Abstract
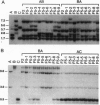
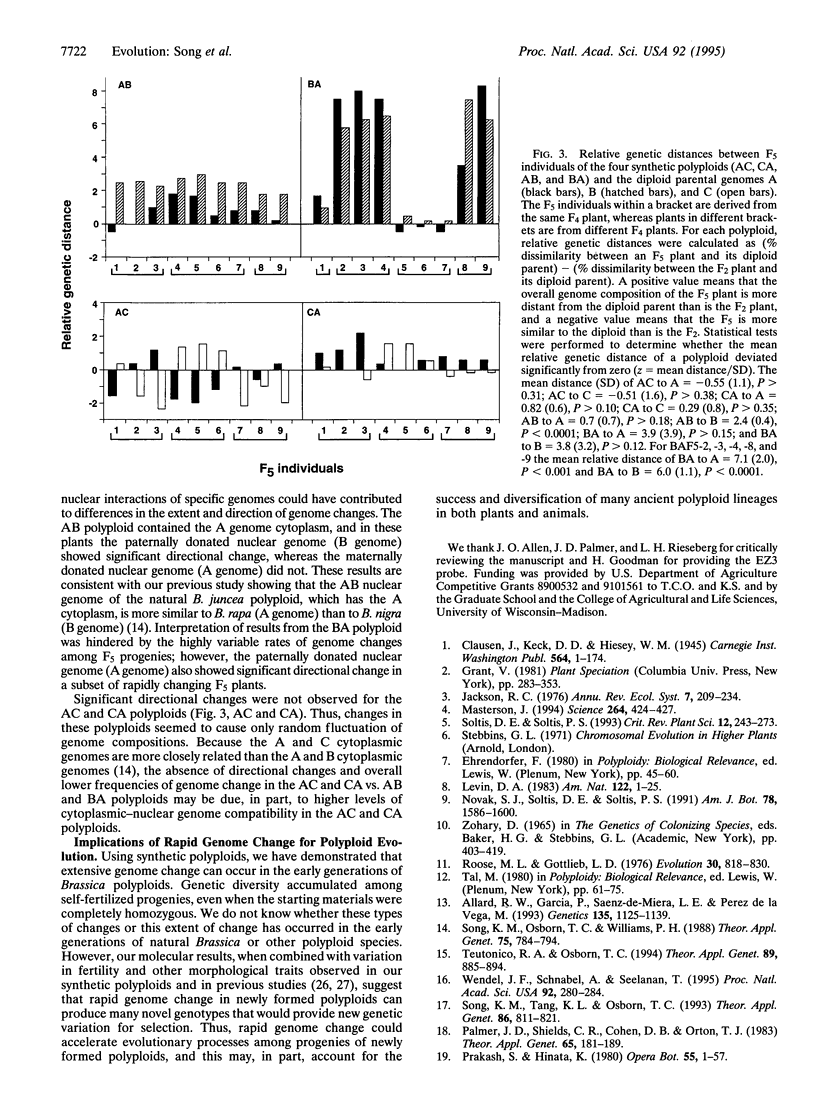
Although the evolutionary success of polyploidy in higher plants has been widely recognized, there is virtually no information on how polyploid genomes have evolved after their formation. In this report, we used synthetic polyploids of Brassica as a model system to study genome evolution in the early generations after polyploidization. The initial polyploids we developed were completely homozygous, and thus, no nuclear genome changes were expected in self-fertilized progenies. However, extensive genome change was detected by 89 nuclear DNA clones used as probes. Most genome changes involved loss and/or gain of parental restriction fragments and appearance of novel fragments. Genome changes occurred in each generation from F2 to F5, and the frequency of change was associated with divergence of the diploid parental genomes. Genetic divergence among the derivatives of synthetic polyploids was evident from variation in genome composition and phenotypes. Directional genome changes, possibly influenced by cytoplasmic-nuclear interactions, were observed in one pair of reciprocal synthetics. Our results demonstrate that polyploid species can generate extensive genetic diversity in a short period of time. The occurrence and impact of this process in the evolution of natural polyploids is unknown, but it may have contributed to the success and diversification of many polyploid lineages in both plants and animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard R. W., García P., Sáenz-de-Miera L. E., Pérez de la Vega M. Evolution of multilocus genetic structure in Avena hirtula and Avena barbata. Genetics. 1993 Dec;135(4):1125–1139. doi: 10.1093/genetics/135.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Naveh-Many T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981 Aug 27;292(5826):860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Harris S., Rudnicki K. S., Haber J. E. Gene conversions and crossing over during homologous and homeologous ectopic recombination in Saccharomyces cerevisiae. Genetics. 1993 Sep;135(1):5–16. doi: 10.1093/genetics/135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Petes T. D. High-frequency meiotic gene conversion between repeated genes on nonhomologous chromosomes in yeast. Proc Natl Acad Sci U S A. 1985 May;82(10):3350–3354. doi: 10.1073/pnas.82.10.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson J. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science. 1994 Apr 15;264(5157):421–424. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- Wendel J. F., Schnabel A., Seelanan T. Bidirectional interlocus concerted evolution following allopolyploid speciation in cotton (Gossypium). Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):280–284. doi: 10.1073/pnas.92.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]