Abstract
Objective: To investigate the role of three genetic polymorphisms of ABC proteins in response to chemotherapy and overall survival of osteosarcoma patients.
Methods: A prospective study was conducted. Genotyping analyses of ABCB1 C3435T, ABCG2 C421A, and ABCC3 C-211T were conducted using the TaqMan methodology. Multivariate Cox proportional hazards models were used to calculate hazard ratio (HR) and 95% CI of effect of each genotype of ABCB1 C3435T, ABCG2 c421A, and ABCC3 C-211T on PFS and OS.
Results: During the follow-up period, 135 patients (74.18%) were alive and 47 died (25.82). The median follow-up periods were 36.7 months. Individuals carrying with ABCB1 3435TT genotype and T allele showed less likely to have a poor response to chemotherapy. Cox regression analysis showed that individuals with ABCB1 TT genotype and T allele were associated with high risk of death from osteosarcoma when compared with wide-type genotype. However, we did not find significant association between ABCG2 C421A and ABCC3 C-211T polymorphisms and overall survival of osteosarcoma.
Conclusion: ABCB1 C3435T polymorphism may be used as a genetic predictor of clinical outcome in osteosarcoma patients treated with chemotherapy. However, no association was found between polymorphisms in ABCG2 C421A and ABCC3 C-211T and clinical outcome of osteosarcoma.
Key Words: APT-binding cassette, Chemotherapy, Clinical outcome, Osteosarcoma
INTRODUCTION
Osteosarcoma is the most common primary malignant bone tumor worldwide, and it accounts for about 20% of all primary sarcomas in bone.1 It is estimated that the incidence of osteosarcoma in youths less than 20 years old is about 8.7 per 10 populations, and osteosarcoma is a leading cause of death of these youths.2,3 Although advanced therapies with aggressive adjuvant chemotherapy and wide tumor resection could improve the overall survival of osteosarcoma,4 patients with metastatic disease always present a shorter survival time.5 It is estimated that about 40% of patients with osteosarcoma show a poor response to chemotherapy, and the five-year survival rate is less than 50%.6 However, patients with same tumor stages show different response to chemotherapy and different survival time. Therefore, genetic factors may influence chemotherapy toxicity and clinical outcome of osteosarcoma.7-9
It is suggested that individualized chemotherapy according to some biomarkers can influence the clinical response to chemotherapeutics and prolong the life of patients with osteosarcoma. The ATP-binding cassette (ABC proteins) transports superfamilies and is responsible for the majority of drug transport.10 It is reported that many drug metabolizers and transporters present genetic variations, which could change the functional expression of the ABC proteins, and thus influence efficacy of chemotherapeutic agents and prognosis of diseases.
We previously reported a study between GSTs polymorphisms and prognosis of osteosarcoma patients treated with chemotherapy,8 and found that the genetic variations could influence the pharmacokinetics and pharmacodynamics of chemotherapeutic drugs. In this study, we planned to investigate the role of three genetic polymorphisms (ABCB1 C3435T, ABCG2 c421A, and ABCC3 C-211T) in response to chemotherapy and overall survival of osteosarcoma patients.
METHODS
Subjects: The study population comprised 182 diagnosed osteosarcoma patients between September 2007 and September 2009 at the first affiliated hospital of Xinxiang Medical University. All cases were newly and histologically diagnosed with primary osteosarcoma. All cases signed an informed consent Form before participating in the study which was approved by the ethics committee of the first affiliated hospital of Xinxiang Medical University.
Osteosarcoma patients received chemotherapy before operation, and the chemotherapy treatment was described in our previous study.8 The treatment response to chemotherapy was classified according to histological extent of tumor necrosis. Poor responders were defined as less than 90% of tumor necrosis, and good responders were defined as 90% of tumor necrosis or more.11 All the patients were followed up until 31th September 2012, with a median follow-up period of 41.5 months (ranged from 2 to 60 months). All patients were followed up by telephone or outpatient service every two months until death or the end of study (31th September 2012). Overall survival (OS) was used as the end point, and the overall survival was calculated from the date of enrolling in this study to the date of death from any cause or last known date alive or last clinical follow-up.
DNA extraction and Genotyping : All study participants were asked to provide 5 ml of peripheral venous blood, and the blood samples were kept at -20ºC. According to the manufacturer’s instructions, genomic DNA was extracted from peripheral venous blood samples with the TIANamp blood DNA kit (Tiangen Biotech, Beijing, China). Genotyping analyses of ABCB1 C3435T, ABCG2 C421A, and ABCC3 C-211T were conducted using the TaqMan methodology in a 384-well plate format on the Sequenom MassARRAY platform and read by Sequence Detection software on the ABI prism 7900 instrument (Sequenom, San Diego, USA), which described in previous study.8 The primers used for ABCB1 C3435T, ABCG2 c421A, and ABCC3 C-211T were designed using Sequenom Assay Design 3.1 software (Sequenom®) according to the manufacturer instructions (Table-I). About 10% randomly chosen subgroup were selected from cases and control subjects, and the results of repeated samples were 100% concordant.
Table-I.
Primers used for ABCB1 C3435T, ABCG2 c421A, and ABCC3 C-211T polymorphisms
| Polymorphism | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
|---|---|---|
| ABCB1 C3435T | TGCTGGTCCTGAAGTTGATCTGTGAAC | ACATTAGGCAGTGACTCGATGAAGGCA |
| ABCG2 c421A | AATGTATTGTCACCTAGTGTTTG | AGTAAATGCCTTCAGGTCAT |
| ABCC3 C-211T | GACTCGACTCCTGGACTGTTAGC | CCCTACCCCAGTGCCTCT |
Statistical analysis : Association between tumor response and genotypes was assessed using logistic regression analysis. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated by comparing genotypes frequencies in good responders versus poor responders. Homozygotes for the most frequent genotype were used as the reference group. Multivariate Cox proportional hazards models were used to calculate hazard ratio (HR) and 95% CI of effect of ABCB1 C3435T, ABCG2 c421A, and ABCC3 C-211T polymorphisms on PFS and OS with or without adjustment for confounding factors. Overall survival times were plotted using Kaplan-Meier product limit method, and the differences between them were estimated by log-rank test. All P values were two-tailed, and P<0.05 was considered as statistically significant, and the SPSS® statistical package, version 11.0 (SPSS Inc., Chicago, IL, USA) for Windows® were used for statistical analyses.
RESULTS
A total of 182 patients with osteosarcoma were enrolled in this study. Table-II shows the clinical characteristics of cases. The mean age of the 182 patients were 16.7 years old (ranged from 9.1 to 58.6 years old), and there were 105 males (57.69%) and 77 females (42.31%). The tumors of 86 patients were at femur (47.25%), and 64 were at tibia/fibula (35.16%). Total 87 patients showed good response to chemotherapy (47.80%), and 95 showed poor response (52.20%).
Table-II.
Clinical and pathological characteristics of included study
| Age at diagnosis, y | Patients, N | % |
|---|---|---|
| Total number of patients | 182 | |
| Median (range) | 16.7 (9.1-58.6) | |
| ≤15 | 79 | 43.4 |
| >15 | 103 | 56.6 |
| Sex | ||
| Male | 105 | 57.7 |
| Female | 77 | 42.3 |
| Tumor location | ||
| Femur | 86 | 47.3 |
| Tibia/fibula | 64 | 35.2 |
| Arm | 14 | 7.7 |
| Central | 18 | 9.9 |
| Necrosis | ||
| Good | 95 | 52.2 |
| Poor | 87 | 47.8 |
| Metastasis | ||
| Absent | 97 | 53.3 |
| At diagnosis | 45 | 24.7 |
| At follow up | 40 | 22.0 |
| Death | ||
| No | 125 | 68.7 |
| Yes | 57 | 31.3 |
Forty patients showed metastasis during the follow-up period, and 45 patients presented metastasis at the time of diagnosis. During the follow-up period, 135 patients (74.18%) were alive and 47 died (25.82%). The median follow-up periods were 36.7 months.
Our results indicated that individuals carrying with ABCB1 3435TT genotype and T allele showed were less likely to have a poor response to chemotherapy, with ORs(95%CI) of 0.38(0.15-0.94) and 0.57(0.37-0.90), respectively (Table-III). However, individuals with ABCG2 C421A and ABCC3 C-211T polymorphisms had no association with response to chemotherapy in patients with osteosarcoma.
Table-III.
Gene polymorphisms association with tumor response to chemotherapy
| Genotype | Patients |
Tumor response
|
OR(95%CI) 1 | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | Poor | % | Good | % | |||||
| ABCB1 C3435T | CC | 77 | 42.31 | 31 | 35.2 | 47 | 49.7 | 1.0(Ref.) | - |
| CT | 68 | 37.36 | 34 | 39.5 | 35 | 37.1 | 0.65(0.32-1.31) | 0.19 | |
| TT | 37 | 20.33 | 22 | 25.3 | 13 | 13.2 | 0.38(0.15-0.94) | 0.02 | |
| C allele | 222 | 60.99 | 96 | 55.0 | 130 | 68.3 | 1.0(Ref.) | - | |
| T allele | 142 | 39.01 | 78 | 45.1 | 60 | 31.8 | 0.57(0.37-0.90) | 0.01 | |
| ABCG2 C421A | CC | 132 | 72.53 | 54 | 62.1 | 62 | 65.2 | 1.0(Ref.) | - |
| CA | 44 | 24.18 | 20 | 23.2 | 21 | 22.1 | 0.90(0.42-1.91) | 0.77 | |
| AA | 6 | 3.30 | 13 | 14.7 | 12 | 12.7 | 0.75(0.24-2.22) | 0.57 | |
| A allele | 308 | 84.62 | 128 | 73.7 | 145 | 76.3 | 1.0(Ref.) | - | |
| G allele | 56 | 15.38 | 46 | 26.3 | 45 | 23.8 | 0.84(0.50-1.42) | 0.49 | |
| ABCC3 C-211T | CC | 85 | 46.70 | 38 | 44.2 | 46 | 48.2 | 1.0(Ref.) | - |
| CT | 64 | 35.16 | 30 | 34.9 | 31 | 32.5 | 0.86(0.43-1.73) | 0.65 | |
| TT | 33 | 18.13 | 18 | 20.9 | 18 | 19.3 | 0.71(0.28-1.74) | 0.41 | |
| C allele | 234 | 64.29 | 107 | 123.3 | 122 | 128.9 | 1.0(Ref.) | - | |
| T allele | 130 | 35.71 | 67 | 76.7 | 68 | 71.1 | 0.81(0.51-1.28) | 0.35 | |
1. Adjusted for sex, age, tumor location and metastasis
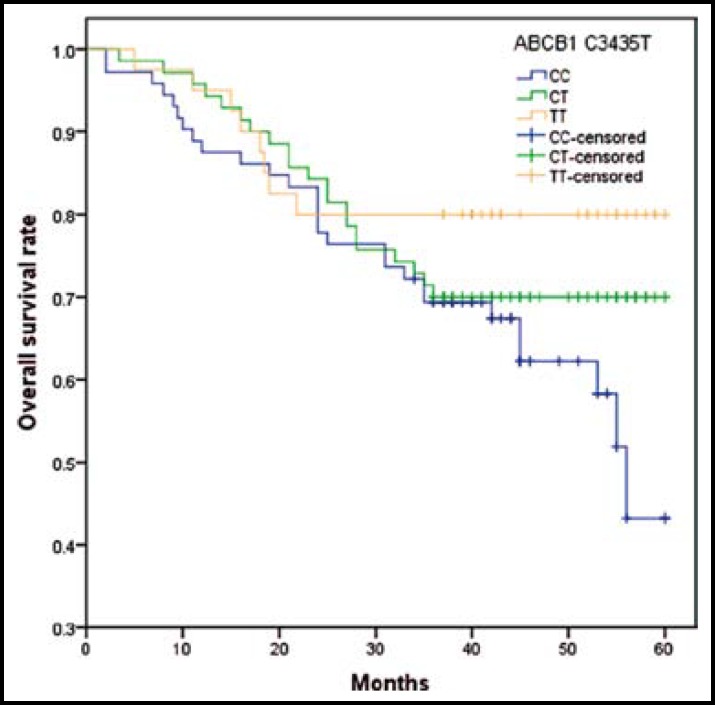
Cox regression analysis of association between ABCB1 C3435T, ABCG2 C421A and ABCC3 C-211T polymorphisms and the survival of osteosarcoma were showed in Table-IV. Log-rank test showed that individuals carrying ABCB1 TT genotype and T allele had longer survival times than CC genotype (Fig.1). The results showed that individuals with ABCB1 TT genotype and T allele were associated with high risk of death from osteosarcoma when compared with wide-type genotype, with HRs(95%CI) of 2.58(1.03-7.28) and 1.65(1.03-2.72), respectively. However, we did not find significant association between ABCG2 C421A and ABCC3 C-211T polymorphisms and overall survival of osteosarcoma.
Table-IV.
Cox regression analysis of ABCB1 C3435T, ABCG2 C421A and ABCC3 C-211T polymorphisms with the survival of osteosarcoma
| Genotype | Months | P value for log-rank test |
Overall survival
|
HR (95%CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Death | % | Alive | % | ||||||
| ABCB1 C3435T | CC | 35.3 | 20 | 35.1 | 62 | 49.6 | 1.0(Ref.) | - | |
| CT | 41.4 | 23 | 40.4 | 46 | 36.8 | 1.48(0.70-3.17) | 0.24 | ||
| TT | 48.6 | 0.03 | 14 | 24.6 | 17 | 13.6 | 2.58(1.03-7.28) | 0.04 | |
| C allele | 39.5 | 63 | 110.5 | 170 | 136 | 1.0(Ref.) | - | ||
| T allele | 46.9 | 0.02 | 51 | 89.5 | 80 | 64 | 1.65(1.03-2.72) | 0.03 | |
| ABCG2 C421A | CC | 38.6 | 35 | 61.4 | 82 | 65.6 | 1.0(Ref.) | - | |
| CA | 42.1 | 13 | 22.8 | 27 | 21.6 | 1.05(0.47-2.49) | 0.83 | ||
| AA | 44.7 | 0.51 | 8 | 14 | 16 | 12.8 | 1.22(0.44-3.77) | 0.65 | |
| A allele | 39.4 | 83 | 145.6 | 191 | 152.8 | 1.0(Ref.) | - | ||
| G allele | 43.2 | 0.42 | 29 | 50.9 | 59 | 47.2 | 1.16(0.67-2.02) | 0.58 | |
| ABCC3 C-211T | CC | 37.1 | 25 | 43.9 | 60 | 48 | 1.0(Ref.) | - | |
| CT | 42.3 | 20 | 35.1 | 41 | 32.8 | 1.14(0.53-2.46) | 0.72 | ||
| TT | 43.5 | 0.63 | 12 | 21.1 | 24 | 19.2 | 1.16(0.47-3.02) | 0.73 | |
| C allele | 39.1 | 70 | 122.8 | 161 | 128.8 | 1.0(Ref.) | - | ||
| T allele | 42.7 | 0.48 | 44 | 77.2 | 89 | 71.2 | 1.11(0.68-1.81) | 0.65 | |
Fig.1.
Kaplan-Meier survival curve of ABCB1 C3435T polymorphisms
DISCUSSION
In this study, we found that common genetic polymorphisms in APT-binding cassette were associated with increased risk of overall survival in a Chinese population. Although the chemotherapy is selected based on the histological type and tumor stage of osteosarcoma patients, genotyping of genomic DNA could guide the therapy and improve efficacy of treatment. Therefore, we investigated that polymorphisms in ABCB1, ABCG2 and ABCC3 could be a predictor for treatment response for osteosarcoma patients.
It is reported that ABCB1 C3435T gene has a role in encoding a plasma membrane pump, P-glycoprotein, and this gene can protect the cells and organs from damages of xenobiotic agents and environmental carcinogens through draining various structurally unrelated anticancer agents and toxins.12,13 Previous studies have indicated that overall expression of ATP binding cassette transporter, such as ABCB1, ABCG2 and ABCC3, has a direct role in resistance to a broad spectrum of chemotherapeutic agents in vitro, such as anthracyclines, paclitaxel and Vinca alkaloids.14 ABCB1 polymorphism can be the one of the main factors in limiting the efficacy of chemotherapeutic agents in several types of cancerr.15-17 Previous three studies have reported the association between ABCB1 polymorphism and response to chemotherapy in patients with osteosarcoma.18-20 Yang et al. reported that ABCB1 and ABCC3 polymorphisms were associated with response to chemotherapy and overall survival in osteosarcoma patients.18 Caronia et al. indicated that ABCB1 and ABCC3 polymorphisms may influence the efficacy of chemotherapy on clinical outcome of steosarcoma patients.19 Our study also reported similar results with previous ones, which indicated that ABCB1 polymorphism can have an important role in the interindividual differences in clinical outcome of osteosarcoma patients.
It is well known that ABCC3 is one kind of the multidrug resistance protein family (MRP), and it is expressed in various organs, such as liver, gallbladder and kidney.21,22 Previous studies have suggested that ABCC3 mRNA is associated with drug resistance, and several studies have reported an association between ABCC3 C-211T polymorphism and clinical outcome of cancer patients after receiving chemotherapy.19,23 However, we did not find a significant association between ABCC3 C-211T polymorphism and clinical outcome of osteosarcoma, which is difference from previous two studies.18,19 The discrepancy of the results may be explained by the differences in ethnicities, sample size and also by chance. Further studies are greatly needed to confirm the association between ABCC3 C-211T polymorphism and clinical outcome of osteosarcoma.
Limitations of the Study: The current study has several limitations. First, the study was conducted in one hospital, and the sample could not better represent other populations. Second, number of cases is relatively small which may decrease the statistical power to find the differences between groups. Thirdly, multiple genes are involved in the clinical outcome of osteosarcoma, and thus other genetic factors should be considered in further studies.
CONCLUSION
ABCB1 3435TT genotype and T allele may be associated with the clinical outcome of osteosarcoma patients in a Chinese population. ABCB1 C3435T polymorphism may be used as a genetic predicator of clinical outcome in osteosarcoma patients treated with chemotherapy.
Authors Contributions:
SXH, LAG, GXL & LY: Designed and performed the study, did statistical analysis & editing of manuscript.
SXH, ZHX & ZYL: Did data collection and manuscript writing.
References
- 1.Duong LA, Richardson LC. Descriptive Epidemiology of Malignant Primary Osteosarcoma Using Population-based Registries, United States, 1999-2008. J Registry Manag. 2013;40(2):59–64. [PMC free article] [PubMed] [Google Scholar]
- 2.Savage SA, Woodson K, Walk E, Modi W, Liao J, Douglass C, et al. Analysis of genes critical for growth regulation identifies Insulin-like Growth Factor 2 Receptor variations with possible functional significance as risk factors for osteosarcoma. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1667–1674. doi: 10.1158/1055-9965.EPI-07-0214. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG. SEER Program (National Cancer Institute (U.S.)). Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. In: Lynn A, Gloecker Ries, et al., editors. Bethesda, MD, USA: National Cancer Institute, SEER Program; 1999. [Google Scholar]
- 4.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(6):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 7.Zhao YL, Yang LB, Geng XL, Zhou QL, Qin H, Yang L, et al. The association of XPG and MMS19L polymorphisms response to chemotherapy in osteosarcoma. Pak J Med Sci. 2013;29(5):1225–1229. doi: 10.12669/pjms.295.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng JW, Yang ZM, Li J, Xu B. Predictive role of Glutathione S-transferases (GSTs) on the prognosis of osteosarcoma patients treated with chemotherapy. Pak J Med Sci. 2013;29(5):1182–1186. doi: 10.12669/pjms.295.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai SB, Chen HX, Bao YX, Luo X, Zhong JJ. Predictive impact of common variations in DNA repair genes on clinical outcome of osteosarcoma. Asian Pac J Cancer Prev. 2013;14(6):3677–3680. doi: 10.7314/apjcp.2013.14.6.3677. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SF, Di YM, Chan E, Du YM, Chow VD, Xue CC, et al. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab. 2008;9(8):738–784. doi: 10.2174/138920008786049302. [DOI] [PubMed] [Google Scholar]
- 11.Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R, et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer. 2003;97(12):3068–3075. doi: 10.1002/cncr.11456. [DOI] [PubMed] [Google Scholar]
- 12.Jamroziak K, Mlynarski W, Balcerczak E, Mistygacz M, Trelinska J, Mirowski M. Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Haematol. 2004;72:314–321. doi: 10.1111/j.1600-0609.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 13.Gervasini G, Carrillo JA, Garcia M, San JC, Cabanillas A, Benitez J. Adenosine triphosphate-binding cassette B1 (ABCB1) (multidrug resistance 1) G2677T/A gene polymorphism is associated with high risk of lung cancer. Cancer. 2006;107:2850–2857. doi: 10.1002/cncr.22332. [DOI] [PubMed] [Google Scholar]
- 14.Hill CR, Jamieson D, Thomas HD, Brown CD, Boddy AV, Veal GJ. Characterisation of the roles of ABCB1, ABCC1, ABCC2 and ABCG2 in the transport and pharmacokinetics of actinomycin D in vitro and in vivo. Biochem Pharmacol. 2013;85(1):29–37. doi: 10.1016/j.bcp.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue AM, Xie ZB, Zhao HF, Guo SP, Shen YH, Wang HP. Associations of ABCB1 and XPC genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis in a Chinese population. Asian Pac J Cancer Prev. 2013;14(5):3085–3091. doi: 10.7314/apjcp.2013.14.5.3085. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi P, Tulsyan S, Agarwal G, Lal P, Agarwal S, Mittal RD, et al. Influence of ABCB1 genetic variants in breast cancer treatment outcomes. Cancer Epidemiol. 2013;37(5):754–761. doi: 10.1016/j.canep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Wei HB, Hu J, Shang LH, Zhang YY, Lu FF, Wei M, et al. A meta-analytic review of ERCC1/MDR1 polymorphism and chemosensitivity to platinum in patients with advanced non-small cell lung cancer. Chin Med J (Engl) 2012;125(16):2902–2907. [PubMed] [Google Scholar]
- 18.Yang J, Wang ZG, Cai HQ, Li YC, Xu YL. Effect of variation of ABCB1 and ABCC3 genotypes on the survival of bone tumor cases after chemotherapy. Asian Pac J Cancer Prev. 2013;14(8):4595–4598. doi: 10.7314/apjcp.2013.14.8.4595. [DOI] [PubMed] [Google Scholar]
- 19.Caronia D, Patiño-Garcia A, Peréz-Martínez A, Pita G, Moreno LT, Zalacain-Díez M, et al. Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: a pharmacogenetic study. PLoS One. 2011;6(10):e26091. doi: 10.1371/journal.pone.0026091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Windsor RE, Strauss SJ, Kallis C, Wood NE, Whelan JS. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: a pilot study. Cancer. 2012;118(7):1856–1867. doi: 10.1002/cncr.26472. [DOI] [PubMed] [Google Scholar]
- 21.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 22.Rost D, Kinig J, Weiss G, Klar E, Stremmel W, Keppler D. Expression and localization of the multidrug resistance proteins MRP2 and MRP3 in human gallbladder epithelia. Gastroenterology. 2001;121(5):1203–1208. doi: 10.1053/gast.2001.28648. [DOI] [PubMed] [Google Scholar]
- 23.Campa D, Vodicka P, Pardini B, Novotny J, Försti A, Hemminki K, et al. Could polymorphisms in ATP-binding cassette C3/multidrug resistance associated protein 3 (ABCC3/MRP3) modify colorectal cancer risk? Eur J Cancer. 2008;44(6):854–857. doi: 10.1016/j.ejca.2008.02.004. [DOI] [PubMed] [Google Scholar]