 | .01 | 6393 |
| Framingham 10-year risk score (≥3% vs <3%) | 0.6 | 0.4–1.0 | .04 | 2882 |
CI = confidence interval; CS = contrast sensitivity; OR = odds ratio.
Factor present at baseline or at any visit during follow-up; the analysis investigates the association of factors at individual visits.
Abnormal CS defined as <logCS 1.5, which corresponds to the lower 2.5th percentile for a normal control population described by Myers and associates.14
Logistic regression, accounting for correlation from multiple visits, adjusted for date of CS measurement.
includes coronary heart disease and peripheral vascular disease.
Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) Study equation.15
Table 4 shows both crude and adjusted comparisons (for factors known to be associated with death) between abnormal CS and mortality. In most comparisons, there was a significantly increased risk of death among individuals with abnormal contrast sensitivity. In the secondary analyses, the risk of death among those with versus those without abnormal contrast sensitivity remained highly significant in the crude and each adjusted model, even after we restricted each analysis to those individuals with no missing data for any of the potential confounders (data not shown); only 19% of visits had no missing data. Figure 3 illustrates the fact that abnormal contrast sensitivity is associated with excess mortality even when the interval since diagnosis of AIDS is taken into account.
TABLE 4.
Relationship Between Abnormal Contrast Sensitivity and Death With Adjustment for Other Potential Risk Factors Among 1706 Study Participants (3395 Eyes) in the Longitudinal Study of the Ocular Complications of AIDS
| Risk Factor | Number of Eye-Deathsa |
Number of Eye-Visitsb |
RRc | 95% CI | P Valued |
|---|---|---|---|---|---|
| Abnormal CSe at baseline | 801 | 3363 | 2.0 | 1.7–2.3 | <.0001 |
| Abnormal CSe at baseline or during follow-up |
814 | 28 319 | 2.7 | 2.2–3.3 | <.0001 |
| Adjusted Model 1f | 683 | 25 848 | 1.9 | 1.4–2.5 | <.0001 |
| Adjusted Model 2g | 547 | 22 464 | 1.9 | 1.4–2.6 | .0002 |
| Adjusted Model 3h | 221 | 11 087 | 1.5 | 0.9–2.5 | .10 |
| Adjusted Model 4i | 67 | 5294 | 2.6 | 1.4–4.8 | .003 |
CI = confidence interval; CS = contrast sensitivity; RR = relative risk.
Defined as death in a study participant per evaluable eye.
Defined as baseline or follow-up visit per evaluable eye.
Relative risk of death.
Cox regression accounting for correlation of eyes within participants.
Abnormal CS defined as <logCS 1.5, which corresponds to the lower 2.5th percentile for a normal control population described by Myers and associates.14
Time-dependent CS adjusted for highly active antiretroviral therapy (HAART), CD4+ T-lymphocyte count,HIV RNA blood level, visual acuity, and date of CS measurement.
Time-dependent CS adjusted for the factors in Adjusted Model 1 plus nadir CD4+ T-lymphocyte count and the presence or absence of hypertension, cardiovascular disease, diabetes mellitus, and stroke.
Time-dependent CS adjusted for the factors in Adjusted Model 2 plus the presence or absence of renal disease.
Time dependent CS adjusted for the factors in Adjusted Model 3 plus Framingham risk score.
FIGURE 3.
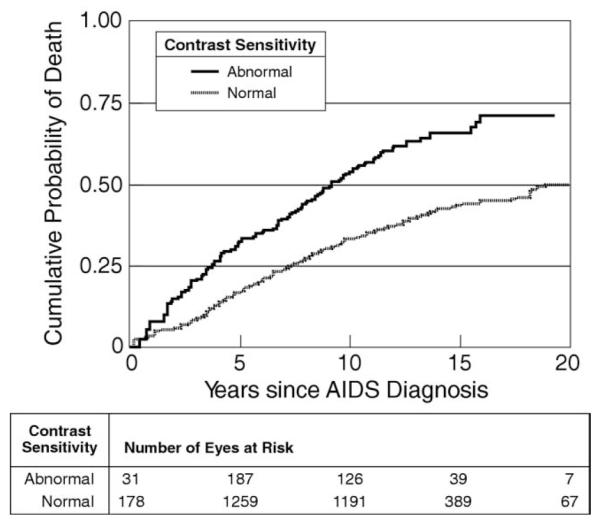
Kaplan-Meier plot showing cumulative probability of death versus interval since diagnosis of AIDS for participants grouped by normal (dotted line) versus abnormal (solid line) CS in the Longitudinal Study of the Ocular Manifestations of AIDS. The risk of death is significantly greater for those with abnormal CS (P < .0001). Abnormal CS was defined as logCS <1.5 (the lower 2.5th percentile for a normal control population described by Myers and associates).14
Death certificates were available for only 32% of study participants who were known to have died (Table 5). Among those with death certificates, death was more often attributed to diseases characterized by microvasculopathy among individuals with abnormal CS in at least 1 eye than among those with normal CS in both eyes, but the association was moderate (P = .06). When specific diseases were considered, both cardiovascular disease and stroke were more common among those with abnormal contrast sensitivity, although the associations were not strong (P = .06 and P = .42, respectively). When considering all individuals who died, regardless of death certificate availability, there was no difference between those with abnormal CS in at least 1 eye and those with normal CS in both eyes in terms of the following factors: the proportion for whom AIDS was listed on death certificates as the cause of death or a contributor to death; or, for those whose death certificates were not available, the proportion for whom CD4+ T-lymphocyte counts measured closest to death were <200 cells/μL. One or both of these factors were present in 79 of 102 individuals (77%) with abnormal CS in at least 1 eye and were present in 232 of 302 individuals (77%) with normal CS in both eyes (P = .90). There were only 3 deaths known to be associated with trauma; all occurred in study participants with normal CS.
TABLE 5.
Causes of Death by Contrast Sensitivity for 404 Study Participants With AIDS
| Abnormal CSa in Either Eye (n=102) | Normal CS in Both Eyes (n=302) | P Valueb | |
|---|---|---|---|
| Participants with death certificates | 31 (30%) | 100 (33%) | |
| Immediate cause of deathc | |||
| Diseases characterized by microvasculopathy | .06d | ||
| Cardiovascular diseasee | 5 (16%) | 5 (5.0%) | .06 |
| Diabetes mellitus | 0 | 1 (1.0%) | |
| Renal disease | 1 (3.2%) | 3 (3.0%) | |
| Stroke | 1 (3.2%) | 1 (1.0%) | .42 |
| Liver disease | 3 (9.7%) | 6 (6.0%) | |
| Other specified diseasesf | 4 (13%) | 14 (14%) | |
| AIDS-related opportunistic infection or malignancy | 9 (29%) | 35 (35%) | |
| AIDS-related, not otherwise specified | 8 (26%) | 33 (33%) | |
| Trauma | 0 | 3 (3.0%) |
CS = contrast sensitivity.
Defined as logCS <1.5, which corresponds to the lower 2.5th percentile for a normal control population described by Myers and associates.14
Fisher exact test.
Listed as cause of death or contributing factor on death certificates.
Compares occurrence of any listed disease characterized by microvasculopathy between groups.
Includes coronary heart disease and peripheral vascular disease.
Includes organ diseases other than those listed in the table; sepsis; and shock.
DISCUSSION
it is well established that hiv-infected individuals have an increased prevalence of abnormal CS.2–4 One of the goals of ophthalmologists interested in AIDS is to identify relationships between HIV-related visual abnormalities, such as decreased CS, and non-ocular disorders.18 This study begins to address that issue. Our results demonstrate that abnormal CS is a risk factor for death among people with AIDS and that this risk is independent of other stage-of-disease clinical indices that are known predictors of increased mortality, such as duration of AIDS, decreased CD4+ T-lymphocyte count, or increased plasma HIV RNA level.
Studies to date have identified links between altered CS and mortality in the setting of other diseases as well. The bases for these associations are poorly understood but appear to be multifactoral. For example, an association between poor CS and increased risk for accidents has been established. Poor CS has been associated with self-reported problems with mobility19 and with increased fall risk.20,21 Falls increase the risk of hip fractures,22,23 and hip fractures increase mortality risk, especially among the elderly.24,25 Poor CS is also associated with self-reported problems with motor vehicle driving,19 and studies have shown a relationship between poor CS and risk of driving-related automobile accidents.26,27 Pedula and associates28 reported that people with poor CS are at greater risk of overall, trauma-related mortality. In contrast, we found no evidence that deaths among LSOCA participants were related directly to abnormal CS because of poor vision and accidents; only 3 study participants were known to have died because of trauma, and all had normal CS.
In addition to the association between poor CS and trauma-related deaths, Pedula and associates28 showed that poor CS was associated with an increased risk for all-cause mortality even after adjusting for potentially confounding factors. Specifically, they found a 39% greater all-cause mortality risk in women with the worst CS values in a multivariate model that adjusted for age, chronic medical problems, and smoking.
Individuals with diabetes mellitus also have abnormal CS,5,6 which can be linked indirectly to death by the positive association of both factors with diabetic retinopathy. Arend and associates5 demonstrated that reduced CS in diabetics was significantly related to increased perifoveal intercapillary area and to an increased foveal avascular zone. Harris and associates6 showed that diabetics who had minimal diabetic retinopathy and normal best-corrected visual acuity (≥20/25) had both prolonged arteriovenous transit times and decreased CS. In addition, they showed that under isocapnic, hyperoxic conditions, CS improved to levels indistinguishable from normal, nondiabetic controls, providing further evidence that abnormal CS is a manifestation of microvascular disease. Diabetic retinopathy is associated with increased all-cause mortality, and specifically with cardiovascular events.7–10,29,30
It has been hypothesized that damage to the neuroretina seen in NRD is caused by the retinal microvasculopathy of HIV disease, and that this microvasculopathy is therefore the basis for abnormal CS in people with AIDS.3 Abnormalities of “color contrast sensitivity” among HIV-infected individuals have been related to cotton-wool spots, which are clinical manifestations of retinal microvascular disease.31 Future studies should seek direct evidence of a relationship between abnormal CS and retinal vascular changes, using fluorescein angiography or other imaging techniques.
The microvasculopathy of HIV disease is ultrastructurally similar to that seen in diabetics,32 which suggests an explanation for the association between abnormal CS and death in people with AIDS. The relationship between abnormal CS and the presence of diabetes, cardiovascular disease, and history of stroke at individual visits (Table 3) supports our hypothesis that CS is a marker for widespread, non-ocular microvasculopathy. The association with markers of renal disease and the weak association with cardiovascular disease as a cause of death also support this hypothesis. The fact that the relationship between abnormal CS and death remains significant despite adjustment for these disorders and for other predictors of increased mortality (Table 4) indicates that abnormal CS is probably not a surrogate for any specific life-threatening disease that is characterized by microvasculopathy.
We found no unique causes of death among study participants with abnormal CS; also, the association between study participants with abnormal CS and death related to diseases characterized by microvasculopathy was weak. These observations suggest that CS may be a marker for acceleration of disease processes common to all people with AIDS. Monitoring of CS might indicate the presence of, or even portend the development of, progressive nonocular microvascular disease, but additional study will be required to determine whether intervention will alter mortality risk in this population.
There are known relationships between CS, visual acuity, and cataracts. Cataracts may be more common among people with AIDS than among age-matched individuals in the general population.2 The relationships between reduced visual acuity or cataracts and mortality among people with AIDS are not known, but a relationship between best-corrected visual acuity of 20/40 or worse and increased mortality has been identified in studies of elderly populations33,34 and diabetics.10 This relationship is believed to reflect an effect of overall state of health on both vision and survival. In diabetics, reduced visual acuity is a marker of both diabetic retinopathy and systemic microvascular diseases, including ischemic heart disease.10 Our results showed that the relationship between abnormal CS and death in people with AIDS was independent of visual acuity. The occurrence of abnormal CS despite normal visual acuity has been described in people with HIV disease3,4 and in diabetics;5,6 CS may, therefore, be a more sensitive marker of mortality risk in persons with these disorders than visual acuity.
There are a number of limitations associated with our data. One should be cautious of interpreting results of analyses based on a subset of study participants (in this study because of missing data), because findings may not be representative of the entire population. Nevertheless, we believe our results are reliable; a major reason for missing data was the fact that certain parameters (systolic and diastolic blood pressure, and histories of smoking, coronary heart disease, peripheral vascular disease, and stroke) were not collected for any participants during the early years of the study. Furthermore, it is unlikely that any systematic bias associated with other missing data would result in similar, yet spurious, results across all of the models shown in Table 4. Death certificates were available for only a minority of study participants who died during the course of the study, and thus the subgroup analyzed for causes of death may not have been representative of the entire population of people with AIDS.
In summary, abnormal CS among people with AIDS is associated with increased mortality, and is independent of other established risk factors for death in this group that are monitored routinely. These findings support our hypothesis that the relationship noted between abnormal CS and mortality reflects the presence of potentially life-threatening microvasculopathies elsewhere in the body. Thus, CS may represent another useful marker of a global increase in risk for death. Continued study of the relationship may have implications for understanding the systemic effects of long-term HIV infection, particularly on organs susceptible to microvascular damage, and may lead to better methods for monitoring the health of HIV-infected individuals.
Acknowledgments
THIS STUDY WAS SUPPORTED BY COOPERATIVE AGREEMENTS FROM THE NATIONAL EYE INSTITUTE, THE NATIONAL Institutes of Health, Bethesda, Maryland, to the Mount Sinai School of Medicine, New York, NY (U10 EY08052), and the Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland (U10 EY 08057). Additional support was provided by the National Center for Research Resources through General Clinical Research Center Grants 5M01 RR 00350 (Baylor College of Medicine, Houston, Texas), 5M01 RR 05096 (Louisiana State University, Baton Rouge, Louisiana/Tulane/Charity Hospital, New Orleans, Louisiana), 5M01 RR00096 (New York University Medical Center, New York, New York), 5M01 RR 00865 (University of California, Los Angeles, California), 5M01 RR00046 (University of North Carolina, Chapel Hill, North Carolina), 5M01 RR00043 (University of Southern California, Los Angeles, California), and ULI RR024996 (Weill Medical College of Cornell University, Ithaca, New York). Support also was provided through cooperative agreements U01 AI 27674 (Louisiana State University/Tulane), U01 AI 27660 (University of California, Los Angeles), U01 AI 27670 (University of California, San Diego, California), U01 AI 27663 (University of California, San Francisco, California), U01 AI25868 (University of North Carolina), and U01 AI32783 (University of Pennsylvania, Philadelphia, Pennsylvania). Additional support was provided by the Skirball Foundation, New York, New York (Dr Holland); The Elizabeth Taylor AIDS Foundation through a gift to the UCLA Herb Ritts, Jr Memorial Vision Fund (Dr Holland); and the Jack H. Skirball Endowed Professorship (Dr Holland).
Funding entities had no role in the conduction or presentation of this study. Involved in study design (G.N.H., P.J.K., M.L.V.N., F.J.P., D.A.J.); data collection (M.L.V.N. and the SOCA Research Group); data management and analysis (M.L.V.N.); data interpretation (G.N.H., P.J.K., M.L.V.N., F.J.P., D.A.J.); preparation of initial draft of manuscript (G.N.H., P.J.K., M.L.V.N.); and review and approval of manuscript (all authors). The study was conducted with approval from the appropriate institutional review boards at each participating institution. Informed consent was obtained from all subjects, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations.
Footnotes
None of the authors have conflicts of interest with any aspect of this study.
THE SOCA RESEARCH GROUP Participating clinical centers can be found in a prior printed publication.1
REFERENCES
- 1.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114:780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 3.Shah KH, Holland GN, Yu F, Van Natta M, Nusinowitz S. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol. 2006;142:284–292. doi: 10.1016/j.ajo.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Freeman WR, Van Natta ML, Jabs D, et al. Vision function in HIV-infected individuals without retinitis: report of the Studies of Ocular Complications of AIDS Research Group. Am J Ophthalmol. 2008;145:453–462. doi: 10.1016/j.ajo.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arend O, Remky A, Evans D, Stuber R, Harris A. Contrast sensitivity loss is coupled with capillary dropout in patients with diabetes. Invest Ophthalmol Vis Sci. 1997;38:1819–1824. [PubMed] [Google Scholar]
- 6.Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol. 1996;80:209–213. doi: 10.1136/bjo.80.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grauslund J, Green A, Sjolie AK. Proliferative retinopathy and proteinuria predict mortality rate in type 1 diabetic patients from Fyn County, Denmark. Diabetologia. 2008;51:583–588. doi: 10.1007/s00125-008-0953-8. [DOI] [PubMed] [Google Scholar]
- 8.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30:292–299. doi: 10.2337/dc06-1747. [DOI] [PubMed] [Google Scholar]
- 9.van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28:1383–1389. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117:1487–1495. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 12.Elliott D, Billimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clinical Vision Sciences. 1991;6:471–475. [Google Scholar]
- 13.Lovie-Kitchin JE, Brown B. Repeatability and intercorrelations of standard vision tests as a function of age. Optom Vis Sci. 2000;77:412–420. doi: 10.1097/00006324-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Myers VS, Gidlewski N, Quinn GE, Miller D, Dobson V. Distance and near visual acuity, contrast sensitivity, and visual fields of 10-year-old children. Arch Ophthalmol. 1999;117:94–99. doi: 10.1001/archopht.117.1.94. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical statistics for medical research. Chapman and Hall; London: 1993. pp. 241–248. [Google Scholar]
- 17.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–681. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 18.Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145:397–408. doi: 10.1016/j.ajo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Rubin GS, Roche KB, Prasada-Rao P, Fried LP. Visual impairment and disability in older adults. Optom Vis Sci. 1994;71:750–760. doi: 10.1097/00006324-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46:58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 21.Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age Ageing. 1991;20:175–181. doi: 10.1093/ageing/20.3.175. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 23.Dargent-Molina P, Favier F, Grandjean H, et al. Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/s0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 24.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone. 2003;32:468–473. doi: 10.1016/s8756-3282(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 26.Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 27.Owsley C, Stalvey BT, Wells J, Sloane ME, McGwin G., Jr Visual risk factors for crash involvement in older drivers with cataract. Arch Ophthalmol. 2001;119:881–887. doi: 10.1001/archopht.119.6.881. [DOI] [PubMed] [Google Scholar]
- 28.Pedula KL, Coleman AL, Hillier TA, et al. Visual acuity, contrast sensitivity, and mortality in older women: Study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1871–1877. doi: 10.1111/j.1532-5415.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 29.Gimeno Orna JA, Castro Alonso FJ, Sanchez Vano R, Latre Rebled B, Lou Arnal LM, Molinero Herguedas E. [Diabetic retinopathy and mortality in type 2 diabetic patients] Med Clin (Barc) 2006;126:686–689. doi: 10.1157/13088771. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in Type 2 diabetic patients. Diabet Med. 2008;25:45–50. doi: 10.1111/j.1464-5491.2007.02327.x. [DOI] [PubMed] [Google Scholar]
- 31.Geier SA, Hammel G, Bogner JR, Kronawitter U, Berninger T, Goebel FD. HIV-related ocular microangiopathic syndrome and color contrast sensitivity. Invest Ophthalmol Vis Sci. 1994;35:3011–3021. [PubMed] [Google Scholar]
- 32.Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 33.Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–249. doi: 10.1001/archopht.124.2.243. [DOI] [PubMed] [Google Scholar]
- 34.Taylor HR, McCarty CA, Nanjan MB. Vision impairment predicts five-year mortality. Trans Am Ophthalmol Soc. 2000;98:91–96. discussion 96–99. [PMC free article] [PubMed] [Google Scholar]