Abstract
Protein kinase C (PKC)-mediated phosphorylation of troponin I (cTnI) at Ser42/44 is increased in heart failure. While studies in rodents demonstrated that PKC-mediated Ser42/44 phosphorylation decreases maximal force and ATPase activity, PKC incubation of human cardiomyocytes did not affect maximal force. We investigated whether Ser42/44 pseudo-phosphorylation affects force development and ATPase activity using troponin exchange in human myocardium. Additionally, we studied if pseudo-phosphorylated Ser42/44 modulates length-dependent activation of force, which is regulated by protein kinase A (PKA)-mediated cTnI-Ser23/24 phosphorylation. Isometric force was measured in membrane-permeabilized cardiomyocytes exchanged with human recombinant wild-type troponin or troponin mutated at Ser42/44 or Ser23/24 into aspartic acid (D) or alanine (A) to mimic phosphorylation and dephosphorylation, respectively. In troponin-exchanged donor cardiomyocytes experiments were repeated after PKA incubation. ATPase activity was measured in troponin-exchanged cardiac muscle strips. Compared to wild-type, 42D/44D decreased Ca2+-sensitivity without affecting maximal force in failing and donor cardiomyocytes. In donor myocardium, 42D/44D did not affect maximal ATPase activity or tension cost. Interestingly, 42D/44D blunted the length-dependent increase in Ca2+-sensitivity induced upon PKA-mediated phosphorylation. Since the drop in Ca2+-sensitivity at physiological Ca2+-concentrations is relatively large phosphorylation of Ser42/44 may result in a decrease of force and associated ATP utilization in the human heart.
Keywords: myofilament function, protein phosphorylation, cardiomyocyte, troponin I, protein kinase C
Introduction
Phosphorylation of myofilament proteins is an important post-translational modification which regulates cardiomyocyte contractility of the heart. Specifically, the functional properties of cardiac troponin I (cTnI) are regulated by phosphorylation and play a vital role in tuning cardiomyocyte performance in health and disease.1 cTnI is the “inhibitor” of the trimeric troponin complex, which together with cardiac troponin C (cTnC, the Ca2+-sensor) and cardiac troponin T (cTnT), controls the position of tropomyosin on the actin filament in response to Ca2+ and thereby regulates actin-myosin interactions and thus cardiomyocyte force development and relaxation.1
cTnI has multiple phosphorylation sites that regulate cardiomyocyte contractility in cardiac health and disease. In the healthy heart upon stress or exercise, beta-adrenergic receptor stimulation activates protein kinase A (PKA).2 PKA phosphorylates cTnI at Ser23/24, thereby reducing myofilament Ca2+-sensitivity and enhancing relaxation of the heart that is needed to maintain cardiac performance at increased heart rates.3–5 In the end- stage failing heart, PKA activity is reduced due to defects in the beta-adrenergic signaling pathway.2 Reduced PKA activity has been associated with decreased cTnI-Ser23/24 phosphorylation,6–9 and increased Ca2+-sensitivity.7, 8 In contrast, expression10, 11 and activity10 of several protein kinase C (PKC) isoforms (α, β1, β2) are increased in heart failure and higher cTnI phosphorylation has been reported at several PKC-sites12 (Ser42 and Ser44,13 Thr143,13 and Ser19913) in end-stage failure when compared to non-failing myocardium. Although PKC-mediated effects of cTnI phosphorylation have been studied in vitro and in vivo in rodents, data on site-specific phosphorylation of cTnI by PKC is scarce in man.
In the present study, we focused on PKC-mediated site-specific phosphorylation of cTnI at Ser42/44 (Ser42/44 in human, Ser43/45 in mice and rats) in human cardiomyocytes, based on evidence that Ser42/44 phosphorylation is increased in heart failure in animal studies.14–16 It has been demonstrated via top-down mass spectrometry (MS) that cTnI-Ser42/44 phosphorylation is induced by pressure overload of the heart (hypertension-induced heart failure) in rats.14 In addition, in a rat model of low myocardial blood flow (impaired myocardial perfusion), increased cTnI phosphorylation at Ser42/44 was detected by LC-MS.15 In a mouse model of myocardial infarction, enhanced phosphorylation of Ser42 was detected using a phospho-specific antibody at 2 and 14 days after myocardial infarction, which returned to control levels after 2 months.16 Studies in failing human hearts showed either no phosphorylation at PKC sites9 or a slightly higher Ser42 and Ser44 phosphorylation compared to non-failing donor hearts.13 The low phosphorylation level or absence of phosphorylation at Ser42/44 in human studies may be explained by transient changes at PKC sites, which are more difficult to trace in samples from patients with advanced stages of cardiac disease than in experimental animal models.
Studies in rodents demonstrated that PKC-mediated phosphorylation at Ser42/44 decreases maximal force17 and maximal ATPase activity.18, 19 However, in human cTnI phosphorylation by the catalytic domain of PKC or by PKCα or PKCε did not affect maximal force in donor and failing cardiomyocytes, even after pretreatment with phosphatases.20, 21 Though it is conceivable that Ser42/44 were not phosphorylated by PKC in these experiments, an alternative explanation may be that the effects of phosphorylation of cTnI-Ser42/44 are species-dependent. Therefore, in the present study we investigated the specific functional effects of Ser42/44 phosphorylation in human cardiomyocytes by exchanging endogenous cTnI with pseudo-phosphorylated cTnI at Ser42/44, mimicked by aspartic acid (42D/44D). Force development and ATPase activity were measured at maximal and submaximal calcium concentrations. In addition, the effect of Ser42/44 phosphorylation on length-dependent activation was studied with and without PKA incubation, since PKA-mediated cTnI phosphorylation at Ser23/24 is an important regulator of the sarcomere length-dependent increase in myofilament Ca2+-sensitivity.22, 23 Data were compared with cells exchanged with recombinant wild-type cTnI (Wt), pseudo-dephosphorylated cTnI at Ser42/44 (mimicked by alanine; 42A/44A) and pseudo-phosphorylated cTnI at Ser23/24. The specific functional effects of Ser42/44 phosphorylation in human cardiomyocytes are compared with previously published results in rodent.
Methods
Expression and purification of recombinant troponin subunits
Recombinant human troponin complex was produced as described in detail previously.5 Briefly, three different cTnI forms were made via site-directed mutations of Ser42/44 and Ser23/24 into aspartic acid (D) to mimic phosphorylation or into alanine (A) to mimic dephosphorylation: 42D/44D, 42A/44A and 23D/24D. cDNA encoding human cardiac isoforms (troponin C (cTnC), myc-tag labeled cTnT (cTnT-myc), cTnI wild-type, and cTnI mutants) were transformed in E. coli Rosetta224 and cultured under carbenicillin/chloramphenicol selection in Overnight Express TB medium (EMD Biosciences). Cultures were harvested by centrifugation, re-suspended in phosphate buffered saline (PBS), and centrifuged at 10000xg. Pellets were stored at −80°C until used. Troponin subunits were purified using fast protein liquid chromatography (AKTA-FPLC System Amersham Biosciences) essentially as described previously.24
Fractions containing equal ratios of cTnT, cTnC and cTnI subunits were pooled, subsequently purified by AKTA-FPLC chromatography using a Resource Q to remove residual uncomplexed troponin subunit and finally dialyzed against 10 mM imidazole, 200 mM KCl, 5 mM MgCl2, 2.5 mM EGTA, 1 mM DTT, 0.1 mM PMSF (pH 6.9; twice, 1L each) prior to concentrating the complexes to a final concentration >2 mg/mL by centrifugation using Centriprep YM-10 centrifugal filters (Millipore).
Exchange of human troponin complex
Exchange of recombinant cTn in human cardiomyocytes was done as described previously.5 Briefly, single cardiomyocytes were mechanically isolated with a glass tissue homogenizer, and permeabilized by Triton X-100 (0.5%; v/v) for 5 minutes. They were subsequently incubated overnight at 4°C in exchange solution containing 1 mg/mL of recombinant human cTn complex with the addition of 4 mM CaCl2, 4 mM DTT, 5 μl/mL protease inhibitor cocktail (Sigma, P8340) and 10 μl/mL phosphatase inhibitor cocktail 2 and 3 (Sigma, P5726 and P0044) (pH 6.9). The next day, cardiomyocytes were washed twice in rigor solution and finally in relaxing solution (5.95 mM Na2ATP, 6.04 mM MgCl2, 2 mM EGTA, 139.6 mM KCl, 10 mM imidazole, pH 7.0). This produces a homogenous distribution of recombinant cTn complex within the exchanged cardiomyocytes.24
Exchange experiments were performed in cardiomyocytes from end-stage failing idiopathic dilated cardiomyopathy (IDCM) hearts (2 males/1 female, left ventricular ejection fraction 16.7 ± 4.4%, age 54.3 ± 1.9 years) or from non-failing donor myocardium that was not required for heart transplantation. The tissue was collected in ice-cold cardioplegic solution and stored in liquid nitrogen. Samples were obtained after informed consent and with approval of the Human Research Ethics Committee of The University of Sydney (#12146). The investigation conforms with the principles outlined in the Declaration of Helsinki (1997). Human cardiac samples used were extensively characterized (cardiomyocyte force characteristics and cTnI phosphorylation) in a previous study.6
Determination of the degree of troponin exchange
To determine the degree of cTn exchange and to assess the protein phosphorylation status, part of the suspension of cells was treated with 2D-clean-up kit (GE Healthcare) as described by the manufacturer protocol after overnight cTn exchange. Subsequently, tissue pellets were homogenized in sample buffer containing 15% glycerol, 62.5 mM Tris (pH 6.8), 1% (w/v) SDS and 2% (w/v) DTT. Protein concentrations (2–4 mg/mL) were measured with RCDC Protein Assay Kit II (BioRad).
Immunoblotting was used to determine the degree of exchange of endogenous cTn for recombinant cTn complex. Recombinant cTnT was labeled with a Myc-tag to allow discrimination between endogenous and recombinant cTn complex. Proteins were separated on a 13% SDS-polyacrylamide gel and blotted (1 hour at 75 V) onto a nitrocellulose membrane (Hybond) using the protocol supplied by the manufacturer. A monoclonal antibody against cTnT (Clone JLT-12, Sigma; dilution 1:1250) was used to detect endogenous and recombinant cTnT by chemiluminescence (ECL, Amersham Biosciences). We have previously demonstrated that the affinity of the cTnT antibody was the same for cTnT compared to cTnT-Myc and that cTnT loading was within the linear range.5
Myofilament protein phosphorylation
The phosphorylation levels of sarcomeric proteins were determined before and after cTn exchange using ProQ Diamond-stained 1D gels as described previously.5 The phosphorylation signals (cardiac myosin-binding protein-C, myosin light chain 2 and desmin) were normalized to the intensities of the SYPRO Ruby-stained myosin light chain 2 bands to correct for small differences in protein loading. The PeppermintStick Phosphoprotein marker (Molecular Probes) was used to correct for differences in staining between gels.25 The ratio of the intensities of ProQ-Diamond and SYPRO Ruby stained ovalbumin band was used to correct for inter-gel differences. The distribution of endogenous phosphorylated species of cTnI was analyzed using Phos-tag™ acrylamide gels (FMS Laboratory; Hiroshima University, Japan) as described before.6, 26
Isometric force measurements
Force measurements in cardiomyocytes exchanged with recombinant cTn were performed as described previously.24 Isometric force was measured at 15°C and myofilament length-dependent activation (LDA) was determined by measuring force at different Ca2+ concentrations, first at a sarcomere length of 1.8 μm and subsequently at 2.2 μm. If indicated, troponin exchanged donor cardiomyocytes were incubated with exogenous PKA for 40 minutes at 20°C in relaxing solution containing the catalytic subunit of PKA (1 U/μl; a total of 100 U/incubation, Sigma). The following parameters were determined: passive force at pCa 9.0 (Fpas), maximal force at pCa 4.5 (Fmax: total force minus Fpas), Ca2+-sensitivity of force development (pCa50) and steepness of the sigmoidal force-pCa relation (nHill).
ATPase measurements
Muscle strips were isolated from the left ventricular free wall from a healthy donor with no history of cardiac disease. All muscle strips were cut parallel to the long axis of the fibers to minimize damage in cold ATPase relaxing solution (1 mM Mg2+, 145 mM KCl, 2 mM EGTA, 4 mM ATP, 10 mM imidazole, pH 7.0). Subsequently, muscle strips were permeabilized overnight in relaxing solution with 1% Triton at 4°C. The next day, the strips were incubated for 4 hours at 4°C in exchange solution (1 mg/mL of recombinant human cTn complex) and then washed as described above. The dimensions of the muscle strips were 1.44 ± 0.04 mm long, 365 ± 8 μm wide and 312 ± 5 μm thick.
ATP utilization and force development in muscle strips after cTn exchange was measured simultaneously. The experimental procedures, solutions and equipment used for functional measurements were as described previously.27 The length of the preparations was adjusted on the basis of passive tension by stretching them until 1–2 kN/m2 was reached, corresponding to a sarcomere length of about 2.2 μm.28 Isometric force and ATP utilization were measured at maximal and submaximal Ca2+-concentrations at 20°C. Force generating capacity was determined after the force signal reached a plateau and normalized to the cross-sectional area (CSA) of the muscle strip to calculate tension (force per CSA). The CSA of the preparation was estimated assuming an elliptical shape, i.e. CSA= (width x depth x π)/4.
ATP utilization, i.e. ATPase activity, was measured using an enzyme coupled assay. In this assay the ATP regeneration from ADP and phosphoenol-pyruvate, catalyzed by the enzyme pyruvate kinase, is coupled to the oxidation of NADH to NAD and the reduction of pyruvate to lactate, catalyzed by L-lactic dehydrogenase. NADH oxidation was photometrically measured from the absorbance at 340 nm. The absorbance signal was calibrated by adding 0.5 nmol of ADP to the chamber. Using this calibration, the rate of ATP utilization by the muscle strip can be derived from the slope of the absorbance signal. The Ca2+-activated ATP utilization was normalized to the volume of the muscle strip. The economy of muscle contraction is expressed as tension cost, i.e. the rate of ATP utilization during force development (ATP utilization/tension).
Data analysis
Data analysis was performed as previously described using the Hill equation to fit force-Ca relations:
Where F is steady-state force, F0 the steady-state force at saturating [Ca2+], nHill the steepness of the relationship, and Ca50 (or pCa50) is the midpoint of the relation. One-way ANOVA followed by Bonferroni post-hoc tests were used to compare the amounts of exchange of the different cTn-complexes. Two-way ANOVA repeated measures followed by a Bonferroni post-hoc test was used to compare groups exchanged with the different cTn complexes (#P<0.05, significant difference compared to control (Wt: Table 2–4)). When two-way ANOVA revealed a significant effect for sarcomere length (P<0.05), paired t-tests were performed to compare cell measurements at two different sarcomere lengths in each cTn-exchange group (*P<0.05, 1.8 vs 2.2 μm). Differences between maximal tension, ATP utilization and tension cost were analyzed with a t-test. To obtain information about tension and ATP utilization at submaximal Ca2+-concentrations two-way ANOVA with Bonferroni’s post hoc corrections were performed. Linear regression analysis was used to determine the correlation between ATP utilization and tension over the entire range of Ca2+-concentrations. Values are given as means ± S.E.M. n denotes myocytes, muscle strips or patients.
Table 2. Force measurements in failing cardiomyocytes after exchange with recombinant cTn at sarcomere length 1.8 and 2.2 μm.
IDCM cardiomyocytes exchanged with Wt, 42D/44D and 42A/44A troponin complex (2 samples; 8 myocytes per complex). Myofilament force was measured at sarcomere lengths of 1.8 and 2.2 μm at different Ca2+-concentrations. Stretching of cardiomyocytes from 1.8 to 2.2 μm significantly increased Ca2+-sensitivity (pCa50), maximal (Fmax) and passive force (Fpas) in all groups. There was no significant effect of sarcomere length on nHill (steepness of the force-pCa curves). Two-way ANOVA repeated measures followed by a Bonferroni post-hoc test revealed a significant decrease in Ca2+-sensitivity in 42D/44D and 42A/44A compared to Wt at both sarcomere lengths (#P<0.05, significant difference compared to Wt). When two-way ANOVA revealed a significant effect for sarcomere length (P<0.05), paired t-tests were performed to compare cell measurements at two different sarcomere lengths in each cTn-exchange group (*P<0.05, 1.8 vs 2.2 μm). Wt data has been published before.23 Values are means ± SEM.
| Wt | 42D/44D | 42A/44A | ||
|---|---|---|---|---|
|
| ||||
| Fmax (kN.m−2) | 1.8 μm | 14.5±0.8 | 10.2±1.4 | 12.2±2.2 |
| 2.2 μm | 18.0±1.0* | 13.7±1.6* | 15.1±2.0* | |
|
| ||||
| Fpas (kN.m−2) | 1.8 μm | 2.2±0.1 | 2.4±0.3 | 1.9±0.3 |
| 2.2 μm | 3.3±0.3* | 3.6±0.4* | 3.1±0.5* | |
|
| ||||
| pCa50 | 1.8 μm | 5.64±0.02 | 5.42±0.01# | 5.56±0.02# |
| 2.2 μm | 5.67±0.02* | 5.45±0.01#,* | 5.60±0.02#,* | |
|
| ||||
| nHill | 1.8 μm | 2.9±0.1 | 2.4±0.2 | 2.5±0.2 |
| 2.2 μm | 2.7±0.1 | 2.4±0.1 | 2.5±0.2 | |
Table 4. Force measurements in donor cardiomyocytes after exchange with recombinant troponin and subsequent PKA treatment.
Donor cardiomyocytes exchanged with 42D/44D, 23D/24D and Wt troponin complex with PKA treatment (4–5 myocytes per complex). Myofilament force was measured at sarcomere lengths of 1.8 and 2.2 μm at different Ca2+-concentrations. Ca2+-sensitivity derived from the midpoint of the force–pCa relationship (pCa50) significantly increased at 2.2 μm compared to 1.8 μm for all complexes, however, significantly less in 42D/44D compared with Wt and 23D/24D. Stretching of cardiomyocytes from 1.8 to 2.2 μm increased maximal (Fmax) and passive force (Fpas) in all groups, without affecting nHill (steepness of the force-pCa curves). Compared to Wt, 42D/44D decreased Ca2+-sensitivity, reaching significance at sarcomere length 2.2 μm (#P<0.05). pCa50 of Wt did not significantly differ from 23D/24D after PKA treatment. No significant differences between the 3 cTn complexes for Fmax, Fpas and nHill were found. When two-way ANOVA revealed a significant effect for sarcomere length (P<0.05), paired t-tests were performed to compare cell measurements at two different sarcomere lengths in each cTn-exchange group (*P<0.05, 1.8 vs 2.2 μm). Wt and 23D/24D data have been published before.23 Values are means ± SEM.
| With | PKA | Wt | 42D/44D | 23D/24D |
|---|---|---|---|---|
|
| ||||
| Fmax (kN.m−2) | 1.8 μm | 12.8±1.1 | 12.5±1.2 | 11.1±1.9 |
| 2.2 μm | 14.9±1.6* | 16.3±0.7* | 16.2±2.2* | |
|
| ||||
| Fpas (kN.m−2) | 1.8 μm | 2.0±0.2 | 2.1±0.2 | 2.6±0.1 |
| 2.2 μm | 2.9±0.2* | 3.3±0.5* | 3.6±0.3* | |
|
| ||||
| pCa50 | 1.8 μm | 5.34±0.06 | 5.25±0.01 | 5.37±0.01 |
| 2.2 μm | 5.44±0.08* | 5.28±0.01*,# | 5.48±0.01* | |
|
| ||||
| nHill | 1.8 μm | 2.1±0.1 | 1.9±0.1 | 1.7±0.1 |
| 2.2 μm | 2.1±0.1 | 1.9±0.3 | 1.7±0.2 | |
Results
Pseudo-phosphorylation of Ser42/44 decreases myofilament Ca2+-sensitivity
As the effect of pseudo-phosphorylated Ser42/44 may be modulated by cTnI phosphorylation at Ser23/24, we first analyzed baseline cTnI phosphorylation in the IDCM samples used in the exchange experiments. Phostag analysis showed relatively low cTnI phosphorylation in IDCM hearts (n = 3): 7.5 ± 0.9% bisphosphorylated, 27.2 ± 4.7% monophosphorylated and 65.3 ± 5.1% unphosphorylated cTnI.
Troponin exchange was determined using immunoblot detection of the myc-tag labeled cTnT. Figure 1A depicts a representative immunoblot loaded with IDCM cardiomyocytes incubated overnight with recombinant cTn containing wild-type cTnI (Wt), pseudo-phosphorylated cTnI at Ser42/44 (42D/44D) and pseudo-dephosphorylated cTnI at Ser42/44 (42A/44A). Myc-tagged cTnT migrates more slowly through the gel compared to endogenous cTnT and therefore two cTnT bands are seen. The percentage of cTn exchange was calculated from the ratio of myc-tagged recombinant cTnT and the total amount of cTnT. The average exchange percentage of the three cTn complexes in IDCM did not differ between the cTn complexes and amounted to 65.9±1.0% (Figure 1B), indicating similar incorporation in the myofilaments. To assess if cTn exchange affected phosphorylation of myofilament proteins other than troponin, ProQ-Diamond staining was performed before and after cTn exchange. In agreement with our previous study,5 no significant differences in protein phosphorylation were induced in the other contractile proteins studied (cardiac myosin-binding protein-C, myosin light chain 2 and desmin) upon cTn exchange (Table 1).
Fig. 1. Quantification of troponin exchange in cardiomyocytes by immunoblotting.
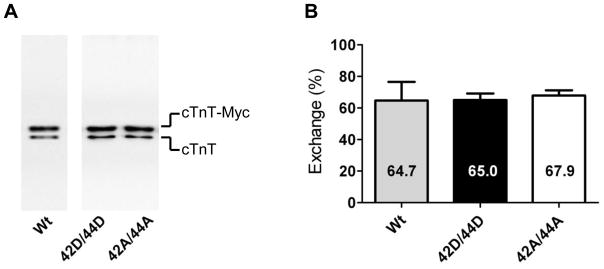
A. Immunoblot stained with an antibody against troponin T (cTnT) that recognizes both endogenous cTnT (lower band) and recombinant Myc-tag labeled cTnT (cTnT-myc; upper band). An example is shown of a suspension of IDCM cardiomyocytes exchanged with recombinant cTn containing wild-type cTnI (Wt), pseudo-phosphorylated cTnI at Ser42/44 (42D/44D) or pseudo-dephosphorylated cTnI at Ser42/44 (42A/44A) at 1 mg/mL. B. The average percentages of cTn exchange in cardioymyocytes after overnight incubation in exchange solution with 1 mg/mL cTn containing the different cTnI species (average values represent cTn exchange experiments in cardiomyocytes isolated from 3 IDCM hearts). No significant differences were found in exchange percentage between the three cTnI complexes.
Table 1. Myofilament protein phosphorylation before and after cTn exchange.
Values are means ± SEM of the ProQ/SYPRO intensity ratio. Shown is myofilament protein phosphorylation determined with ProQ-diamond staining before and after exchange in three IDCM samples. Troponin exchange did not affect myofilament protein phosphorylation (by one-way ANOVA comparing before exchange to the exchanged groups). cMyBP-C, cardiac myosin-binding protein-C; MLC2, myosin light chain 2; A, alanine substitution; D, aspartic acid substitution; Wt, wild-type.
| cMyBP-C | Desmin | MLC2 | |
|---|---|---|---|
| Before exchange | 0.43±0.01 | 0.58±0.15 | 0.40±0.10 |
| Wt | 0.41±0.03 | 0.59±0.07 | 0.34±0.04 |
| 42D/44D | 0.41±0.05 | 0.63±0.20 | 0.37±0.10 |
| 42A/44A | 0.41±0.04 | 0.62±0.20 | 0.47±0.18 |
To determine the effects of Ser42/44 phosphorylation on myofilament force development at optimal overlap of the myofilaments, force measurements were performed at 2.2 μm sarcomere length in failing cardiomyocytes exchanged with 42D/44D. Data were compared to cells exchanged with 42A/44A, where alanines mimic dephosphorylation at Ser42/44, and unphosphorylated Wt cTnI. Myofilament Ca2+-sensitivity was significantly decreased after exchange with pseudo-phosphorylated cTnI, evident from the right shift of the force-pCa curve for 42D/44D compared to Wt (Figure 2A). Pseudo-dephosphorylation at Ser42/44 (42A/44A), which blocks Ser42/44 phosphorylation and was used as an additional control, also significantly reduced Ca2+-sensitivity compared to Wt (Figure 2A). The midpoint of the force-pCa relation (pCa50) was significantly lower in cells exchanged with 42D/44D and 42A/44A compared to Wt (Figure 2B). Therefore, alanine mutations at Ser42/44 do not function as phospho-null mutations. Moreover, pCa50 was significantly lower in 42D/44D compared to 42A/44A (Figure 2B).
Fig. 2. Ca2+-sensitivity is decreased upon pseudo-phosphorylation of Ser42/44 in cardiomyocytes from failing human hearts.
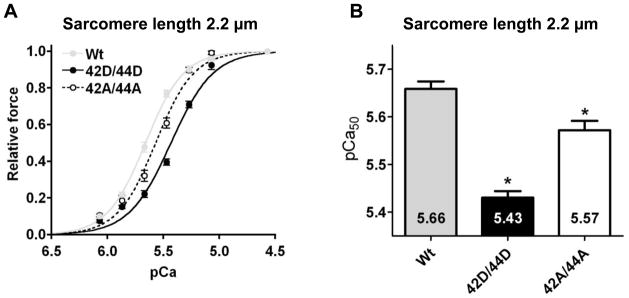
Myofilament force development measured at a sarcomere length of 2.2 μm at various [Ca2+] in permeabilized cardiomyocytes from idiopathic dilated cardiomyopathy (IDCM) patients in which endogenous troponin complex was partially exchanged (65.9±1.0%) with 1 mg/mL of the recombinant myc-tag labeled troponin complexes (12 cardiomyocytes from 3 IDCM hearts per group). A. Compared to wild-type cTnI (Wt), the relative force plotted against pCa demonstrates a shift of the curve to the right upon exchange with pseudo-phosphorylated cTnI at Ser42/44 (42D/44D) and upon exchange with cTnI pseudo-dephosphorylated at Ser42/44 (42A/44A). B. Compared to Wt, Ca2+-sensitivity derived from the midpoint of the force–pCa relationship (pCa50) was significantly decreased after exchange with 42D/44D and 42A/44A. pCa50 was significantly lower in 42D/44D compared to 42A/44A. *, p<0.05, Wt versus 42D/44D, 42A/44A in post-test Bonferroni analyses of one-way ANOVA.
Pseudo-phosphorylation of Ser42/44 does not affect length-dependent activation in failing cardiomyocytes
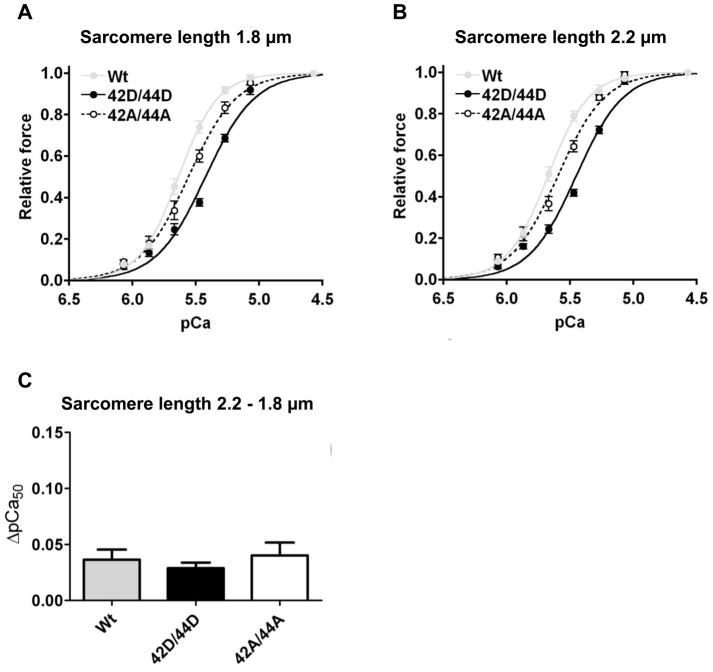
To study the effect of Ser42/44 phosphorylation on length-dependent myofilament activation (LDA), force measurements were performed in IDCM cardiomyocytes at sarcomere lengths of 1.8 μm (Figure 3A) and 2.2 μm (Figure 3B) in cTn exchanged cardiomyocytes from two IDCM hearts (8 cells for each group, 68.6±4.3% exchange). The effect of sarcomere lengthening on myofilament Ca2+-sensitivity is displayed as the ΔpCa50, which is the difference in pCa50 at a sarcomere length of 1.8 and 2.2 μm. For all cTn complexes, Ca2+-sensitivity significantly increased upon cardiomyocyte lengthening. The length-dependent increase in pCa50 did not differ among groups (ΔpCa50 Wt; 0.04 ± 0.01, 42D/44D; 0.03 ± 0.01, 42A/44A; 0.04 ± 0.01; Figure 3C). Sarcomere lengthening from 1.8 to 2.2 μm significantly increased Fmax and Fpas in all cTn-exchanged groups in accordance with the well-known length-force relationship,29 while nHill was not affected (Table 2). No significant differences in Fmax, Fpas and nHill were found between cells exchanged with the three cTn complexes at both sarcomere lengths (Table 2). Overall, these data show that phosphorylation at Ser42/44 decreases myofilament Ca2+-sensitivity, but does not alter LDA of the sarcomeres in failing cardiomyocytes.
Fig. 3. Pseudo-phosphorylation of Ser42/44 does not affect length-dependent activation in cardiomyocytes from failing human hearts.
Length-dependent regulation of Ca2+-sensitivity was studied by measuring myofilament force at various [Ca2+] at a sarcomere length of 1.8 (A) and 2.2 (B) μm (2 IDCM hearts; 8 cells for each group, 68.6±4.3% exchange) after cTn exchange. C. Sarcomere lengthening significantly increased Ca2+-sensitivity for all 3 cTn complexes. Pseudo-phosphorylation of Ser42/44 (42D/44D) did not significantly alter the length-dependent increase in myofilament Ca2+-sensitivity (ΔpCa50: 2.2-1.8 μm) compared to Wt or 42A/44A.
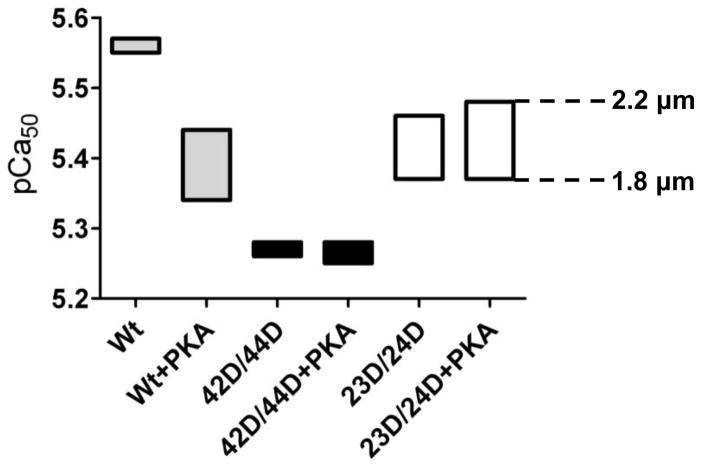
Pseudo-phosphorylation of Ser42/44 blunts the enhanced length-dependent increase in myofilament Ca2+-sensitivity upon PKA incubation in donor cardiomyocytes
The absence of an effect on LDA of Ser42/44 phosphorylation in failing cells may be due to a blunted LDA of failing myocardium, as a reduced length-dependent increase in myofilament Ca2+-sensitivity has been reported in failing compared to donor cardiac tissue.30 We recently reported a length-dependent increase in pCa50 of 0.10 ± 0.01 (1.8 versus 2.2 μm sarcomere length) in six donor samples,31 which is significantly larger than the value found in the IDCM samples used in this study without cTn exchange (ΔpCa50 = 0.04 ± 0.01). Such a blunted length-dependent increase in Ca2+-sensitivity in failing cardiomyocytes may be the result of the low baseline cTnI phosphorylation, since it has been demonstrated that phosphorylation of cTnI-Ser23/24 enhances the length-dependent increase in Ca2+-sensitivity,22, 23 but could also be related to changes in myofilament proteins other than troponin. Therefore, we studied the effects of Ser42/44 pseudo-phosphorylation in combination with PKA-mediated effects on LDA in non-failing donor cardiomyocytes. Wt exchange was used as a control because alanine mutations at Ser42/44 did not function as phospho-null mimetics in failing cardiomyocytes (Figure 2). Pseudo-phosphorylation at Ser23/24 (23D/24D) was used to mimic the effects of PKA-mediated phosphorylation at Ser23/24. Wt, 42D/44D and 23D/24D were studied without and with treatment with exogenous PKA. The average cTn exchange in donor cardiomyocytes amounted to 84.1 ± 2.8 % and did not differ between the three complexes.
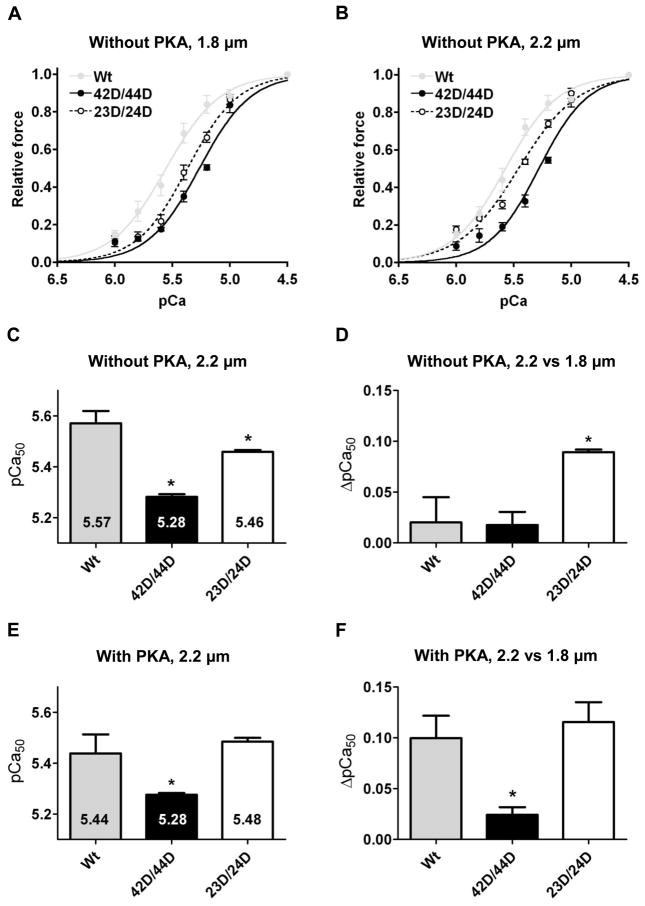
Similar to IDCM, 42D/44D significantly decreased Ca2+-sensitivity compared to Wt in donor cardiomyocytes at both sarcomere lengths (Figure 4A–C). 23D/24D also significantly reduced Ca2+-sensitivity compared to Wt, however, to a lesser extent than 42D/44D (Figure 4A–C). No difference was found in the length-dependent change in Ca2+-sensitivity (ΔpCa50) between Wt and 42D/44D, while it was significantly higher in cells exchanged with 23D/24D (Figure 4D and Table 3). No differences were observed in Fmax, Fpas and nHill among the three groups (Table 3).
Figure 4. Phosphorylation of Ser42/44 blunts the length-dependent increase in Ca2+-sensitivity in donor cardiomyocytes.
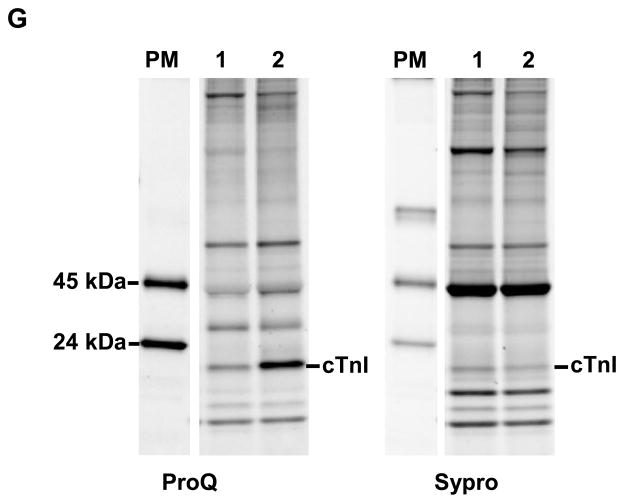
Myofilament force development was measured at a sarcomere length of 1.8 (A) and 2.2 μm (B) at various [Ca2+] in donor cardiomyocytes in which endogenous troponin complex was partially exchanged (84.1±2.8%) with recombinant myc-tag-labeled troponin complexes (4–5 cardiomyocytes per group). C. Compared to unphosphorylated wild-type cTnI (Wt), Ca2+-sensitivity derived from the midpoint of the force–pCa relationship (pCa50) was significantly decreased after exchange with pseudo-phosphorylated cTnI at Ser42/44 (42D/44D) and at Ser23/Ser24 (23D/24D) at 2.2 μm. D. The length-dependent increase in Ca2+-sensitivity (ΔpCa50) did not differ between 42D/44D and Wt. However, exchange with 23D/24D significantly increased the length-dependent Ca2+-sensitivity increase compared to Wt. E, F. Troponin-exchanged donor cardiomyocytes were incubated with exogenous PKA. E. PKA treatment decreased Ca2+-sensitivity in cells exchanged with Wt compared to untreated cardiomyoctes after exchange, but had no effect in 42D/44D and 23D/24D cells (Figure 4E versus Figure 4C). F. PKA treatment significantly increased the length-dependent increase in Ca2+-sensitivity for Wt compared to untreated cells after exchange (Figure 4D versus Figure 4F). However, PKA treatment did not significantly increase the length-dependent increase in Ca2+-sensitivity for 42D/44D (Figure 4D versus Figure 4F). After PKA treatment, ΔpCa50 was significantly lower in 42D/44D compared with Wt. G. Myofilament proteins of cardiomyocytes exchanged with 42D/44D without (lane 1) and with (lane 2) PKA treatment were separated by 1D-gel electrophoresis. Staining differences between gels were corrected with phosphorylated ovalbumin of the peppermint marker (PM). Phosphorylation signal of cTnI on ProQ Diamond-stained gels was divided by the SYPRO signal of cTnI; to correct for minor differences in loading. cTnI phosphorylation was 5.4±0.5 times higher (n = 3) after PKA treatment. *P<0.05, Wt versus 42D/44D and 23D/24D complexes in post-test Bonferroni analyses of one-way ANOVA. PKA treated cardiomyocytes were compared to untreated cardiomyocytes after exchange with the same complex via a student’s t-test. Abbreviations: cTnI, cardiac troponin I.
Table 3. Force measurements in donor cardiomyocytes after exchange with recombinant troponin without PKA treatment.
Donor cardiomyocytes exchanged with 42D/44D, 23D/24D and Wt troponin complex (4–5 myocytes per complex). Myofilament force was measured at sarcomere lengths of 1.8 and 2.2 μm at different Ca2+-concentrations. Ca2+-sensitivity derived from the midpoint of the force–pCa relationship (pCa50) increased at 2.2 μm compared to 1.8 μm for all complexes, however, the difference was only significant in 23D/24D. Stretching of cardiomyocytes from 1.8 to 2.2 μm increased maximal (Fmax) and passive force (Fpas) in all groups. There was no significant effect of sarcomere length on nHill (steepness of the force-pCa curves). Compared to Wt, 42D/44D and 23D/24D significantly decreased Ca2+-sensitivity at both sarcomere lengths (#P<0.05). No significant differences between the 3 cTn complexes for Fmax, Fpas and nHill were found. When two-way ANOVA revealed a significant effect for sarcomere length (P<0.05), paired t-tests were performed to compare cell measurements at two different sarcomere lengths in each cTn-exchange group (*P<0.05, 1.8 vs 2.2 μm). Wt and 23D/24D data have been published before.23 Values are means ± SEM.
| Without | PKA | Wt | 42D/44D | 23D/24D |
|---|---|---|---|---|
|
| ||||
| Fmax (kN.m−2) | 1.8 μm | 12.4±0.5 | 10.3±1.9 | 11.7±1.1 |
| 2.2 μm | 16.2±0.5* | 16.3±0.8* | 16.3±0.9* | |
|
| ||||
| Fpas (kN.m−2) | 1.8 μm | 2.2±0.3 | 2.1±0.5 | 2.9±0.3 |
| 2.2 μm | 3.9±0.6* | 3.0±0.7* | 4.0±0.3* | |
|
| ||||
| pCa50 | 1.8 μm | 5.55±0.05 | 5.26±0.01# | 5.37±0.01# |
| 2.2 μm | 5.57±0.05 | 5.28±0.01# | 5.46±0.01*,# | |
|
| ||||
| nHill | 1.8 μm | 1.9±0.1 | 2.0±0.2 | 2.0±0.1 |
| 2.2 μm | 1.9±0.1 | 2.1±0.3 | 1.7±0.1 | |
The relatively low ΔpCa50 in Wt and 42D/44D compared to 23D/24D-exchanged cells (Figure 4D) may be explained by removal of phosphorylated endogenous cTnI upon exchange with recombinant unphosphorylated cTn complex. It is known that donor cardiomyocytes are relatively highly phosphorylated at cTnI-Ser23/24.5, 6 Phostag analysis showed 52.7% bisphosphorylated, 35.9% monophosphorylated and 11.4% unphosphorylated cTnI at baseline in the donor tissue used in this study. Treatment of cTn-exchanged donor cardiomyocytes with exogenous PKA reduced Ca2+-sensitivity in Wt (Figure 4E) compared to cTn-exchanged cells without PKA treatment (Figure 4C). Moreover, PKA treatment increased ΔpCa50 in Wt (Figure 4F versus Figure 4D). However, PKA had no significant effect on pCa50 and ΔpCa50 in 42D/44D-exchanged cells. ProQ-Diamond staining illustrated that, although PKA did not affect pCa50 and ΔpCa50 in 42D/44D-exchanged cells, cTnI phosphorylation was 5.4±0.5 fold higher (n = 3) in 42D/44D-exchanged donor cells treated with PKA compared to untreated 42D/44D-exchanged donor cells (Figure 4G). This suggests that phosphorylation of Ser42/44 blunts the PKA-mediated length-dependent increase in Ca2+ sensitivity (Figure 4F).
In line with our experiments in IDCM cardiomyocytes, a sarcomere length increase from 1.8 to 2.2 μm in donor cardiomyocytes without (Table 3) or with (Table 4) PKA treatment increased Fmax and Fpas for all cTn complexes, without changing nHill. Also between PKA-treated groups (Wt, 42D/44D and 23D/24D), no significant differences were found for Fpas and nHill (Table 4). Additionally, PKA treatment did not affect maximal force in pseudo-phosphorylated Ser42/44 compared to Wt (Table 4).
Overall, our data in non-failing human cardiomyocytes show that cTnI pseudo-phosphorylation at the PKC sites Ser42/44 blunts the enhanced length-dependent increase in Ca2+-sensitivity upon PKA-mediated phosphorylation of Ser23/24, without significantly affecting the length-dependent increase in maximal force.
Pseudo-phosphorylation of Ser42/44 does not affect maximal ATPase activity and tension cost in human cardiomyocytes
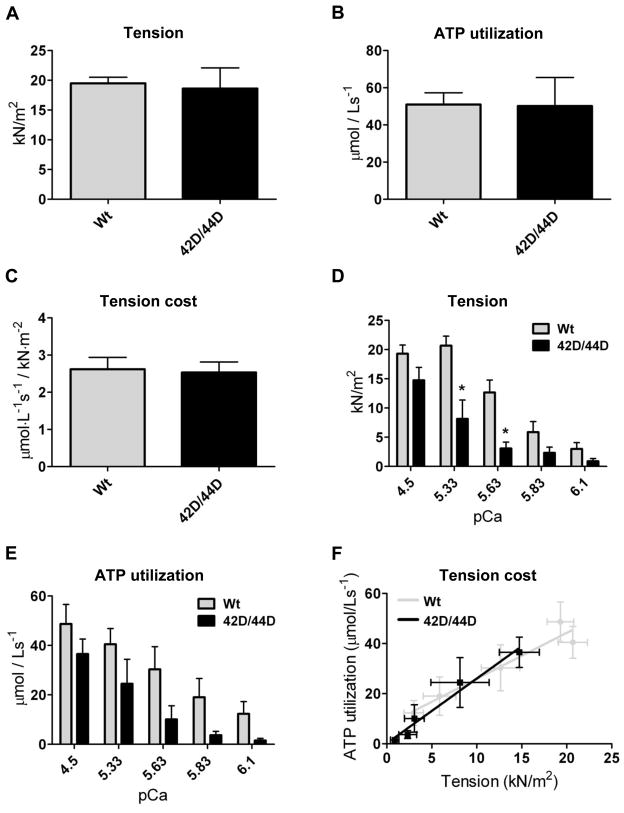
The effects of phosphorylation at Ser42/44 on tension, ATP utilization and tension cost were determined by exchanging Wt and 42D/44D in Triton-permeabilized multicellular muscle strips. The average amount of exchange in the muscle strips was 67.9 ± 11.6%. Tension (Figure 5A), ATP utilization (Figure 5B) and tension cost (Figure 5C) at maximal Ca2+ concentrations were not different between Wt and 42D/44D.
Figure 5. Pseudo-phosphorylation of Ser42/44 does not affect tension cost in donor myocardium.
ATPase measurements were performed in human donor muscle strips after Wt and 42D/44D exchange (average 67.9±11.6%). Tension (A), ATP utilization (B) and tension cost (C) at maximal Ca2+ concentrations did not differ between Wt and 42D/44D (n=6 muscle strips). Additional measurements at maximal and submaximal Ca2+ concentrations (n=4 muscle strips) demonstrated a reduction in force (D) and ATP utilization (E) at submaximal Ca2+ concentrations in 42D/44D compared to Wt. Tension cost at the entire range of Ca2+ concentrations is represented by the slope of the relation plotted between ATP utilization and tension (F). The slope of 42D/44D did not significantly differ from Wt (Wt: y=1.8x+7.9; R2 =0.93; 42D/44D: y=2.6x+0.3; R2=0.97). Basal ATP utilization, which corresponds to the y-intercepts, also did not significantly differ between 42D/44D and Wt (Wt: 7.9±6.3; 42D/44D: 0.3±0.8).
Force was reduced at submaximal Ca2+ concentrations in 42D/44D compared to Wt (Figure 5D). In line with force, ATP utilization at submaximal Ca2+ concentrations was lower in 42D/44D compared to Wt (Figure 5E). Tension cost for the entire range of Ca2+ concentrations is represented by the slope plotted between ATP utilization and tension (Figure 5F). The slope of 42D/44D did not significantly differ from Wt (Wt: y = 1.8x + 7.9; R2 = 0.93; 42D/44D: y = 2.6x + 0.3; R2 = 0.97). Basal ATP utilization, which corresponds to the y-intercepts, also did not significantly differ between 42D/44D and Wt (Wt: 7.9 ± 6.3; 42D/44D: 0.3 ± 0.8).
Discussion
This study demonstrates that cTnI pseudo-phosphorylation at the PKC-sites Ser42/44 induces a relatively large decrease in myofilament Ca2+-sensitivity in human failing and non-failing cardiomyocytes without affecting maximal force development. In addition, maximal ATPase activity and tension cost were not affected by pseudo-phosphorylation at Ser42/44. Interestingly, pseudo-phosphorylation at Ser42/44 largely blunted the increased length-dependence of myofilament Ca2+-sensitivity observed when Ser23/24 are phosphorylated. Since the drop in Ca2+-sensitivity at physiological Ca2+ levels is relatively large, these data indicate that phosphorylation of Ser42/44 may result in a reduced force development and associated ATP utilization in the human heart. Results of the present study in human cardiomyocytes will be discussed and compared with previous studies performed in rodents.
Larger reduction in Ca2+-sensitivity upon pseudo-phosphorylation of PKC sites Ser42/44 compared to Ser23/24
Our exchange experiments in human cardiomyocytes show that pseudo-phosphorylation of Ser42/44 (42D/44D) mainly affects Ca2+-sensitivity of force development without an effect on the maximal force generating capacity. PKC-mediated phosphorylation of cTnI at Ser42/44 has been implicated in several forms of cardiac disease in rodents and humans.13–16 An increase in Ser42/44 phosphorylation has been reported in rodent models of pressure-overload of the heart,14 low myocardial blood flow15 and myocardial infarction.16 In human, phosphorylation was not9 or slightly13 increased in ischemic and dilated end-stage failing hearts compared to non-failing donor hearts. Exchange with 42D/44D significantly reduced myofilament Ca2+-sensitivity both in failing and in non-failing cardiomyoctyes. Interestingly, the reduction in Ca2+-sensitivity in 42D/44D was larger compared with the reduction in Ca2+-sensitivity upon PKA-mediated phosphorylation of Ser23/24 or with pseudo-phosphorylation at Ser23/24 (23D/24D; Figure 4A–C, 4E).
The present study relies on the assumption that aspartic acid incorporation mimics phosphorylated serines. It has been previously demonstrated that pseudo-phosphorylation of Ser42/44 mimics function (maximal force and Ca2+-sensitivity) of PKC-mediated phosphorylation of cTnI.17, 32 In addition, as we discussed before,5 previous studies have indicated that phosphorylation and pseudo-phosphorylation by aspartic acid structurally and functionally behave the same at cTnI-Ser23/24.
Ser42/44 pseudo-phosphorylation blunts the enhanced length-dependent increase in Ca2+-sensitivity upon PKA incubation
PKA-mediated cTnI phosphorylation at Ser23/24 is a regulator of LDA, since it has been previously demonstrated that cTnI-Ser23/24 phosphorylation enhances the length-dependent increase in myofilament Ca2+-sensitivity.22, 23 Pseudo-phosphorylation of Ser42/44 largely blunted this increased length-dependence of myofilament Ca2+-sensitivity observed upon PKA-mediated phosphorylation of Ser23/24. An increase in sarcomere length in the working range of the heart enhances the maximal force generating capacity and the sensitivity of the myofilaments to calcium.33, 34 Length-dependent myofilament activation thus represents an important cellular mechanism to adjust cardiac performance in response to increased preload of the heart and is believed to be the cellular basis of the Frank-Starling mechanism.35, 36 In IDCM cardiomyocytes exchanged with Wt and 42D/44D, the length-dependent increase in Ca2+-sensitivity was relatively small and was not affected by pseudo-phosphorylation at Ser42/44. The same was found after Wt and 42D/44D exchange in donor cardiomyocytes. The relatively small length-dependent increase in Ca2+-sensitivity might be attributed to the low phosphorylation levels at cTnI-Ser23/24 in failing cardiomyocytes or in donor cardiomyocytes after exchange with recombinant unphosphorylated cTn. In agreement with this, PKA treatment after exchange with unphosphorylated Wt cTn complex enhanced the length-dependent increase in Ca2+-sensitivity (Figure 4F versus 4D, Figure 6). However, PKA treatment after 42D/44D exchange did not enhance the length-dependent increase in Ca2+-sensitivity (Figure 4F versus 4D, Figure 6), although cTnI was phosphorylated by PKA (Figure 4G). This suggests that phosphorylation of cTnI at Ser42/44 blunts the PKA-mediated enhancement of length-dependent activation in human cardiomyocytes.
Figure 6. Schematic representation of changes in length-dependent Ca2+-sensitivity upon phosphorylation of Ser42/44 and/or Ser23/24.
Data obtained in troponin-exchanged donor cells without and with treatment with exogenous PKA (data from Figure 4 and Table 3 and 4) were combined to illustrate the range at which myofilament Ca2+-sensitivity (pCa50) may vary in response to phosphorylation at the PKC sites Ser42/44 and at Ser23/24. Abbreviations: wild-type (Wt); pseudo-phosphorylated 42/44 (42D/44D); pseudo-phosphorylated Ser23/24 (23D/24D). Boxes represent the range of Ca2+-sensitivity measured at a sarcomere length of 1.8 (bottom line) and 2.2 μm (upper line). This figure demonstrates that the sarcomere length-dependent shift in Ca2+-sensitivity is relatively small in Wt and 42D/44D without PKA (i.e. when cTnI phosphorylation at Ser23/24 is low). PKA treatment of Wt and Ser23/24 pseudo-phosphorylation enhance the length-dependent increase in Ca2+-sensitivity. However, PKA treatment of 42D/44D does not increase the Ca2+-sensitivity range at which the sarcomere is operating between sarcomere lengths of 1.8 and 2.2 μm.
Phosphorylation of Ser23/24 decreases Ca2+-sensitivity most likely by weakening the interaction of the N-extension of cTnI with the N-lobe of cTnC, accompanied by a reduction in Ca2+-affinity of the N-lobe (enhanced off rate for Ca2+ exchange).37 This increases the binding affinity of cTnI to actin37 that promotes blockage of myosin-binding sites via tropomyosin. Myofilament protein structure is different at long compared to short sarcomere length.33 At the Ser23/24 phosphorylation background, an increase in sarcomere length may putatively change the interaction between cTnI and cTnC and increase actin-myosin interactions (resulting from uncovering of myosin-binding sites on actin). Ser42 and Ser44 are located in the N-terminal region of cTnI that interacts with the C-lobe of cTnC,38 and Ser44 has been suggested to interact with Glu-10 in the N-domain of cTnC.39 Pseudo-phosphorylation of Ser42/44 has been demonstrated to alter C-lobe metal ion affinities and perturb Ca2+/Mg2+-dependent protein-protein interactions, which may decrease Ca2+-binding affinity to cTnC.40 This may result in a stronger binding between cTnI and actin which may become less sensitive to changes in sarcomere length.
Regulation of maximal force by pseudo-phosphorylation at Ser42/44
In our study, no significant effect of Ser42/44 pseudo-phosphorylation was found on maximal force. Studies in rodent myocardium indicate central roles for cTnI and cTnT phosphorylation in reducing maximal force upon PKC activation.17, 41, 42 In a previous study in mouse cardiac fibers, maximal force at saturating Ca2+ concentrations was significantly reduced upon cTn exchange pseudo-phosphorylated at Ser42/44 compared to Wt, either by substitution into aspartic acid (Fmax reduced by 20%) or glutamic acid (Fmax reduced by 27%).17 In another study it was demonstrated that the PKC agonist phenylephrine (PE), reduced the steady force developed by mouse papillary muscles.42 However, in mouse papillary muscles expressing cTnI-42A/44A, the drop in steady developed force upon PE was smaller, suggesting a role for PKC-mediated Ser42/44 phosphorylation in the reduction of force development in intact mouse myocardium.42 The finding that maximal force is not affected by pseudo-phosphorylation at Ser42/44 in human cardiomyocytes is in line with a previous study in human cardiomyocytes treated with the catalytic domain of PKC.21 The catalytic domain of PKC may exert different effects than the various PKC isoforms present in the heart due to target selectivity. However, incubation of human cardiomyocytes with the isoforms PKCε20 or PKCα,20, 43 which have been demonstrated to phosphorylate cTnI at Ser42/44,19 also did not affect maximal force. One possible explanation for the discrepancies between rodent and human studies may reside in species differences in isoform composition of myosin heavy chain (MyHC). In adult rodent hearts (mouse, rat) the fast α-MyHC isoform is predominantly expressed, while the slow β-isoform is the predominant isoform expressed in large mammals such as human.44 Since we do not find a decrease in maximal force in human cardiomyocytes exchanged with pseudo-phosphorylated cTnI-Ser42/44, our data suggest that the slow β-MyHC isoform may be less sensitive (i.e. more protected) to changes that affect strong-binding cross-bridge formation and developed force. Alternatively, as PKC phosphorylates multiple cellular targets involved in myofilament contractility and calcium handling, a more complex protein phosphorylation pattern may underlie the observed differences in experimental models. Our study ensured high levels of phosphorylation at Ser42/44 via cTn exchange, demonstrating that specific pseudo-phosphorylation at Ser42/44 in human failing and non-failing cardiomyocytes did not affect maximal force development.
Maximal ATPase activity and tension cost are not affected by pseudo-phosphorylation of Ser42/44 in human myocardium
In human cardiac muscle strips, 42D/44D did not affect maximal force or maximal ATPase activity compared to Wt. This suggests that phosphorylation of Ser42/44 in human cardiomyocytes does not affect maximal tension cost. At submaximal Ca2+ concentrations, in line with the measurements in single cardiomyocytes, a large decrease in force development (Figure 5D) was found in 42D/44D compared to Wt. Likewise, ATPase activity at submaximal Ca2+ concentrations was reduced in 42D/44D compared to Wt (Figure 5E). As both force and ATPase activity were decreased at submaximal Ca2+ concentrations tension cost remained unaffected by pseudo-phosphorylation of Ser42/44 over the whole range of Ca2+ concentrations (Figure 5F). Previous studies performed in rodents, using alanine substitutions at Ser42/44, demonstrated that PKC-mediated phosphorylation of Ser42/44 reduces the maximal Ca2+-stimulated activity of the MgATPase18, 19 and suggested that tension cost may be affected by phosphorylation at these sites.45 Therefore, the effects of cTnI phosphorylation at Ser42/44 on ATPase activity and tension cost may be species dependent. Overall our data indicate that PKC-mediated phosphorylation of Ser42/44 does not affect economy of force development in human myocardium.
Alanine mutations at cTnI-Ser42/44 do not function as null-mutation
Mimetics are commonly used for the unphosphorylated or phosphorylated states of specific phosphorylation sites of interest. Pseudo-phosphorylation (aspartic acid, glutamate) mimics phosphorylation by introducing a negative charge, which is similar to that of a phosphate group. Another type of mutation that is often used at phosphorylation sites is the phospho-null analog alanine, which is favored as a substitution for serine due to their structural similarities. Alanine cannot be phosphorylated upon stimulation with agonists/kinases and prevents the introduction of a negative charge at a specific phosphorylation site. In the present study, it became clear that alanine mutations at Ser42/44 do not function as phospho-null mutations because 42A/44A induced a significant reduction in Ca2+ sensitivity compared to Wt (ΔpCa50 = 0.09) at a sarcomere length of 2.2 μm. This decrease in Ca2+-sensitivity is almost as large as found upon pseudo-phosphorylation of Ser23/24 (ΔpCa50 = 0.11 pCa units).5 In contrast to 42A/44A, alanine mutations at Ser23/24,5 and at PKC sites Thr14323 and Ser199 (unpublished data from our group) all displayed the same Ca2+-sensitivity compared to Wt. These data indicate that the region of cTnI around Ser42/44 is more sensitive to minor changes in structure caused by the introduction of an alanine compared to Ser23/24, Thr143 and Ser199. The finding that alanine mutations at Ser42/44 affect cTn function is consistent with a recent publication46 where 42A/44A and Wt cTnI were expressed in intact rat cardiomyocytes via viral-based gene transfer. In intact rat cardiomyocytes expressing 42A/44A, a diminished peak shortening amplitude was found and an accelerated re-lengthening.46 In addition, a decrease in Ca2+-sensitivity was found in skinned rat cardiomyocytes expressing 42A/44A compared to Wt.46 Our study demonstrates that care should be taken to interpret the results of studies where alanine mutations were used as a phospho-null mutation at Ser42/44, either in combination with agonist stimulation/enzyme incubations,42, 45–48 or in in vivo animal models.42, 45, 47, 48
Clinical implications of phosphorylation at Ser42/44
A small increase in phosphorylation of cTnI at Ser42 and Ser44 in human end-stage heart failure compared to non-failing donor hearts was recently reported.13 However, other studies performed in human heart failure tissue showed low levels of cTnI phosphorylation7,8,26 and little7,26 or no phosphorylation at PKC sites.9 The relatively low phosphorylation at Ser42/44 may be explained by transient changes in phosphorylation as was demonstrated in a mouse model of myocardial infarction.16 PKC-mediated cTnI-Ser42/44 phosphorylation may determine myofilament function also upon an acute myocardial insult in human. Although challenging, future studies could aim to determine dynamic phosphorylation changes at Ser42/44 using receptor-mediated activation of PKC in intact human preparations.
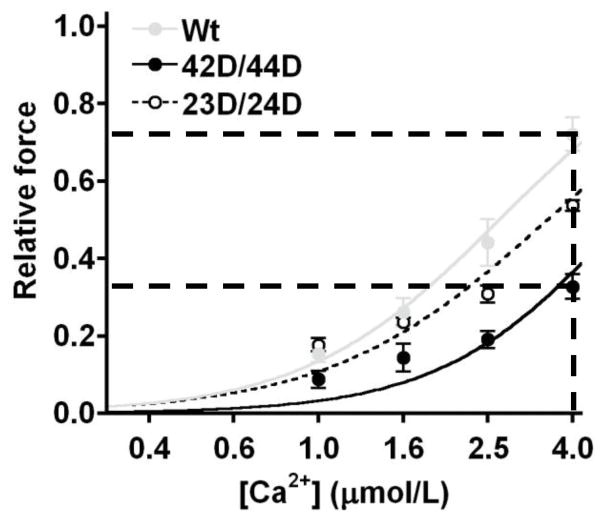
Based on our results, cTnI phosphorylation at Ser42/44 reduces Ca2+-sensitivity of the myofilaments and blunts the enhanced length-dependent increase in Ca2+-sensitivity mediated by PKA (Figure 6). This may result in a decrease in force development in vivo, since the maximal intracellular Ca2+ concentration is ~pCa 5.4 (= 3.98 μM).49 Figure 7 demonstrates that at the range of physiological Ca2+ concentrations, force development is much lower in 42D/44D compared to unphosphorylated Wt due to the relatively large reduction in Ca2+-sensitivity upon pseudo-phosphorylation of Ser42/44. While the low force development at physiological Ca2+ concentrations may reduce cardiac pump function, PKC-mediated Ser42/44 phosphorylation may exert beneficial effects. The decrease in force development coincides with a decrease in ATP utilization by the myofilaments, which may benefit the energy status of the failing heart. Moreover, the decrease in myofilament Ca2+-sensitivity may also improve diastolic function, as was suggested in a previous study.46
Figure 7. Force development upon Ser42/44 and Ser23/24 bisphosphorylation at physiological Ca2+ concentrations.
Data obtained in troponin-exchanged donor cells at a sarcomere length of 2.2 μm (data from Figure 4B and Table 3) plotted at the physiological range of intracellular Ca2+ concentrations. This plot shows that at maximal physiological (intracellular) Ca2+ concentrations, force is ~2-fold reduced by pseudo-phosphorylation of Ser42/44 compared to unphosphorylated Wt troponin, while an intermediate effect is found upon pseudo-phosphorylation of Ser23/24.
In conclusion, our study demonstrates that Ser42/44 phosphorylation may be detrimental for cardiac pump function by reducing force development at physiological Ca2+-concentrations and blunting of the enhanced myofilament length-dependent activation mediated by PKA. However, whether cTnI-Ser42/44 phosphorylation is beneficial or detrimental in patients may depend on the stage and underlying cause of cardiac disease.
Highlights.
cTnI-Ser42/44 pseudo-phosphorylation (42D/44D) reduces myofilament Ca2+-sensitivity
42D/44D does not affect maximal force and ATPase activity in human myocardium
42D/44D blunts enhanced length-dependent activation in response to protein kinase A
At physiological [Ca2+] Ser42/44 phosphorylation may decrease force and ATP utilization
Acknowledgments
Grants
This work was supported by National Institute of health (NIH) R01 HL063038 and NIH R01 HL76038, the Netherlands organization for scientific research (NWO; VIDI grant) and a European Society of Cardiology Research Grant.
Non-standard abbreviations and Acronyms
- A
alanine
- cMyBP-C
cardiac Myosin-Binding Protein-C
- cTnC
cardiac Troponin C
- cTnI
cardiac Troponin I
- cTnT
cardiac Troponin T
- D
aspartic acid
- Fmax
maximal force
- Fpas
passive force
- IDCM
idiopathic dilated cardiomyopathy
- LDA
length-dependent activation
- MLC2
myosin light chain 2
- MS
mass spectrometry
- MyHC
myosin heavy chain
- nHill
steepness of the force-pCa relation
- pCa50
−log10 of the calcium concentration at which 50% of maximal force is reached
- PE
phenylephrine
- PKA
protein kinase A
- PKC
protein kinase C
- PM
peppermint marker
- Wt
wild-type
Footnotes
Disclosures
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vasco Sequeira, Email: v.sequeiraoliveira@vumc.nl.
E. Rosalie Witjas-Paalberends, Email: e.paalberends@vumc.nl.
D. Brian Foster, Email: dbrianfoster@jhmi.edu.
Cristobal G. dos Remedios, Email: cris.dosremedios@sydney.edu.au.
Anne M. Murphy, Email: murphy@jhmi.edu.
Ger J.M. Stienen, Email: g.stienen@vumc.nl.
Jolanda van der Velden, Email: j.vandervelden@vumc.nl.
Reference List
- 1.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 2.van der Velden J. Diastolic myofilament dysfunction in the failing human heart. Pflugers Arch. 2011 Jul;462(1):155–63. doi: 10.1007/s00424-011-0960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–7. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 4.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004 Mar 5;94(4):496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 5.Wijnker PJM, Foster DB, Tsao AL, Frazier AH, dos Remedios CG, Murphy AM, Stienen GJM, van der Velden J. Impact of site-specific phosphorylation of protein kinase A sites Ser23 and Ser24 of cardiac troponin I in human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013 Jan 15;304(2):H260–H268. doi: 10.1152/ajpheart.00498.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdani N, Borbely A, Veenstra SP, Kooij V, Vrydag W, Zaremba R, dos Remedios CG, Niessen HW, Michel MC, Paulus WJ, Stienen GJM, van der Velden J. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J Muscle Res Cell Motil. 2010 Dec;31(4):289–301. doi: 10.1007/s10974-010-9231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007 Jan;42(1):247–59. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJM. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003 Jan;57(1):37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Guy MJ, Norman HS, Chen YC, Xu Q, Dong X, Guner H, Wang S, Kohmoto T, Young KH, Moss RL, Ge Y. Top-down quantitative proteomics identified phosphorylation of cardiac troponin I as a candidate biomarker for chronic heart failure. J Proteome Res. 2011 Sep 2;10(9):4054–65. doi: 10.1021/pr200258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999 Jan 26;99(3):384–91. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004 Aug 24;110(8):982–7. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 12.Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989 Dec 5;264(34):20778–85. [PubMed] [Google Scholar]
- 13.Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation. 2012 Oct 9;126(15):1828–37. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong X, Sumandea CA, Chen YC, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. Augmented phosphorylation of cardiac troponin I in hypertensive heart failure. J Biol Chem. 2012 Jan 6;287(2):848–57. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christopher B, Pizarro GO, Nicholson B, Yuen S, Hoit BD, Ogut O. Reduced force production during low blood flow to the heart correlates with altered troponin I phosphorylation. J Muscle Res Cell Motil. 2009;30(3–4):111–23. doi: 10.1007/s10974-009-9180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2010 Jun;48(6):1180–6. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003 Mar 28;278(13):11265–72. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 18.Noland TA, Jr, Guo X, Raynor RL, Jideama NM, veryhart-Fullard V, Solaro RJ, Kuo JF. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca(2+)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995 Oct 27;270(43):25445–54. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 19.Noland TA, Jr, Raynor RL, Jideama NM, Guo X, Kazanietz MG, Blumberg PM, Solaro RJ, Kuo JF. Differential regulation of cardiac actomyosin S-1 MgATPase by protein kinase C isozyme-specific phosphorylation of specific sites in cardiac troponin I and its phosphorylation site mutants. Biochemistry. 1996 Nov 26;35(47):14923–31. doi: 10.1021/bi9616357. [DOI] [PubMed] [Google Scholar]
- 20.Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios CG, Stienen GJM, van der Velden J. Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol. 2010 Mar;105(2):289–300. doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJM. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006 Mar 1;69(4):876–87. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol. 2003 Mar 15;547(Pt 3):951–61. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijnker PJM, Sequeira V, Foster DB, Li Y, Dos Remedios CG, Murphy AM, Stienen GJM, van der Velden J. Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol Heart Circ Physiol. 2014 Apr 15;306(8):H1171–1181. doi: 10.1152/ajpheart.00580.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, dos Remedios CG, Jaquet K, Foster DB, Murphy AM, Van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJM. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006 Oct 27;99(9):1012–20. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 25.Zaremba R, Merkus D, Hamdani N, Lamers JM, Paulus WJ, dos Remedios CG, Duncker DJ, Stienen GJM, van der Velden J. Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomics Clin Appl. 2007 Oct;1(10):1285–90. doi: 10.1002/prca.200600891. [DOI] [PubMed] [Google Scholar]
- 26.Messer AE, Gallon CE, McKenna WJ, dos Remedios CG, Marston SB. The use of phosphate-affinity SDS-PAGE to measure the cardiac troponin I phosphorylation site distribution in human heart muscle. Proteomics Clin Appl. 2009 Dec;3(12):1371–82. doi: 10.1002/prca.200900071. [DOI] [PubMed] [Google Scholar]
- 27.Potma EJ, Stienen GJM, Barends JP, Elzinga G. Myofibrillar ATPase activity and mechanical performance of skinned fibres from rabbit psoas muscle. J Physiol. 1994 Jan 15;474(2):303–17. doi: 10.1113/jphysiol.1994.sp020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narolska NA, van Loon RB, Boontje NM, Zaremba R, Penas SE, Russell J, Spiegelenberg SR, Huybregts MA, Visser FC, de Jong JW, van der Velden J, Stienen GJM. Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue. Cardiovasc Res. 2005 Jan 1;65(1):221–9. doi: 10.1016/j.cardiores.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–40. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 30.Schwinger RH, Bohm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res. 1994 May;74(5):959–69. doi: 10.1161/01.res.74.5.959. [DOI] [PubMed] [Google Scholar]
- 31.Sequeira V, Wijnker PJM, Nijenkamp LL, Kuster DWD, Najafi A, Witjas-Paalberends R, Regan JA, Boontje N, Ten CF, Germans T, Carrier L, Sadayappan S, van Slegtenhorst M, Zaremba R, Foster DB, Murphy A, Poggesi C, dos Remedios CG, Stienen GJM, Ho CY, Michels M, van der Velden J. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013 Mar;112:1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- 33.de Tombe PP, Mateja RD, Tachampa K, Ait MY, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010 May;48(5):851–8. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs F, Martyn DA. Length-dependent Ca(2+) activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil. 2005;26(4–5):199–212. doi: 10.1007/s10974-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 35.Nowak G, Pena JR, Urboniene D, Geenen DL, Solaro RJ, Wolska BM. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end-systolic pressure-volume relation. J Muscle Res Cell Motil. 2007;28(7–8):415–9. doi: 10.1007/s10974-008-9136-y. [DOI] [PubMed] [Google Scholar]
- 36.ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980 May;46(5):703–14. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- 37.Solaro RJ, Rosevear P, Kobayashi T. The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation. Biochem Biophys Res Commun. 2008 Apr 25;369(1):82–7. doi: 10.1016/j.bbrc.2007.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda S, Yamashita A, Maeda K, Maéda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Dong WJ, Burkart EM, Cheung HC, Solaro RJ. Effects of protein kinase C dependent phosphorylation and a familial hypertrophic cardiomyopathy-related mutation of cardiac troponin I on structural transition of troponin C and myofilament activation. Biochemistry. 2004;43:5996–6004. doi: 10.1021/bi036073n. [DOI] [PubMed] [Google Scholar]
- 40.Finley NL, Rosevear PR. Introduction of negative charge mimicking protein kinase C phosphorylation of cardiac troponin I. Effects on cardiac troponin C. J Biol Chem. 2004;279:54833–54840. doi: 10.1074/jbc.M408304200. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery DE, Chandra M, Huang Q, Jin J, Solaro RJ. Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force. Am J Physiol Heart Circ Physiol. 2001 Mar;280(3):H1011–H1018. doi: 10.1152/ajpheart.2001.280.3.H1011. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery DE, Wolska BM, Pyle WG, Roman BB, Dowell JC, Buttrick PM, Koretsky AP, Del NP, Solaro RJ. Alpha-Adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. Am J Physiol Heart Circ Physiol. 2002 Jun;282(6):H2397–H2405. doi: 10.1152/ajpheart.00714.2001. [DOI] [PubMed] [Google Scholar]
- 43.Kooij V, Zhang P, Piersma SR, Sequeira V, Boontje NM, Wijnker PJM, Jiménez CR, Jaquet KE, dos Remedios C, Murphy AM, Van Eyk JE, van der Velden J, Stienen GJM. PKCα-specific phosphorylation of the troponin complex in human myocardium: a functional and proteomics analysis. PLoS One. 2013 Oct 7;8(10):e74847. doi: 10.1371/journal.pone.0074847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mercadier JJ, Bouveret P, Gorza L, Schiaffino S, Clark WA, Zak R, Swynghedauw B, Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res. 1983 Jul;53(1):52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- 45.Pyle WG, Sumandea MP, Solaro RJ, de Tombe PP. Troponin I serines 43/45 and regulation of cardiac myofilament function. Am J Physiol Heart Circ Physiol. 2002 Sep;283(3):H1215–H1224. doi: 10.1152/ajpheart.00128.2002. [DOI] [PubMed] [Google Scholar]
- 46.Lang SE, Robinson DA, Wu HC, Herron TJ, Wahr PA, Westfall MV. Myofilament incorporation and contractile function after gene transfer of cardiac troponin I Ser43/45Ala. Arch Biochem Biophys. 2013 Jul 1;535(1):49–55. doi: 10.1016/j.abb.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacGowan GA, Du C, Cowan DB, Stamm C, McGowan FX, Solaro RJ, Koretsky AP, Del Nido PJ. Ischemic dysfunction in transgenic mice expressing troponin I lacking protein kinase C phosphorylation sites. Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H835–H843. doi: 10.1152/ajpheart.2001.280.2.H835. [DOI] [PubMed] [Google Scholar]
- 48.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circ Res. 2002 Apr 5;90(6):649–56. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 49.Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–97. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]