Abstract
Breast cancer mortality is principally due to tumor recurrence; however, the molecular mechanisms underlying this process are poorly understood. We now demonstrate that the suppressor of cytokine signaling protein SPSB1 is spontaneously upregulated during mammary tumor recurrence and is both necessary and sufficient to promote tumor recurrence in genetically engineered mouse models. The recurrence-promoting effects of SPSB1 result from its ability to protect cells from apoptosis induced by HER2/neu pathway inhibition or chemotherapy. This, in turn, is attributable to SPSB1 potentiation of c-MET signaling, such that preexisting SPSB1-overexpressing tumor cells are selected for following HER2/neu downregulation. Consistent with this, SPSB1 expression is positively correlated with c-MET activity in human breast cancers and with an increased risk of relapse in patients with breast cancer in a manner that is dependent upon c-MET activity. Our findings define a novel pathway that contributes to breast cancer recurrence and provide the first evidence implicating SPSB proteins in cancer.
Significance
The principal cause of death from breast cancer is recurrence. This study identifies SPSB1 as a critical mediator of breast cancer recurrence, suggests activation of the SPSB1/c-MET pathway as an important mechanism of therapeutic resistance in breast cancers, and emphasizes that pharmacological targets for recurrence may be unique to this stage of tumor progression.
Keywords: SPSB1, c-MET, breast cancer, recurrence, apoptosis
Introduction
Breast cancer is both common and treatable. As a consequence, it is by far the most prevalent cancer with nearly 10 million women worldwide currently harboring this diagnosis. Since recurrent breast cancer is typically an incurable disease, the propensity of breast cancers to recur following surgery and adjuvant treatment is the most important determinant of clinical outcome. Recurrent breast cancers arise from the pool of local and disseminated residual tumor cells, termed minimal residual disease, that survive in a presumed dormant state following treatment of the primary cancer. However, while tumor dormancy and recurrence are responsible for the vast majority of breast cancer deaths, the mechanisms underlying these critical stages of cancer progression are largely unknown.
Since residual neoplastic cells constitute the reservoir from which recurrent cancers arise, the lack of therapeutic approaches targeting these cells, combined with our lack of understanding of their biology, constitute major obstacles to the successful treatment of breast cancer. Accordingly, the development of molecularly targeted therapies that are specifically designed to eliminate residual cancer cells, or maintain them in a dormant state, represents an attractive approach to preventing tumor recurrence. Such an approach, however, would require a far more detailed understanding of the biology of minimal residual disease, tumor dormancy, and recurrence than currently exists.
To better define the molecular and cellular events that contribute to breast cancer recurrence, we have developed a series of conditional mouse models for HER2/neu, c-MYC and Wnt1-overexpressing breast cancers that display key features of human breast cancer progression, including metastasis, minimal residual disease, and recurrence (1-5). Following oncogene induction with doxycycline, bitransgenic mice from each of these models develop invasive mammary adenocarcinomas in a manner that is highly penetrant, mammary-specific, and absolutely dependent upon transgene induction by doxycycline. Strikingly, the majority of these tumors regress to a non-palpable state following oncogene down-regulation induced by doxycycline withdrawal via a phenomenon referred to as oncogene addiction.
Notably, the majority of tumors in conditional transgenic mice that regress to a non-palpable state following oncogene down-regulation spontaneously recur over periods of up to a year in the absence of expression of the initiating oncogene (1-7). This observation is consistent with clinical studies demonstrating that neoadjuvant treatment with trastuzumab plus chemotherapy converts HER2/neu-amplified breast cancers to HER2-negative residual disease in more than 40% of cases (8). Thus, primary breast cancer cells likely have the ability to survive therapy and recur via HER2/neu-independent pathways in mice as well as humans. These parallels suggest that mouse models can enable mechanistic approaches to elucidating the molecular and cellular pathways that contribute to the survival and recurrence of residual cancer cells. This goal is of paramount importance for the development of more effective therapies aimed at preventing – as well as treating – breast cancer recurrence.
The SPSB protein family is characterized by a central SPRY (p1A/ryanodine receptor) protein-binding domain and a C-terminal SOCS (suppressor of cytokine signaling) box-containing domain. While the biological functions of these proteins have yet to be elucidated, some SPSB binding partners have been identified, including inducible nitric oxide synthase (iNOS), the c-MET receptor tyrosine kinase, and the prostate apoptosis response protein-4 (PAR-4), for which we have recently reported a role in mammary tumor recurrence (9-12). While SPSB proteins have been reported to promote iNOS degradation, their functions with respect to PAR-4 and c-MET are unknown.
In the present study, we have identified a functional role for SPSB1 in breast cancer recurrence. We show that Spsb1 is spontaneously up-regulated during mammary tumor recurrence in multiple genetically engineered mouse models and that Spsb1 is both necessary and sufficient to promote tumor recurrence. Furthermore, we demonstrate that the recurrence-promoting effects of Spsb1 are due to its ability to protect cells from apoptosis induced by chemotherapy as well as by HER2/neu pathway inhibition, and that the pro-survival effects of Spsb1 are attributable to its ability to potentiate HGF-induced c-MET signaling. Notably, the effects of SPSB1 and c-MET were found to be interdependent in that SPSB1 is required for c-MET activation and tumor recurrence in mice, whereas c-MET is required for SPSB1 to exert its anti-apoptotic effects following HER2/neu down-regulation or chemotherapy.
In support of the relevance of this mechanism to breast cancer relapse in humans, we find that elevated SPSB1 expression in primary tumors is associated with an increased risk of recurrence in breast cancer patients independently of classical prognostic factors. Consistent with our biochemical observations, SPSB1 expression was positively correlated with c-MET activity in human breast cancers, and SPSB1 was associated with an increased risk of relapse specifically in women with whose tumors expressed high levels of c-MET. Finally, while the association of SPSB1 expression with decreased relapse-free survival was independent of classical prognostic factors, it was dependent on the association between SPSB1 and c-MET activity, suggesting that c-MET pathway activation mediates the effect of SPSB1 on relapse in breast cancer patients. Taken together, our observations provide the first evidence for a role for the SPSB family of proteins in cancer and identify a novel pathway involved in breast cancer recurrence.
Results
Spsb1 is spontaneously up-regulated during mammary tumor recurrence
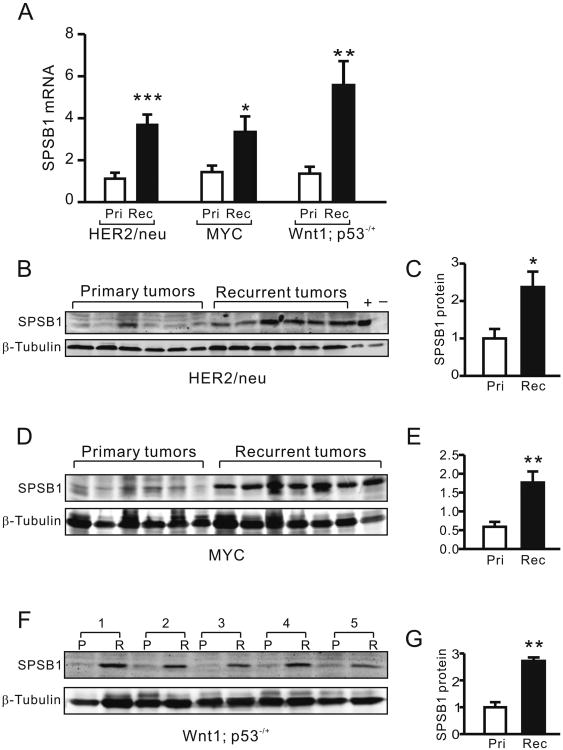
To explore the potential function of SPSB proteins in tumor progression, we performed quantitative real-time RT-PCR (qRT-PCR) for Spsb1, Spsb2, Spsb3 and Spsb4 in primary and recurrent tumors harvested from MMTV-rtTA;TetO-HER2/neu, MMTV-rtTA;TetO-MYC, and MMTV-rtTA;TetO-Wnt1;p53-/+ mice (1-4). Expression of Spsb1 was consistently up-regulated in recurrent tumors compared to primary tumors in each of the models tested (Fig. 1A). No alterations in mRNA expression were detected for Spsb2 or Spsb3 in any of the mouse models and Spsb4 expression was decreased in recurrent tumors from MMTV-rtTA;TetO-HER2/neu mice, but unchanged in recurrent tumors from MYC and Wnt1;p53+/− mice (Sup. Fig 1 A-C).
Figure 1. Spsb1 is spontaneously up-regulated in recurrent mammary tumors.
A. qRT-PCR analysis of Spsb1 mRNA expression in primary (open bar) and recurrent (closed bar) tumors from MMTV-rtTA;TetO-HER2/neu (primary, n=7; recurrent n=15), MMTV-rtTA;TetO-MYC (primary, n=7; recurrent, n=7) and MMTV-rtTA;TetO-Wnt1;p53+/- (primary, n=10; recurrent, n=10) mouse models. Relative Spsb1 expression was normalized to the expression of Tbp, which serves as a loading control. B, D and F. Western blot analysis of Spsb1 protein expression in primary and recurrent tumors from MMTV-TetO-HER2/neu (B), MMTV-rtTA;TetO-MYC (D) and MMTV-rtTA;TetO-Wnt1;p53+/- (F) mice. β-tubulin is shown as a loading control. NMuMG parental and Spsb1-overexpressing cell lysates are shown as negative (-) and positive (+) controls, respectively. C, E and G. Quantification of Spsb1 protein expression normalized to β-tubulin in primary (open bar) and recurrent tumors (closed bar) from MMTV-TetO-HER2/neu (C), MMTV-rtTA;TetO-MYC (E) and MMTV- rtTA;TetO-Wnt1;p53+/- (G) mice. *p<0.05, **p<0.01. p values are calculated by Mann Whitney test (A); and student t-test (C, E and G).
Consistent with its up-regulation at the mRNA level, Spsb1 protein expression was also elevated in recurrent HER2/neu tumors (Fig. 1B, C), recurrent MYC tumors (Fig. 1D, E) and recurrent Wnt1;p53-/+ tumors (Fig. 1F, G). In addition, Spsb1 mRNA levels were strongly correlated with Spsb1 protein levels, suggesting that mRNA levels are generally indicative of protein levels (Sup. Fig. 1D).
Spsb1 is necessary and sufficient to promote mammary tumor recurrence in vivo
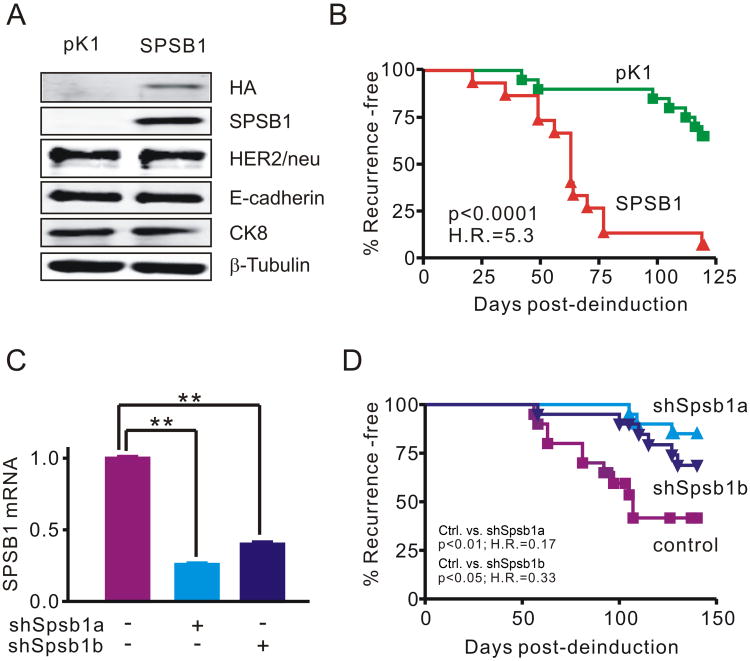
Our finding that Spsb1 is up-regulated in mouse models for breast cancer recurrence induced by three different oncogenic pathways suggested the possibility that this protein might functionally contribute to tumor recurrence. To address this possibility, we first transduced doxycycline-dependent mammary tumor cells derived from an MMTV-rtTA;TetO-HER2/neu primary tumor with a retrovirus expressing HA-tagged Spsb1 (SPSB1) or an empty vector control (pK1). We previously reported that the transcription repressor Snail promotes epithelial- to-mesenchymal transition (EMT) and breast cancer recurrence in a HER2/neu independent manner (5). Unlike Snail, Spsb1-expressing primary tumor cells retained an epithelial morphology indistinguishable from control cells (Sup. Fig. 2A) and expressed HER2/neu, E-cadherin and Cytokeratin 8 (Ck8) at levels comparable to controls (Fig. 2A). Furthermore, Spsb1 expression did not induce endogenous Snail expression in primary mammary tumor cells and Snail expression, if anything, modestly down-regulated endogenous Spsb1 (Sup. Fig. 2B, C). Thus, Spsb1 does not induce Snail expression or EMT in mouse primary mammary tumor cells.
Figure 2. Spsb1 is necessary and sufficient to promote mammary tumor recurrence in vivo.
A. Western blot analysis of HA-tagged Spsb1, HER2/neu, E-cadherin, Cytokeratin 8 (Ck8) and β-tubulin expression in pK1 and SPSB1, doxycycline-dependent HER2/neu-induced primary tumor cells grown in the presence of doxycycline. B. Recurrence-free survival for mice harboring fully regressed orthotopic tumors derived from pK1 or Spsb1-expressing cells. The experiment was repeated three times and a representative result is shown. C. qRT-PCR analysis of endogenous Spsb1 mRNA expression in primary mouse HER2/neu-induced tumor cells expressing Spsb1-specific hairpins (shSpsb1a and shSpsb1b) or a non-silencing control. Error bars indicate standard error of the mean. p<0.05, **p<0.01. p values are calculated by student t-test. D. Recurrence-free survival of mice bearing fully regressed tumors derived from Spsb1-knockdown or control cells. The experiment was repeated three times and a representative result is shown. p-values and hazard ratios (H.R.) as determined by Kaplan-Meier survival analysis.
Next, we employed a previously validated orthotopic model (5) to determine whether Spsb1 expression is sufficient to promote mammary tumor recurrence in vivo, as we hypothesized might be the case based upon the spontaneous up-regulation of Spsb1 in recurrent mammary tumors. H2B-EGFP-labeled control (pK1) or Spsb1-expressing primary tumor cells were injected into the mammary fat pads of nu/nu mice maintained on doxycycline to permit tumor outgrowth in the presence of HER2/neu expression. Similar to the behavior of tumors in intact transgenic mice, doxycycline withdrawal-induced down-regulation of HER2/neu resulted in orthotopic tumor regression to a non-palpable state irrespective of Spsb1 expression status. Strikingly, within 120 days of doxycycline withdrawal, 93% (14 of 15) of Spsb1-transduced tumors had recurred, whereas only 33% (5 of 15) of control tumors had recurred (H.R.=5.3; p < 0.0001) (Fig. 2B). All recurrent tumors were GFP-positive, confirming that they were derived from injected primary tumor cells, and recurrent tumors derived from Spsb1-transduced cells maintained Spsb1 expression (Sup. Fig. 2D, 2E). Neither pK1 nor Spsb1-derived recurrent tumors exhibited HER2/neu up-regulation based on qRT-PCR or western blot analysis, indicating that recurrent tumors did not arise as a consequence of doxycycline-independent reactivation of the HER2/neu transgene or activation of endogenous HER2/neu (data not shown). This result demonstrates that Spsb1 up-regulation can promote mammary tumor recurrence in a HER2/neu-independent manner.
Next, to determine whether Spsb1 is required for mammary tumor recurrence following HER2/neu down-regulation, we knocked down endogenous Spsb1 expression in HER2/neu-induced mouse primary tumor cells using either of two hairpins, shSpsb1a or shSpsb1b, that down-regulated endogenous Spsb1 expression by 60-75% (Fig. 2C). Next, shSpsb1 and control shRNA-expressing doxycycline-dependent primary tumor cells were injected into the mammary fat pads of nu/nu mice maintained on doxycycline to permit primary tumor outgrowth in the presence of HER2/neu expression.
As before, down-regulation of HER2/neu induced by doxycycline withdrawal resulted in the rapid regression of orthotopic tumors to a non-palpable state irrespective of whether they were derived from control or Spsb1 knockdown cells (data not shown). However, consistent with the spontaneous up-regulation of Spsb1 observed in recurrent mammary tumors, the rates of recurrence of mammary tumors derived from Spsb1 knockdown cells were dramatically reduced compared to the control tumors (Fig. 2D, shSpsb1a vs. control H.R=0.17, p<0.01; shSpsb1b vs. control H.R. = 0.34, p<0.05). These results demonstrate that endogenous Spsb1 is required for mammary tumor recurrence following HER2/neu down-regulation. Consistent with this conclusion, Spsb1 expression levels were restored in recurrent tumors derived from shSpsb1 knockdown cells to levels comparable to those seen in recurrent tumors derived from control cells (Sup. Fig. 2F), suggesting that Spsb1 up-regulation in tumor cells is strongly selected for during the process of recurrence. In aggregate, our findings indicate that Spsb1 is both necessary and sufficient to promote mammary tumor recurrence.
Spsb1 protects tumor cells from apoptosis induced by HER2/neu down-regulation
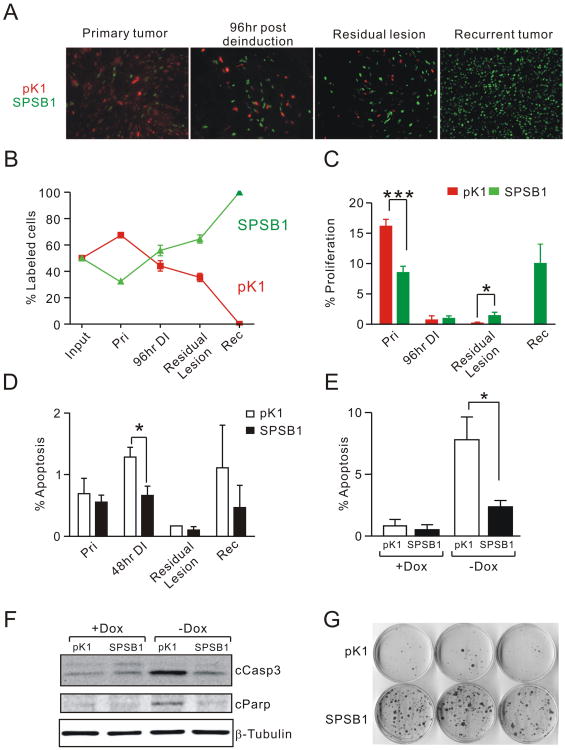
To delineate the cellular mechanism by which Spsb1 promotes mammary tumor recurrence, we stably transduced pK1 and SPSB1 primary tumor cells with H2B-ECFP and H2B-EGFP, respectively. Equal numbers of ECFP-labeled pK1 and EGFP-labeled SPSB1 cells were admixed and injected into the mammary fat pads of nu/nu mice maintained on doxycycline. Primary orthotopic tumors, regressing tumor grafts (96 h post doxycycline withdrawal), residual lesions (32 d post doxycycline withdrawal) and recurrent tumors were harvested and the number of EGFP and ECFP-positive cells was determined as a percentage of the total number of fluorescently-labeled tumor cells present at each time point (Fig. 3A, B).
Figure 3. Spsb1 protects cells from apoptosis induced by oncogene down-regulation.
A. Representative images of pK1-H2B-ECFP (red) or SPSB1-H2B-EGFP (green) cells harvested from primary orthotopic tumors growing in the presence of doxycycline, regressing tumor grafts 96 h post-oncogene deinduction, residual lesions formed from primary tumors 32 d post-oncogene deinduction, and recurrent tumors growing in the absence of doxycycline. B. Fraction of pK1-H2B-ECFP (red) or SPSB1-H2B-EGFP (green) cells as a percentage of total fluorescent cells present at each of the indicated time points in panel A (Pri, primary orthotopic tumor, n=8; 96-h DI, 96-h post-deinduction, n=6; Residual lesion, n=8; and Rec, recurrent tumors, n=3). C. Quantification of Ki-67-positive pK1-H2B-ECFP (red) or SPSB1-H2B-EGFP (green) cells at the same time points as in panel A. D. Quantification of cleaved capspase-3-positive pK1 (open bars) or Spsb1-expressing cells (closed bars) in primary tumors (Pri, n=4); 48 h post-doxycycline withdrawal (48 h DI, n=6); Residual lesions (n=3); and recurrent tumors (Rec, n=4). E. Quantification of cleaved capspase-3-positive pK1 (open bars) and Spsb1-expressing cells (closed bars) in the presence and absence of doxycycline. Percentages shown represent the means from 4 independent experiments. F. Western blot analysis of cleaved caspase-3 (cCasp3), cleaved Parp (cParp), and β-tubulin in pK1 and SPSB1 cells in the presence and absence of doxycycline. G. Clonogenic assay for survival and outgrowth of pK1 and SPSB1 cells in the absence of doxycycline for 20 days. The experiment was performed five times and a representative result is shown. Error bars indicate standard error of the mean. p values are calculated by one-sample t-test (B) or Mann Whitney test (C, D, and E). *p<0.05, **p<0.01, ***p<0.001.
Despite being injected in equal numbers, SPSB1 cells comprised only 30% of labeled tumor cells in primary orthotopic tumors, suggesting that SPSB1 cells are at a selective disadvantage compared to control cells in the presence of HER2/neu expression (Fig. 3A, B). However, within 96 h of doxycycline withdrawal this trend reversed as SPSB1 cells were markedly enriched compared to control cells. Selection for SPSB1 cells was further accentuated in residual tumor lesions 32 d following doxycycline withdrawal, at which time EGFP-positive SPSB1 cells constituted ∼65% of tumor cells. Strikingly, recurrent tumors were composed almost exclusively of Spsb1-transduced cells (Fig. 3A, B). To rule out differential growth effects of H2B-EGFP and H2B-ECFP, the experiment was repeated after swapping fluorescent protein labels between pK1 and SPSB1 cells. Similar results were observed (data not shown). These data demonstrate that Spsb1 expression confers a strong selective advantage to tumor cells in which HER2/neu activity has been down-regulated.
To investigate the basis for the observed selection for Spsb1-expressing cells following HER2/neu down-regulation, we determined proliferation rates in tumors derived from control cells or Spsb1-expressing cells during the course of recurrence. Consistent with the observed selection against Spsb1-expressing cells during primary tumor outgrowth, SPSB1 cells in primary tumors expressing HER2/neu exhibited lower proliferation rates compared to control cells (Fig. 3C). Following doxycycline withdrawal, proliferation rates fell dramatically and did not differ between SPSB1 and control pK1 cells (Fig. 3C). In residual lesions proliferation rates were extremely low, although SPSB1 cells exhibited slightly elevated proliferation rates compared to pK1 control cells (Fig. 3C). Proliferation rates for SPSB1 cells in recurrent tumors were restored to levels similar to those observed in primary tumors. Too few pK1 control cells were present in recurrent tumors to ascertain their proliferation rates. Repeat experiments conducted after swapping fluorescent protein labels between pK1 and SPSB1 cells yielded similar results (data not shown).
Since selection for SPSB1 cells within 96 h following HER2/neu down-regulation could not be explained by differences in proliferation rates between these two populations of cells, we next compared apoptosis rates in orthotopic tumors derived from pK1 or SPSB1 cells 48 h following doxycycline withdrawal. As predicted based on prior results (4), pK1 control tumor cells exhibited a marked increase in cleaved-caspase-3 staining 48 h after doxycycline withdrawal (Fig. 3D). In contrast, apoptotic rates for Spsb1-expressing tumor cells remained low despite oncogene down-regulation (Fig. 3D). These findings suggest that Spsb1 expression protects tumors cells from apoptosis induced by acute HER2/neu down-regulation and, more broadly, that this survival advantage forms the basis for their selection relative to control cells in the context of HER2/neu down-regulation.
To confirm the cell-intrinsic survival advantage of SPSB1 cells observed in vivo, we withdrew doxycycline from the above pK1 and SPSB1 cells cultured in vitro. Consistent with our in vivo observations, acute HER2/neu down-regulation resulted in a dramatic increase in apoptotic rates in pK1 cells, as evidenced by immunofluorescence for cleaved caspase-3, whereas apoptotic rates in SPSB1 cells remained low (Fig. 3E). This finding was confirmed by immunoblotting analysis for cleaved caspase-3 and cleaved Parp (Fig. 3F). In contrast, proliferation rates did not differ between SPSB1 and control cells regardless of HER2/neu expression status (Sup. Fig. 3A). As a result, there was a net growth advantage for SPSB1 cells compared to control cells when grown in the absence of doxycycline (Sup Fig. 3B). Additionally, SPSB1 cells were far more efficient than control cells at forming colonies in a clonogenic assay in the absence of HER2/neu expression (Fig. 3G). Together, these findings demonstrate that SPSB1 confers a strong selective advantage for tumor cell survival following HER2/neu down-regulation.
SPSB1 protects human breast cancer cells from apoptosis induced by HER2/neu inhibition or treatment with chemotherapeutic agents
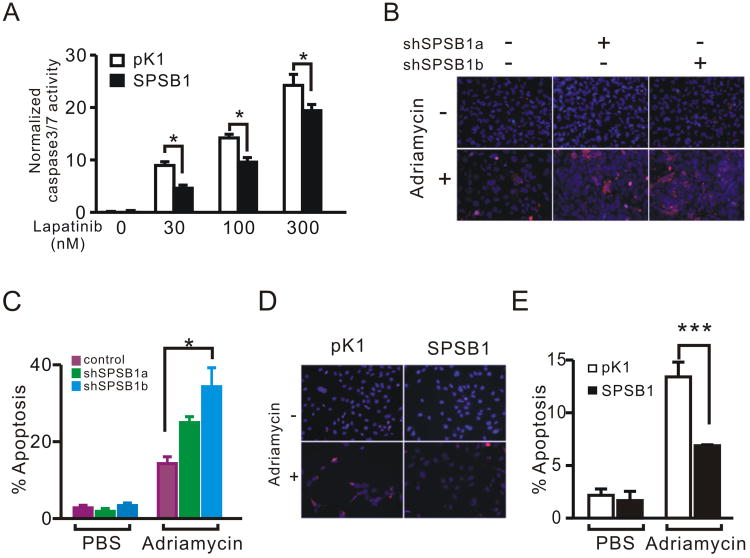
To extend our observations from mouse mammary tumors to human breast cancer, we overexpressed SPSB1 in BT474 cells, a HER2/neu-amplified human breast cancer cell line (13) that expresses relatively low levels of SPSB1 (Sup. Fig. 3C). In agreement with our observations in mouse tumor cells, SPSB1-expressing BT474 cells treated with Lapatinib exhibited lower caspase-3/7 activity and less cell death than control cells (Fig. 4A, Sup. Fig. 3D).
Figure 4. SPSB1 protects human breast cancer cells from apoptosis induced by oncogene down-regulation or Adriamycin treatment.
A. Relative caspase-3/7 activity in BT474 cells treated with vehicle control or the indicated concentrations of Lapatinib. The experiment was performed in triplicate for each condition and repeated twice. Representative results are shown. B. Immunofluorescence staining for cleaved caspase-3 in Hs578T control and SPSB1 knockdown cells treated with PBS or 10 μM Adriamycin for 24 h. C. Quantification of cleaved caspase-3 staining in B. D. Immunofluorescence staining for cleaved caspse-3 in MDA-MB-231 control and SPSB1-expressing cells treated with PBS or 10 μM Adriamycin for 24 h. E. Quantification of cleaved caspase-3 staining in D. Percentages shown represent the means from 4 independent experiments. p values are calculated by two-way ANOVA (A, C); and two-way ANOVA on log-transformed data (E). *p<0.05 and **p<0.01.
Next, we tested whether SPSB1 could also protect human breast cancer cells from apoptosis induced by treatment with chemotherapeutic agents, which are more commonly used as anti-neoplastic agents in breast cancer patients than targeted therapies directed against HER2. We used two different hairpins targeting human SPSB1 (Sup. Fig. 3E; shSPSB1a and shSPSB1b) to knock down endogenous SPSB1 expression in Hs578T cells, a HER2/neu-negative human breast cancer cell line that expresses high levels of endogenous SPSB1 (Sup. Fig. 3C). HS578T cells expressing SPSB1 hairpins exhibited increased levels of apoptosis following treatment with the chemotherapeutic agents Adriamycin and etoposide, as indicated by increased staining for cleaved-caspase 3 (Fig. 4B, C and Sup. Fig. 3F). Similarly, Hs578T cells with SPSB1 knockdown were less efficient at forming colonies in a clonogenic assay (Sup. Fig. 3G). Conversely, ectopic expression of SPSB1 in MDA-MB-231 cells, which are HER2/neu-negative and express low levels of SPSB1 (Sup. Fig. 3C), reduced the rate of apoptosis following Adriamycin treatment by almost 50% (Fig. 4D, E). Together, our data indicate that SPSB1 plays an essential role in protecting human breast cancer cells from apoptosis induced either by HER2/neu down-regulation or treatment with chemotherapeutic agents.
SPSB1 potentiates c-MET signaling in vitro
To explore the molecular mechanism underlying SPSB1's anti-apoptotic effects, we considered two proteins reported to bind to SPSB1, PAR-4 and the c-MET receptor tyrosine kinase, each of which has been implicated in breast cancer. While human PAR-4 has been reported to bind to multiple SPSB proteins, mouse Par-4 does not contain an Spsb-binding motif (11). Consistent with this, we were unable to detect an interaction between Spsb1 and Par-4 in mouse primary tumor cells by co-immunoprecipitation, nor did we observe a difference in Par-4 expression between pK1 and Spsb1-expressing tumor cells (Sup. Fig. 4A). Like PAR-4, c-MET has been reported to bind to all four SPSB proteins, but only SPSB1 has been reported to potentiate HGF-induced ERK signaling when transiently overexpressed in HEK293 cells (14). Given that Spsb1, but not other Spsb family members, is up-regulated in recurrent tumors and that recurrent mammary tumors in our mouse models frequently activate c-Met (Sup Fig. 4B), we considered the possibility that the anti-apoptotic effects of Spsb1 might be mediated by c-Met.
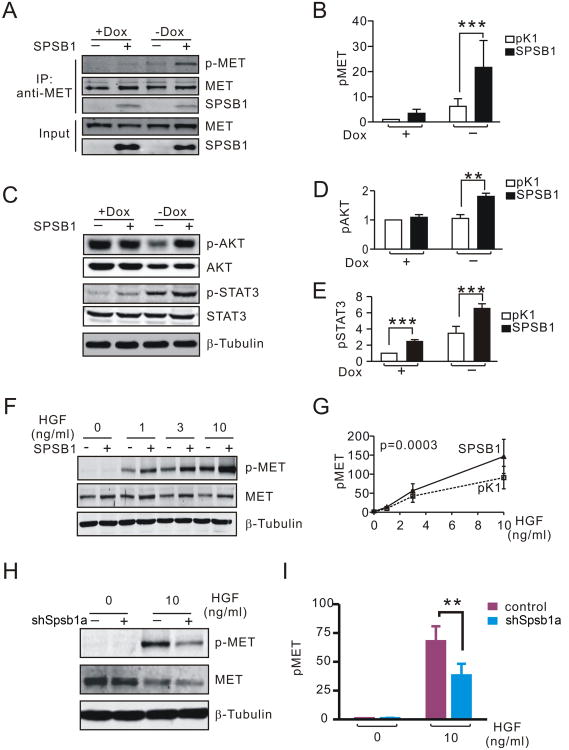
First, we determined that Spsb1 can stably bind to endogenous c-Met in mouse mammary tumor cells in the presence or absence of HER2/neu expression (Fig. 5A). Next, we investigated the effect of Spsb1 on c-Met activity. In cells expressing HER2/neu, c-Met phosphorylation was low irrespective of Spsb1 expression status (Fig. 5A, B). However, following HER2/neu down-regulation, c-Met phosphorylation was markedly up-regulated in Spsb1-expressing cells, but remained low in pK1 control cells (Fig. 5A, B). Consistent with the observed increase in c-Met phosphorylation following HER2/neu down-regulation, downstream effectors of the c-Met pathway, phospho-Akt (Ser473) and phospho-Stat3 (Tyr705), were also up-regulated in Spsb1-expressing cells compared to control cells following doxycycline withdrawal (Fig. 5C-E). However, contrary to a prior report no difference in phospho-Erk activity was detected between SPSB1 cells and pK1 control cells under the same conditions (Sup. Fig. 4C).
Figure 5. SPSB1 potentiates c-MET signaling.
A. Immunoprecipitation and western blot analysis of c-Met (T1234/1235) phosphorylation and Spsb1:Met binding in pK1 and Spsb1-expessing primary MMTV-rtTA;TetO-HER2/neu tumor cells in the presence and absence of doxycycline. B. Quantification of c-Met (T1234/1235) phosphorylation in pK1 and SPSB1 cells in the presence and absence of doxycycline. Results represent the average of 5 independent experiments. C-E. Western blot analysis (C) and quantification (D, E) of phospho-Akt (S473) and phospho-Stat3 (T706) in pK1 and SPSB1 cells in the presence and absence of doxycycline. Results represent the average of 5 independent experiments. F, G. Western blot analysis (F) and quantification (G) of c-Met phosphorylation in pK1 and Spsb1expressing cells treated with HGF in the absence of doxycycline. Results represent the average of 6 independent experiments. H and I. Western blot analysis (H) and quantification (I) of c-Met (T1234/1235) phosphorylation in control and Spsb1-knockdown cells treated with 10 ng/ml HGF in the absence of doxycycline. Results represent the average of 8 independent experiments. Quantification of c-Met activity was performed by normalizing the level of phopho-c-Met to total c-Met expression. Error bars represent the standard error of the mean. p values are calculated by one sample t-test (B, D, E and H) or two-way ANOVA (G). *p<0.05, **p<0.0, ***p<0.001.
Next, we wished to determine whether Spsb1 could potentiate HGF-induced c-Met signaling. In mouse mammary tumor cells subjected to acute HER2/neu down-regulation in vitro, Spsb1-expressing cells exhibited more pronounced c-Met activation in response to HGF treatment than pK1 control cells (Fig. 5F, G). Similarly, ectopic expression of SPSB1 in human MDA-MB-231 breast cancer cells also enhanced HGF-induced c-MET activation (Sup. Fig. 4D). Conversely, knockdown of endogenous SPSB1 impaired HGF-induced c-MET activation in human Hs578T breast cancer cells and in mouse HER2/neu primary mammary tumor cells subjected to HER2/neu down-regulation (Fig. 5H, I and Sup. Fig. 4E). In aggregate, these data confirm that SPSB1 is a c-MET binding partner and demonstrate that SPSB1 can potentiate c-MET signaling in both mouse and human breast cancer cells.
c-MET is required for SPSB1-mediated tumor cell survival
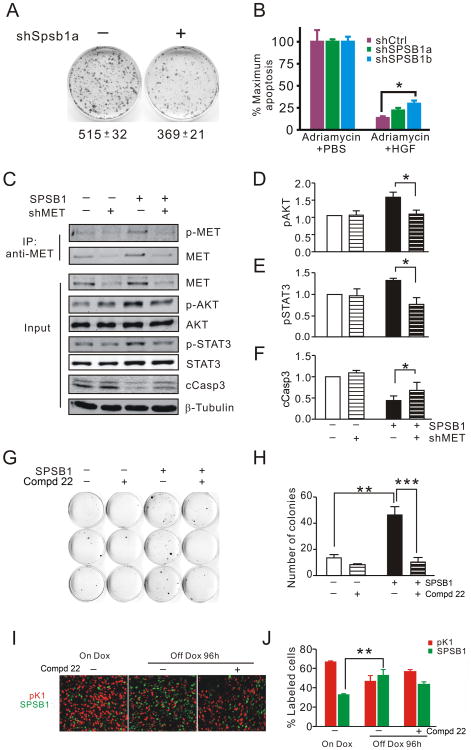
Given that c-MET signaling is a well-documented cell survival pathway and that SPSB1 potentiates c-MET signaling, we reasoned that the anti-apoptotic effects exerted by SPSB1 might be mediated by c-MET. Consistent with this hypothesis, we found that mouse tumor cells with impaired HGF-induced c-Met activation due to Spsb1 knockdown were less efficient at forming colonies than control cells when grown in the presence of HGF following HER2/neu down-regulation (Fig. 6A, p<0.01). In an analogous manner, HGF-mediated rescue from Adriamycin-induced apoptosis was less efficient in Hs578T cells in which SPSB1 had been knocked down (Fig. 6B).
Figure 6. c-MET is required for SPSB1-mediated tumor cell survival.
A. Clonogenic assay for survival and outgrowth of control and Spsb1-knockdown cells treated with 10 ng/ml HGF growing in the absence of doxycycline. The numbers of colonies in each plate was quantified using Cell Profiler (24). Experiments were performed in triplicate and repeated. A representative result is shown. B. HGF-mediated rescue of Adriamycin induced apoptosis in HS578T cells expressing control and SPSB1 specific hairpins. Maximum levels of apoptosis were defined as caspase-3/7 activity in the same cells treated with Adriamycin in the absence of HGF. C-F. Immunoprecipitation and Western blot analysis (A) and quantification (B-D) of c-Met (T1234/1235) phosphorylation, Akt (S473) phosphorylation, Stat3 (T706) phosphorylation and cleaved caspase-3 (cCasp3) levels in pK1 and SPSB1 cells expressing an anti-c-Met shRNA (shMET) or non-silencing control in the absence of doxycycline. Results represent the average of 4 independent experiments. Quantification of c-Met, Akt or Stat3 activity was performed by normalizing the levels of each phospho-protein to its corresponding total protein. The experiment was performed in triplicate for each condition and repeated twice. Representative results are shown. G and H. Clonogenic assay (G) and quantification (H) of pK1 and SPSB1 cells growing in the absence of doxycycline and treated with c-MET inhibitor for 14 days. I and J. Photomicrographs (I) and quantification (J) of pK1-H2B-mCherry (red) or SPSB1-H2B-EGFP (green) cells harvested from primary orthotopic tumors growing in the presence of doxycycline and regressing tumor grafts 96 h post-oncogene deinduction treated with vehicle or c-MET inhibitor. On Dox, primary orthotopic tumor (n=4); off Dox (96-h post-deinduction) with vehicle, n=4; off Dox with c-MET inhibitor, n=6. Error bars represent the standard error of the mean. p-values are calculated by one-sample t-test (B-D), student t-test (E, F), one way ANOVA (H), or Kruskal Wallis test with Dunn's multiple comparison test (J). *p<0.05 and **p<0.01.
To determine whether c-MET is required for SPSB1-mediated cell survival, we knocked down endogenous c-Met expression in pK1 and Spsb1-expressing cells and subjected cells to acute HER2/neu down-regulation. Consistent with our findings above, Spsb1-expressing cells exhibited elevated levels of phospho-Met, phospho-Akt and phospho-Stat3, as well as diminished levels of cleaved-caspase 3, compared to control cells following acute HER2/neu down-regulation (Fig. 6C-F). By comparison, Spsb1-expressing cells in which c-Met had been knocked down exhibited decreased levels of phospho-Met, phospho-Akt and phospho-Stat3, as well as a corresponding increase in levels of cleaved-caspase 3, following acute HER2/neu down-regulation (Fig. 6C-F). Consistent with these findings, c-Met knockdown in Spsb1 expressing cells resulted in decreased cell viability as well as a decrease in cell number following HER2/neu down-regulation (Sup. Fig. 5 A, B).
To demonstrate directly that c-Met is required for Spsb1-mediated cell survival following HER2/neu down-regulation, we first performed a clonogenic assay in which pK1 or Spsb1 expressing cells were treated with either a c-MET small molecule inhibitor, compound 22 (Sup. Fig. 6 A-D and (15)) or vehicle control. Consistent with our previous finding, Spsb1 expressing cells formed colonies far more efficiently than control cells following HER2/neu downregulation (Figure 6G, H). Supporting the notion that Spsb1's recurrence-promoting effect is Met-dependent, treatment with the c-Met inhibitor compound 22 markedly reduced the number of colonies formed by Spsb1 expressing cells in the absence of doxycycline (Figure 6G, H). This observation is consistent with a model in which c-Met activity is required for Spsb1-mediated tumor cell survival following HER2/neu down-regulation in vitro.
Next, we tested the functional importance of c-Met activity for Spsb1-mediated tumor cell survival in vivo. Consistent with our observations in fluorescent cell competition assays (Figure. 3), Spsb1 expressing cells comprised only 30% of fluorescently-labeled cells in primary orthotopic tumors in the presence of HER2/neu expression, and HER2/neu down-regulation resulted in rapid selection for Spsb1 expressing cells within regressing tumor grafts within 96 h following doxycycline withdrawal (Figure 6I, J). However, treatment of tumor-bearing mice with compound 22 impaired the selection for Spsb1 expressing cells following HER2/neu down-regulation, such that in the presence of the c-MET inhibitor significant enrichment of Spsb1-expressing cells relative to primary tumors growing in the presence of HER2/neu was no longer observed (Figure 6I, J). Together, these findings suggest that c-Met is required for Spsb1-mediated tumor cell survival following HER2/neu down-regulation.
SPSB1 is associated with increased risk of relapse in breast cancer patients independently of classical prognostic factors
In light of our finding that Spsb1 is necessary and sufficient to promote mammary tumor recurrence in mice, we hypothesized that women with primary breast cancers expressing high levels of SPSB1 would relapse at a faster rate than women whose breast cancers expressed lower levels of SPSB1. To test this hypothesis, we assembled 1710 unique patient profiles from nine published human primary breast cancer microarray data sets of mixed ER status, each of which contained more than 100 patients and provided information on relapse-free survival as well as SPSB1 mRNA expression.
For each data set, the effect size of the association between SPSB1 mRNA expression and relapse-free survival was estimated using two different methods: the hazard ratio from Cox proportional hazard regression in which SPSB1 expression was modeled as a continuous variable; and the concordance index (c-index) (16). To derive an overall estimate and statistical significance of the association between SPSB1 expression and relapse-free survival while accounting for heterogeneity among data sets, each type of effect size estimate was combined across data sets by meta-analysis using the inverse-variance weighting method (17). In the presence of significant heterogeneity (p-value for Cochran's Q statistic < 0.05), a random-effect model (18) was used for meta-analysis. Otherwise a fixed-effect model (19) was used.
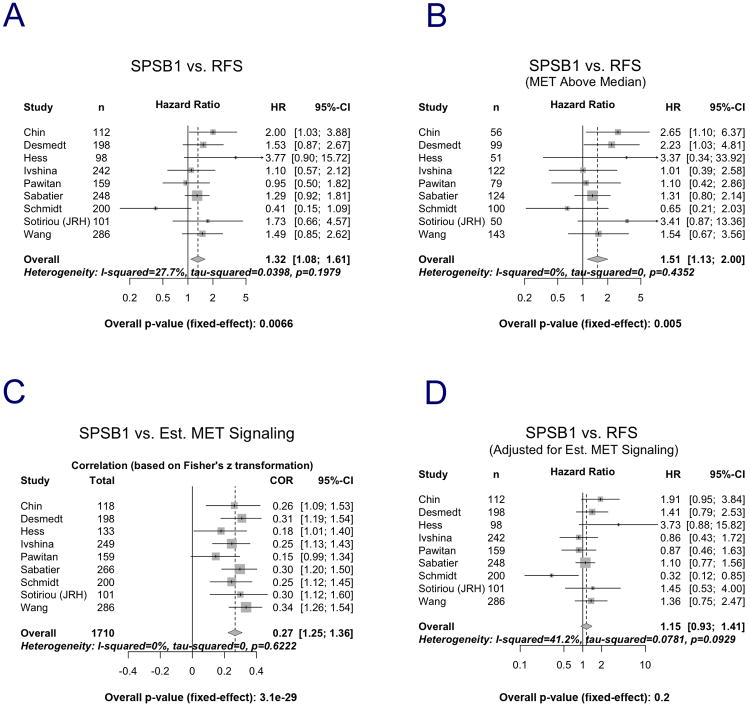
Among the 1710 patients studied, information on relapse-free survival was available for 1644. Across all patients, a significant positive association was observed between elevated SPSB1 expression and increased risk of relapse within 5 years of diagnosis (Fig. 7A, H.R. = 1.32 [1.08-1.61], p=0.0066; c-index=0.54 [0.51-0.57], p=0.01, plot not shown).
Figure 7. Elevated SPSB1 expression in primary tumors correlates with increased risk of relapse and high c-MET activity in women with breast cancer.
Forest plot representation of 5-year survival estimates and hazard ratios for relapse-free survival for breast cancer patients in individual datasets representing either all 1644 breast cancer patients (A), or restricted to patients with c-MET expression above the median (B). C. Forest plot representation of the correlation between SPSB1 mRNA expression and estimated c-MET pathway activity. D. Forest plot representation of 5-year survival estimates and hazard ratios for relapse-free survival for breast cancer patients in individual datasets after adjusting for estimated c-MET activity. Names and sizes of data sets are shown on the left, hazard ratio (center of square) and 95% confidence interval (horizontal line) are shown for each individual dataset on the right. Sizes of squares are proportional to weights used in meta-analysis. The overall hazard ratios (dashed vertical lines) and associated confidence intervals (lateral tips of diamonds) are shown for the fixed-effect model. Solid vertical line indicates no effect. The I-squared statistic describes the percentages of variation across studies that are due to heterogeneity rather than chance. The tau-squared value represents the estimated between-study variance. The p-value after tau-squared is derived from a chi-squared test of homogeneity on the Cochran's Q statistic.
Next, we assessed the association between SPSB1 expression and common clinicopathologic prognostic factors for breast cancer, including estrogen receptor (ER) status, progesterone receptor (PR) status, HER2/neu status, tumor size, tumor grade, lymph node status and intrinsic molecular subtype. Among these, SPSB1 expression was positively and significantly associated only with ER-negative status, PR-negative status and basal-like subtype (Sup. Fig. 7 A-C).
Since ER-negative, PR-negative and basal-like subtype tumors are each generally associated with a worse prognosis in breast cancer patients (20), we wished to determine whether the poor prognosis observed in patients with breast cancers expressing high levels of SPSB1 was an indirect reflection of the association between elevated SPSB1 expression and any of these pathological variables. To address this question, we performed multivariate Cox proportional hazards regression in combination with meta-analysis. The result demonstrated that the association between elevated SPSB1 expression and decreased relapse-free survival was unaffected, and remained significant, after adjusting for ER status and the basal-like subtype using multivariate Cox proportional hazards regression in combination with meta-analysis (Sup. Fig. 8 A, B).
Together, these data indicate that SPSB1 is preferentially expressed in aggressive subsets of human breast cancer, but that the association between elevated SPSB1 expression and decreased relapse-free survival is not attributable to this pattern of expression. Rather, it raises the intriguing possibility that the aggressive behavior of these breast cancer subsets may be due, at least in part, to SPSB1.
SPSB1 expression is correlated with increased c-MET pathway activity in human breast cancers, which mediates its association with increased risk of relapse
In light of our genetic and pharmacological experiments indicating that c-MET is required for SPSB1-mediated tumor cell survival, we hypothesized that the association of SPSB1 expression with relapse-free survival in breast cancer patients would be more pronounced in tumors expressing high levels of c-MET than in those expressing low levels of c-MET. After stratifying samples by median c-MET mRNA expression, we found that SPSB1 mRNA expression in primary human breast tumors was significantly associated with relapse-free survival in patients whose tumors expressed levels of c-MET mRNA higher than the median (Fig. 7B, H.R.=1.51 [1.13-2.00], p=0.005; c-index=0.56 [0.51-0.60], p=0.0078, plot not shown), but not in patients whose tumors expressed levels of c-MET mRNA lower than the median (Sup. Fig. 9). This observation was consistent with our in vitro findings that the biochemical effects of SPSB1 on tumor cell survival require the presence of c-MET, and that c-MET knockdown or c-MET pharmacological inhibition abrogate the effects of SPSB1 on tumor cell survival. These findings further suggest that c-MET expression levels may be rate limiting for the effects of SPSB1.
Given our biochemical findings that SPSB1 potentiates c-MET signaling, we hypothesized that c-MET pathway activity would be more likely to be elevated in human breast cancers expressing high levels of SPSB1. Since c-MET activity data are not available for these data sets, we generated a 24-gene c-MET signature with a previously described scoring algorithm (21). Using this system, we assigned each of 1644 human breast cancer samples a relative c-MET activity score; consistent with our in vitro biochemical findings that SPSB1 expression potentiates c-MET activation, we observed a highly significant positive correlation between SPSB1 mRNA expression and estimated c-MET activity in human breast cancers (Fig. 7C, r=0.27, p=3.1e–29). The magnitude of this correlation was comparable to that observed for c-MET mRNA and estimated c-MET activity in human breast cancers from the same data set (data not shown).
Based on our in vivo and in vitro findings to this point, particularly those in mouse models for mammary tumor recurrence, we hypothesized that the association of SPSB1 with relapse-free survival in human breast cancers might be attributable to its potentiation of c-MET pathway activity. Consistent with our hypothesis, the association between SPSB1 expression and relapse-free survival in breast cancer patients was dependent upon the association between SPSB1 expression and estimated c-MET pathway activity (Fig. 7D). That is, after adjusting for estimated c-MET activity, the association between SPSB1 expression and relapse-free survival was no longer evident. These results suggest that the recurrence-promoting effects of SPSB1 are mediated by its ability to potentiate c-MET signaling.
In aggregate, our analyses demonstrate that SPSB1 expression in human breast cancers is associated with decreased relapse-free survival in breast cancer patients independently of the fact that SPSB1 is preferentially expressed in aggressive subsets of human breast cancers. Moreover, using a c-MET gene expression signature as surrogate marker for c-MET pathway activity, our findings identified a positive correlation between SPSB1 expression and c-MET activity in human breast cancers that appeared to account for the observed association between SPSB1 expression and relapse-free survival. Together, these findings are compatible with a model in which SPSB1 promotes breast cancer recurrence in humans and does so by virtue of its ability to potentiate c-MET pathway activity.
Discussion
For many women with breast cancer, even those with early stage disease, disseminated tumor cells are already present at the time of diagnosis. These residual cells, termed minimal residual disease, have the ability to survive in a presumed dormant state following treatment and linger unrecognized for more than a decade before emerging as recurrent disease. As a result, breast cancers that appear cured by surgery, radiation, and adjuvant therapy may resurface as local or distant recurrences 10 or 20 years later.
In light of these considerations, understanding the biology of minimal residual disease and elucidating the molecular pathways that contribute to tumor dormancy and recurrence is a critical priority in breast cancer research. Using genetically engineered mouse models for breast cancer recurrence, human breast cancer cell lines and interrogation of multiple patient data sets, we have identified SPSB1 as a critical mediator of breast cancer recurrence. In aggregate, our findings identify a novel molecular pathway that contributes to the survival and recurrence of residual cancer cells, define a subset of human breast cancers with a high likelihood of recurrence, and provide the first evidence for a role for the SPSB family of proteins in cancer.
Using three different genetically engineered mouse models, we found that Spsb1 is up-regulated during mammary tumor recurrence. Consistent with its up-regulation in recurrent tumors, enforced expression of Spsb1 in primary mammary tumor cells promoted tumor recurrence, whereas knockdown of endogenous Spsb1 expression inhibited recurrence, demonstrating a requirement for Spsb1 in this process. Cellular analyses indicated that the recurrence-promoting effects of SPSB1 were due to its ability to promote survival in human and mouse breast cancer cells subjected to chemotherapy or HER2/neu pathway down-regulation, and that therapy selected for pre-existing Spsb1 overexpressing cells in primary tumors. Biochemical analyses revealed that the requirement for Spsb1 in tumor cell survival was attributable to, and dependent upon, its ability to potentiate c-MET signaling. In agreement with these findings, endogenous SPSB1 was also required for the survival of human breast cancer cells treated with chemotherapeutic agents.
In support of the potential functional importance of SPSB1 in human breast cancer recurrence, SPSB1 expression was associated with a high risk of relapse in breast cancer patients and SPSB1 expression was associated with aggressive subtypes of human breast cancer, including ER-negative, PR-negative, and basal-like tumors. However, SPSB1 expression was associated with a high risk of recurrence in women independently of its association with these clinicopathological parameters. This suggests that SPSB1 expression is an independent prognostic factor for human breast cancer recurrence and raises the possibility that SPSB1 itself may contribute to the aggressive behavior of human breast cancers.
In agreement with our findings in preclinical models that Spsb1 promotes recurrence by potentiating c-MET pathway activity, the association between SPSB1 expression and relapse-free survival was evident in patients whose breast cancers expressed high levels of c-MET, but not in those whose breast cancers expressed low levels of c-MET. Also consistent with our findings in preclinical models, SPSB1 expression in human primary breast cancers was positively correlated with estimated c-MET pathway activity and this association was primarily responsible for mediating the association of SPSB1 expression with risk of relapse in breast cancer patients.
Tumor heterogeneity poses a major stumbling block to the effective treatment of human cancers as therapeutic interventions frequently result in the selection and outgrowth of preexisting resistant variants. Data from fluorescent cell competition assays indicated that Spsb1-expressing cells exhibit a pronounced survival advantage following HER2/neu down-regulation and are further selected for during recurrent tumor outgrowth. Based on these findings, we propose that cells with elevated Spsb1 expression pre-exist in HER2/neu-induced mouse primary tumors and are selected for in the context of HER2/neu down-regulation and recurrent tumor outgrowth. If true, this model would explain the observed up-regulation of Spsb1 expression in recurrent mouse mammary tumors and could also explain the association of elevated SPSB1 expression in human primary breast cancers with increased rates of relapse, since primary tumors with higher SPSB1 expression may harbor a greater number of apoptosis-resistant, SPSB1-expressing cells, which are more likely to survive therapy and recur. Together, these findings emphasize the fact that clinically important properties of cancers – namely those that determine resistance and recurrence – may arise from small, yet biologically critical, subsets of cells.
Our laboratory recently reported that Par-4 down-regulation can promote mammary tumor recurrence and PAR-4 has been reported to bind to SPSB1 (9). However, consistent with the fact that mouse Par-4 lacks the protein motif responsible for mediating this interaction (11), we did not observe an interaction between Par-4 and Spsb1 in mouse mammary tumor cells. Instead, we found that activation of another SPSB1 binding protein, c-MET, is enhanced by the presence of SPSB1 and is required for SPSB1-mediated tumor cell survival. As it is likely that multiple mechanisms of therapeutic resistance contribute to tumor recurrence, further investigation will be required to determine whether Par-4 and Spsb1 escape pathways are operative within the same tumors, or in the same cells within those tumors.
Tumor recurrence in the conditional transgenic HER2/neu mouse model employed here takes place following targeted down-regulation of the oncogenic pathway driving tumor growth. In contrast, the recurrence of HER2/neu-amplified breast cancers in patients is presumed to occur in the presence of continued HER2/neu signaling. Nevertheless, as it has become increasingly evident that neoadjuvant therapy with trastuzumab may convert HER2-positive breast cancers to HER2-negative residual disease (8), the molecular mechanisms of tumor escape underlying tumor recurrence in these mouse models may be clinically relevant. Indeed, based upon our observations that Spsb1 is spontaneously up-regulated in recurrent mouse mammary tumors induced by oncogenic pathways other than HER2/neu, that SPSB1 provides a survival advantage to non-HER2/neu-amplified human breast cancer cells treated with chemotherapeutic agents, and that SPSB1 is associated with recurrence-free survival across all breast cancer patients (i.e. not simply those that are HER2/neu-amplified), SPSB1-mediated activation of survival pathways may constitute a general mechanism of therapeutic resistance in cancer patients. As such, while conditional mouse models cannot accurately model all biological contexts relevant to tumor recurrence as it occurs in patients, we anticipate that pharmacological agents that target pathways activated by SPSB1 may have clinical utility.
Small molecule inhibitors of the epidermal growth factor receptor (EGFR) are effective in treating non-small cell lung cancers (NSCLC) harboring activating mutations in EGFR. However, their clinical utility is limited by the near universal development of acquired resistance to these agents. Beyond secondary mutations in EGFR itself, amplification of c-MET has been identified as an important mechanism of resistance (22). In addition, HGF-induced c-MET activation has been shown to lead to drug resistance by selecting for pre-existing c-MET-amplified clones in NSCLC cell lines (23). As described here, we have found that c-MET activation induced by SPSB1 up-regulation constitutes a potent in vivo mechanism of escape for mammary tumors in which activity of the EGFR family member, HER2/neu, has been down-regulated. However, distinct from these prior examples, c-MET pathway activation does not arise via amplification of c-MET, by rather by up-regulation of a binding partner that potentiates ligand-induced c-MET signaling.
Finally, it is interesting to note that while our findings reveal that SPSB1 plays a critical role in the survival and recurrence of residual tumor cells, it does not appear to play an important role in the growth of primary HER2/neu-induced mammary tumors. In fact, SPSB1 expressing cells were selected against during primary tumor outgrowth. This observation raises the important possibility that pharmacological targets for preventing or treating cancer recurrence may be unique to this stage of disease progression. If true, this would necessitate that such targets be identified and their roles elucidated in the specific clinical context of tumor dormancy and recurrence. In this regard, our findings suggest that the use of genetically engineered mouse models to identify compensatory pathways of tumor escape from targeted therapies and chemotherapy in mice will enable a better understanding on the molecular underpinnings of cancer recurrence.
Experimental Procedures
Animals, tissues, immunostaining, and molecular analyses
All mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. MMTV-rtTA;TetO-HER2/neu (MTB/TAN), MMTV-rtTA;TetO-MYC (MTB/TOM), and MMTV-rtTA;TetO-Wnt1;p53+/- (MTB/TWNT;p53-/+) mice were housed, induced with 2 mg/ml doxycycline, monitored for tumor development, and sacrificed as described (2, 4). Details of tissue fixation, immunofluorescence, immunohistochemistry, western blotting, and quantitative RNA analyses are provided in Supplemental Experimental Procedures.
Cancer Cell lines
Hs578T, BT474 and MDA-MB-231 were obtained as part of the NCI60 panel available at American Type Culture Collection (ATCC) and were grown under recommended conditions at 37°C in 5% CO2. Overexpression of HER2 was confirmed by western blot analysis in BT-474 cells. Primary tumor cells harvested from tumors arising in MMTV-rtTA;TetO-HER2/neu mice maintained on doxycycline were cultured as described [4].
Tumor grafting and retroviral transduction
Details for culture of tumor cells harvested from MMTV-rtTA;TetO-HER2/neu mice, retroviral transduction, and tumor grafting are provided in the Supplemental Experimental Procedures.
Human breast cancer data sets
Descriptions of human breast cancer microarray data sets that were interrogated and methods used for data analysis, including survival analysis, calculation of hazard ratios, and estimation of the association between SPSB1 expression and established prognostic factors, are described in detail in the Supplemental Experimental Procedures.
Statistical analyses
Details for statistical analysis are included in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Jianping Wang for technical support for immunofluorescence studies and Rick Kendall and Isabelle Dussault (Amgen) for provision of compound 22.
These studies were supported in part by grants from the National Institutes of Health (CA164490, CA98371, CA143296, CA148774 and CA10549 to L.A.C.), the Department of Defense Breast Cancer Research Program (L.A.C.), the Breast Cancer Research Foundation (L.A.C.), and the 2-PREVENT Breast Cancer Translational Center of Excellence at the Abramson Cancer Center (L.A.C.).
Abbreviation list
- qRT-PCR
Quantitative Reverse Transcription Polymerase Chain Reaction
- MMTV
Mouse Mammary Tumor Virus
- rtTA
reverse tetracycline-controlled Transactivator
- TetO
Tet-On
- EMT
Epithelial-to-mesenchymal Transition
- PR
Progesterone Receptor
- ER
Estrogen Receptor
- HGF
Hepatocyte Growth Factor
- EGFP
Enhanced Green Fluorescent Protein
- ECFP
Enhanced Cyan Fluorescent Protein
- SPSB
SPRY domain-containing SOCS BOX
- NSCLC
Non-small Cell Lung Cancers
- EGFR
Epidermal Growth Factor Receptor
Footnotes
Author Contributions: L.A.C and Y.F. designed the study and wrote the manuscript. Y.F. and K.C. performed the molecular, cellular, and animal experiments. J.R. quantified clonogenic assays. T.C.P and D.P. performed bioinformatics analyses. All authors discussed the results and commented on the manuscript.
Author Information: Reprints and permissions information is available at http://cancerdiscovery.aacrjournals.org/.
The authors declare no competing financial interests related to this manuscript.
The authors declare that there are no conflicts of interest related to this work.
Supplementary Information: is linked to the online version of the paper at http://cancerdiscovery.aacrjournals.org/.
References
- 1.Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 2003;17:488–501. doi: 10.1101/gad.1051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–92. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 3.D'Cruz CM, Gunther EJ, Boxer RB, Hartman JL, Sintasath L, Moody SE, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7:235–9. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 4.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 5.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Jang JW, Boxer RB, Chodosh LA. Isoform-specific ras activation and oncogene dependence during MYC- and Wnt-induced mammary tumorigenesis. Mol Cell Biol. 2006;26:8109–21. doi: 10.1128/MCB.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–86. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831–8. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 9.Woo JS, Suh HY, Park SY, Oh BH. Structural basis for protein recognition by B30.2/SPRY domains. Mol Cell. 2006;24:967–76. doi: 10.1016/j.molcel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Li Z, Messing EM, Wu G. The SPRY domain-containing SOCS box protein 1 (SSB-1) interacts with MET and enhances the hepatocyte growth factor-induced Erk-Elk-1-serum response element pathway. J Biol Chem. 2005;280:16393–401. doi: 10.1074/jbc.M413897200. [DOI] [PubMed] [Google Scholar]
- 11.Kuang Z, Lewis RS, Curtis JM, Zhan Y, Saunders BM, Babon JJ, et al. The SPRY domain-containing SOCS box protein SPSB2 targets iNOS for proteasomal degradation. J Cell Biol. 2010;190:129–41. doi: 10.1083/jcb.200912087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez JV, Pan TC, Ruth J, Feng Y, Zhou A, Pant D, et al. Par-4 Downregulation Promotes Breast Cancer Recurrence by Preventing Multinucleation following Targeted Therapy. Cancer Cell. 2013;24:30–44. doi: 10.1016/j.ccr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 15.Boezio AA, Berry L, Albrecht BK, Bauer D, Bellon SF, Bode C, et al. Discovery and optimization of potent and selective triazolopyridazine series of c-Met inhibitors. Bioorg Med Chem Lett. 2009;19:6307–12. doi: 10.1016/j.bmcl.2009.09.096. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy A, Mondry A, Holmes CC, Altman DG. Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med. 2008;5:e184. doi: 10.1371/journal.pmed.0050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–77. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 20.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 21.Wertheim GB, Yang TW, Pan TC, Ramne A, Liu Z, Gardner HP, et al. The Snf1-related kinase, Hunk, is essential for mammary tumor metastasis. Proc Natl Acad Sci U S A. 2009;106:15855–60. doi: 10.1073/pnas.0906993106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 23.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.