Abstract
Purpose
Extrahepatic cholangiocarcinoma is an uncommon but lethal malignancy. We analyzed the role of definitive chemoradiotherapy for patients with nonmetastatic, locally advanced extrahepatic cholangiocarcinoma treated at a single institution.
Methods and Materials
This retrospective analysis included 37 patients who underwent external beam radiation therapy (EBRT) with concurrent chemotherapy and/or brachytherapy (BT) for locally advanced extrahepatic cholangiocarcinoma. Local control (LC) and overall survival (OS) were assessed, and univariate regression analysis was used to evaluate the effects of patient- and treatment-related factors on clinical outcomes.
Results
Twenty-three patients received EBRT alone, 8 patients received EBRT plus BT, and 6 patients received BT alone (median follow-up of 14 months). Two patients were alive without evidence of recurrence at the time of analysis. Actuarial OS and LC rates at 1 year were 59% and 90%, respectively, and 22% and 71%, respectively, at 2 years. Two patients lived beyond 5 years without evidence of recurrence. On univariate analysis, EBRT with or without BT improved LC compared to BT alone (97% vs. 56% at 1 year; 75% vs. 56% at 2 years; p = 0.096). Patients who received EBRT alone vs. BT alone also had improved LC (96% vs. 56% at 1 year; 80% vs. 56% at 2 years; p = 0.113). Age, gender, tumor location (proximal vs. distal), histologic differentiation, EBRT dose (≤ or >50 Gy), EBRT planning method (two-dimensional vs. three-dimensional), and chemotherapy were not associated with patient outcomes.
Conclusions
Patients with locally advanced extrahepatic cholangiocarcinoma have poor survival. Long-term survival is rare. The majority of patients treated with EBRT had local control at the time of death, suggesting that symptoms due to the local tumor effect might be effectively controlled with radiation therapy, and EBRT is an important element of treatment. Novel treatment approaches are indicated in the therapy for this disease.
Keywords: Biliary cancer, Radiotherapy, 5-Fluorouracil, Adjuvant therapy
INTRODUCTION
Carcinoma of the extrahepatic biliary system is a rare malignancy, with approximately 3,000 patients diagnosed in the United States each year (1, 2). Surgery is the only potentially curative treatment modality. However, resection rates are low, ranging from 10% to 35% (3, 4). The majority of patients with biliary cancers present with locally advanced or metastatic disease, leading to treatment with palliative intent. Overall survival in all patient groups is poor, ranging from 2 to 3 months in patients receiving medical management alone, 6 to 12 months for those undergoing surgical palliation, and 12 to 22 months for resected patients (3–6). Overall 5-year survival is dismal at less than 10%. The role of radiation therapy and chemotherapy (ChT) in patients with unresectable, nonmetastatic disease is poorly defined. An analysis of patients with nonmetastatic and unresectable cholangiocarcinoma undergoing radiation therapy and ChT was undertaken to clarify the roles of these treatment modalities.
METHODS AND MATERIALS
This retrospective study was approved by the Duke University institutional review board. From 1992 to 2006, 83 patients were identified with the diagnosis of nonmetastatic adenocarcinoma of the extrahepatic biliary ducts. Patients with carcinoma of the intrahepatic bile ducts, liver, gallbladder, pancreas, ampulla of Vater, and duodenum were excluded, including that tumors invaded the extrahepatic biliary ducts from surrounding structures. The distinction of tumor origin was made based on radiologic and endoscopic studies, as well as the surgeon’s operative notes for patients who underwent exploratory laparotomy. Additionally, patients with extrahepatic bile duct tumors undergoing surgery with curative intent were excluded. As a result, 37 patients were included in the current study. These patients were treated with external beam radiotherapy (EBRT) and/or brachytherapy (BT), usually with concurrent ChT.
Histologic confirmation of malignancy was obtained in all patients prior to initiation of therapy. Resectability was determined by individual surgeons, but generally, patients were considered unresectable for cure if the tumor was in proximity to vasculature such that an anatomic resection and/or adequate margins were unachievable, as determined at the time of laparotomy or abdominal imaging. All patients underwent palliative endoscopic biliary stent placement, percutaneous transhepatic drain placement, or palliative surgical biliary bypass, usually due to progressive biliary obstruction. No patient had evidence of distant metastases by imaging or at the time of exploratory surgery. Seventeen patients underwent laparotomy, which was determined by physician judgment. Of these, 10 patients underwent biliary bypass, and 7 patients underwent biopsy only.
Multifield external beamtechniques were used to treat gross disease to a median dose of 45 Gy (range, 30–62 Gy) using 1.8 to 3 Gy daily fractions. Field arrangements included anterior-posterior/posterior-anterior and anterior-posterior/posterior-anterior with lateral fields. BT using iridium-192 (dose rate, 45–150 cGy/hr) prescribed to a depth of 5 to 10 mm via percutaneous catheters was administered to 6 patients (median dose, 40 Gy; range, 25–50 Gy) without external beam treatment and as a boost to 8 patients who received EBRT (median BT dose, 25 Gy, range, 20–35 Gy; median combined radiation dose, 70 Gy, range, 64–81 Gy). Fluoropyrimidine-based ChT was delivered concurrently with radiation therapy in 32 patients (13 patients received continuous infusion, 15 patients received a bolus, and 4 patients received oral ChT). Four patients received adjuvant ChT following chemoradiotherapy. Adjuvant regimens included capecitabine, gemcitabine/irinotecan, and 5-fluorouracil (5-FU)/mitomycin C. Local, regional, and distant failure patterns were established by follow-up clinical examination, radiographic imaging, endoscopy, and autopsy data. Patients were usually evaluated every 3 months by physical examination, liver function tests, and tumor markers, as well as chest, abdominal, and pelvic computerized tomography scans following treatment. Acute and long-term toxicities were assessed using the NCI Common Terminology Criteria for Adverse Events version 3.0.
The date on which observations for endpoints was started was the date on which radiation therapy was initiated. The Kaplan-Meier method was used to estimate overall survival, median survival, disease-free survival, metastasis-free survival, and local control (LC) rates for all 37 patients. In addition, univariate regression analysis was used to evaluate the influence of selected variables (age >60, gender, tumor location [proximal vs. distal to the cystic duct], histologic differentiation, EBRT dose >50 Gy, two-dimensional vs. three-dimensional treatment planning, use of BT, and use of concurrent ChT) on clinical outcome measures.
RESULTS
Thirty-seven patients (20 male, 17 female) underwent biliary stenting or surgical biliary decompression followed by radiation therapy with or without ChT for unresectable disease (21 distal, 16 proximal to the cystic duct) (Table 1). Median age was 66 (range, 40–81) years. Twenty-three patients received EBRT without BT, 8 patients received EBRT plus BT, and 6 patients received BT alone.
Table 1.
Tumor and treatment characteristics
| Characteristic | No. of patients | % of total |
|---|---|---|
| Gender | ||
| Male | 20 | 54 |
| Female | 17 | 46 |
| Bile duct segment | ||
| Proximal | 16 | 43 |
| Distal | 21 | 57 |
| Tumor grade | ||
| I | 5 | 14 |
| II | 18 | 49 |
| III | 14 | 37 |
| Planning method | ||
| EBRT, two-dimensional | 21 | 57 |
| EBRT, three-dimensional | 10 | 27 |
| Brachytherapy only | 6 | 16 |
| Treatment delivered | ||
| EBRT only | 1 | 3 |
| EBRT plus ChT | 22 | 59 |
| EBRT plus BT | 1 | 3 |
| EBRT plus BT plus ChT | 7 | 19 |
| BT only | 3 | 8 |
| BT plus ChT | 3 | 8 |
| Concurrent 5-FU-based ChT | 32 | |
| Bolus | 15 | 47 |
| Continuous infusion | 13 | 31 |
| Oral | 4 | 12 |
Abbreviations: EBRT = external beam radiation therapy; BT = brachytherapy; ChT = chemotherapy; 5-FU = 5-fluorouracil.
Median follow-up for all patients was 14 months (range, 4–65 months). The tumors of 5 (14%) patients were classified as well differentiated, 18 (49%) patients had moderately differentiated tumors, and 14 (37%) patients had poorly differentiated tumors. Twenty-three (62%) patient deaths were documented as cancer-related deaths, while 3 (8%) patients died of other causes (1 patient died of previously treated, recurrent colon cancer; 1 patient died of Clostridium difficile-infected colitis; and 1 patient died of cardiovascular disease), and the cause of death was not assessable in 9 patients. Two patients are alive without evidence of recurrence at last follow-up, 0.7 and 5.4 years after diagnosis. The latter patient was a 65-year-old male who initially presented with choluria. Endoscopic retrograde cholangiopancreatography revealed a common bile duct stricture and biliary ductal dilation. A stent was placed, and brushing tissue samples from the procedure were nondiagnostic. Exploratory laparotomy was performed, and a 2-cm lymph node in the porta hepatis was resected, and a biopsy sample was taken from the primary mass. A palliative Roux-en-Y hepaticojejunostomy was performed. Both biopsy specimens revealed cholangiocarcinoma. The patient then received EBRT (without BT) to the primary tumor and regional lymph nodes to a total dose of 45 Gy, followed by an additional 9 Gy to the primary mass and enlarged lymph node, with concurrent 5-FU ChT and without adjuvant systemic therapy. The patient continued to be without evidence of disease at last follow-up.
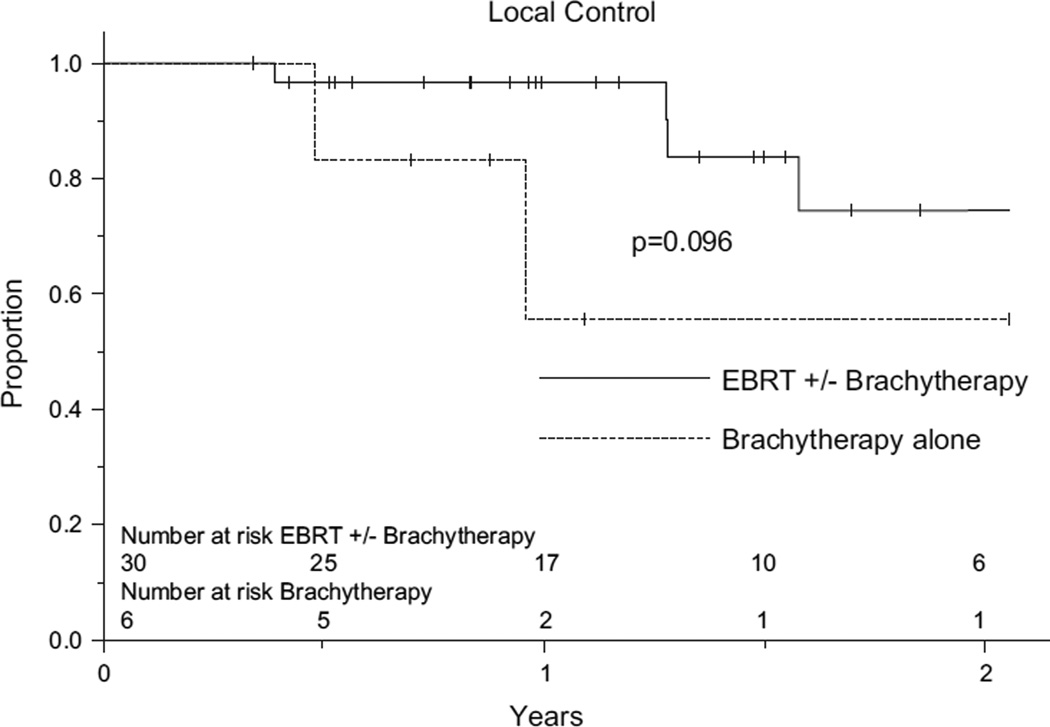
Median overall survival for the entire group was 14 months. One-year actuarial survival and LC rates were 59% and 90%, respectively. At 2 years, these figures were 22% and 71%, respectively. On univariate analysis, patients who received EBRT with or without BT had improved LC compared to those who received BT alone (97% vs. 56% at 1 year; 75% vs. 56% at 2 years; p = 0.096). Patients who received EBRT alone vs. those who received BT alone also had improved LC (96% vs. 56% at 1 year; 80% vs. 56% at 2 years; p = 0.113). Outcomes were similar for patients receiving EBRT with BT vs. EBRT alone (Fig. 1). Age (≤ or > 60), gender, location (proximal vs. distal), histologic differentiation (grade I, II, or III), EBRT dose (≤ or > 50 Gy), EBRT planning method (two-dimensional vs. three-dimensional), and use of concurrent ChT were not found to significantly affect patient outcomes. Multivariate analysis was not performed due to small patient numbers.
Fig.
Probability of LC by radiation treatment modality.
Twenty-one (57%) patients had documented disease progression prior to death at a median time of 8 (range, 2–39) months after treatment. Distant failure dominated in this cohort (17 patients), with 13 patients developing distant disease as the only site of failure at a median time of 8 months (range, 1–39 months). The most common sites of distant failure were liver and peritoneal cavity. Seven (19%) patients experienced locoregional failure at a median time of 10 (range, 2–22) months, 3 patients had no evidence of distant failure, and 4 patients experienced locoregional failure in conjunction with distant disease progression. Only 1 patient with distant failure subsequently developed local failure prior to death. Seven of 32 (22%) evaluable patients for whom long-term biliary patency rates were available required restenting procedures following therapy.
Generally, chemoradiotherapy was reasonably well tolerated, and all patients completed the intended treatment course. Fourteen (38%) patients developed no significant acute treatment-related toxicity. Twenty-three (62%) patients experienced grade I or higher acute treatment-related toxicities (during therapy and within 3 months of completion); these toxicities included nausea, vomiting, anorexia, fatigue, dehydration, mucositis/stomatitis, diarrhea, and dermatitis. Four (11%) patients developed grade 2 acute toxicities, and 5 (14%) patients developed grade 3 acute toxicities. Grade 3 toxicities included dehydration requiring intravenous fluids for >24 hr (3 patients) and confluent mucositis/stomatitis (2 patients). No acute grade 4 or 5 toxicities were observed. Two patients developed acute cholangitis during the course of chemoradiotherapy that prolonged the overall treatment time. Long-term treatment-related adverse effects (>3 months following therapy completion) were challenging to assess, given the fact that many late symptoms were potentially attributable to local or distant disease progression (which occurred in most patients). The two patients who remained disease-free did not endorse any long-term treatment-related adverse effects.
DISCUSSION
The clinical outcome of patients with extrahepatic cholangiocarcinoma is poor. Because the majority of patients with biliary cancers present with unresectable or metastatic disease, overall 5-year survival rates have been reported at 10% or less (3, 4). Although surgery is the only curative treatment modality, only a minority of patients are surgical candidates at presentation. For unresectable patients, the goal of treatment is prevention of locoregional disease progression to enhance survival and quality of life.
In patients with unresectable disease, palliative irradiation following biliary decompression has been shown to prolong survival. Table 2 provides a summary of selected published reports. Most studies have demonstrated improvements in survival outcomes with the use of radiation therapy versus supportive measures only, although long-term survival is uncommon. However, many studies are limited by small sample size, lack of concurrent ChT administration in some patients, possible group imbalance with regard to extent of disease, adverse histologic features, and variable radiation therapy techniques. In the current study, median survival was 14 months, with 2-year survival for 22% of patients, and a 2-year LC rate of 71%, which are similar to outcomes in other series. In our series, the majority of patients treated with EBRT had LC at the time of death, suggesting that symptoms due to local tumor effects might be effectively controlled with radiation therapy. However, given the relative rarity of this disease and the significant heterogeneity among studies, it is difficult to draw firm conclusions regarding the role of definitive radiotherapy.
Table 2.
Results of RT for unresectable biliary cancers
| Author (ref) | No. of patients |
RT | RT dose range (Gy)* |
Median survival (months) |
2-year survival (%) |
Local control (%) |
|---|---|---|---|---|---|---|
| Current study | 37 | Yes | 25–81 | 14 | 22 | 71** |
| Farley et al. (26) | 103 | Yes‡ | NS | NS | 9† | NS |
| Grove et al. (27) | 19 | Yes | 12.6–64 | 12.2 | 10 | NS |
| 9 | No | – | 2.2 | NS | NS | |
| Veeze-Kuijpers et al. (28) | 42 | Yes | 30–65 | 10 | 14 | NS |
| Crane et al. (13) | 52 | Yes | 30–85 | 10 | 13 | 41¶ |
| Bruner et al. (29) | 25 | Yes | 30.4–55.8§ | 16.5 | NS | NS |
| 39 | No | – | 9.3 | NS | NS |
Abbreviation: NS = not stated.
Sum of EBRT plus brachytherapy dose.
3-year survival rates.
In some patients but details not given.
1-year local control.
EBRT-only doses.
2-year local control.
The role of ChT alone or in combination with radiation therapy for localized bile duct carcinomas remains ill-defined. The use of 5-FU–based ChT in combination with radiation is extrapolated from the survival benefit demonstrated with other gastrointestinal malignancies, including pancreatic cancer (7–9). A summary of selected studies is listed in Table 3 (note, many of these contain resected patients) (10–12). The number of patients reported to be receiving combined therapy is too small to draw definitive conclusions about the benefit of concurrent ChT delivery, with reports showing conflicting results. In the present series, no differences in outcomes were seen in patients who received radiation therapy alone versus those who received combined modality therapy, although the number receiving radiotherapy alone was small. Based on the lack of significant added toxicity from ChT in most studies and the proven benefit in other gastrointestinal malignancies, the use of combined chemoradiotherapy is recommended in biliary tract cancers (13).
Table 3.
Concurrent chemoradiotherapy for biliary carcinomas
| Author (ref.) | No. of patients | Chemotherapy | Median survival (months) | 2-year survival (%) |
|---|---|---|---|---|
| Current study | 32 | 5-FU | 15 | 23 |
| Minsky et al. (33)¶ | 12 | 5-FU + MMC | 17 | 36‡ |
| Alden and Mohiuddin (11)¶ | 19 | 5-FU* | NS | 30 |
| Kopelson et al. (34) | 13 | 5-FU | 12.7 | NS |
| Deodata et al. (35)¶ | 22 | 5-FU† | 23 | 41 |
Abbreviations: 5-FU = 5-fluorouracil; MMC = mitomycin-C; NS = not stated.
5-FU alone or in combination with doxorubicin or mitomycin-C.
One patient did not receive 5-FU.
4-year survival rate.
Includes resected patients.
The optimal radiation dose in the definitive treatment of biliary malignancies is unknown. Published reports describing dose response are also generally nonrandomized, single-institution experiences that are subject to selection bias, similar to the current study. Patients with higher performance status may receive higher radiation doses. Reports of patients receiving external radiotherapy for bile duct carcinoma have suggested that doses of ≥40 to 45 Gy result in improved survival outcomes compared to lower doses (14); however, results of these and other reports are likely confounded by varying disease stages and patient performance status with respect to dose selection. Despite this, many patients with bile duct cancer succumb to local tumor progression with biliary obstruction. Conventional doses of EBRT, with or without ChT, are often unable to eradicate all disease. Potential advantages of intraluminal BT include administration of high radiation doses with rapid dose fall-off over a short distance from the radioactive source, thus sparing adjacent normal tissues and localizing dose to the tumor and peritumoral tissues, although dose to extramural disease may also be limited. Some investigators have reported a correlation of improvement in survival with the use of BT (11), while others have shown no obvious benefit (15) (Table 4). Occasionally, reports have described long-term survival in unresectable patients with the use of EBRT and transcatheter BT boost. In our study, local disease control in patients undergoing BT alone was inferior to that of patients receiving EBRT as a portion of their treatment. Additionally, we found no significant difference in disease outcomes in patients undergoing EBRT alone versus those undergoing EBRT and BT. However, selection bias (i.e., patients with a large burden of disease and poor performance status selected for a shorter course of therapy or even delivery of BT in patients with more advanced, bulky disease) could clearly influence these results, as could our limited patient numbers.
Table 4.
Outcome of EBRT with or without intraluminal BT for biliary cancers
| Author (ref.) | No. of patients | Brachytherapy | Median survival (months) | 5-year survival (%) | Local control (%) |
|---|---|---|---|---|---|
| Current study | 8 | Yes | 14.6 | 13 | 67 |
| 23 | No | 10.7 | 5 | 53 | |
| Foo et al. (12) | 24 | Yes | 12.8 | 14 | 67 |
| Valek et al. (30)* | 21 | Yes | 12.9 | ns | ns |
| 21 | No | 9.9 | ns | ns | |
| Montemaggi et al. (31) | 12 | Yes | 14 | ns | ns |
| Eschelman et al. (17) | 11 | Yes | ns | 22.6† | ns |
| Fritz et al. (18) | 30 | Yes | ns | 8 | ns |
| Kamada et al. (32) | 54 | Yes | 12.4 | 6 | ns |
| Shinchi et al. (20) | 51 | Yes | 13 | 57‡ | ns |
| Takamura et al. (19)¶ | 93 | Yes | 11.9 | 4 | 56 |
Randomized study of EBRT plus BT vs. stent only.
2-year survival rate with noncurative resection.
1-year survival rate.
Some patients had metastatic disease at time of RT.
EBRT, with or without intraluminal BT, has also been reported to provide durable palliation (16–18), including maintenance of stent patency for patients with locally advanced biliary carcinoma (17, 19, 20). In our study, all patients initially underwent endoscopic biliary stent placement, percutaneous drainage tube placement, or a palliative surgical bypass procedure. Of the total, 22% of evaluable patients required restenting procedures following therapy completion for symptomatic biliary tract obstruction, which compares favorably to other reports.
Despite the use of combined modality approaches, the overwhelming majority of locally advanced cholangiocarcinoma patients will relapse and die of their disease. While radiation approaches may improve LC rates, local failure remains common despite contemporary approaches and dose escalation techniques. Similarly, distant disease relapse is common and frequently results in disease-related mortality. Therefore, study of novel treatment approaches is indicated. Further investigation of dose escalation techniques using highly conformal, noninvasive methods including intensity-modulated radiation therapy, stereotactic body radiation therapy, as well as proton radiotherapy are ongoing (21, 22). The use of predictive molecular markers also remains an area of promise in the treatment of this otherwise refractory disease (23, 24). Similarly, the integration of novel systemic agents in the treatment of this disease is being actively investigated, including the use of “conventional,” newer generation chemotherapeutics such as gemcitabine, oxaliplatin, and capecitabine, as well as the use of “targeted” therapeutics such as small molecule and antibody-based epidermal growth factor receptor inhibitors, vascular endothelial growth factor inhibitors, and multitargeted kinase inhibitors. A recent randomized trial using the combination of cisplatin with gemcitabine in patients with advanced or metastatic biliary tumors showed significant improvements in overall survival and progression-free survival compared to the use of gemcitabine alone (25). Many of the above-mentioned agents are also established radiation sensitizers, and the combination of these agents with radiation therapy using advanced radiation therapy delivery techniques would be a rational topic of investigation.
CONCLUSIONS
Extrahepatic cholangiocarcinoma is a rare malignancy. Patients with locally advanced disease have poor survival. Long-term survival is possible but rare. In our series, the majority of patients treated with EBRT had local control at the time of death, suggesting that symptoms due to local tumor effects might be effectively controlled with radiation therapy. EBRT is an important element of treatment. 5-FU-based ChT administered concurrently with radiation therapy may be beneficial. The role of intraluminal BT remains undefined. Novel treatment approaches are indicated in the therapy for this disease.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 2.Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156–176. doi: 10.1002/1098-2388(200009)19:2<156::aid-ssu8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Yee K, Sheppard BC, Domreis J, et al. Cancers of the gallbladder and biliary ducts. Oncology (Williston Park) 2002;16:939–950. [PubMed] [Google Scholar]
- 4.Daines WP, Rajagopalan V, Grossbard ML, et al. Gallbladder and biliary tract carcinoma: A comprehensive update, Part 2. Oncology (Williston Park) 2004;18:1049–1060. [PubMed] [Google Scholar]
- 5.Todoroki T, Gunderson L, Nagorney D. Biliary tract IORT. Totowa, NJ: Humana Press; 1999. [Google Scholar]
- 6.Schoenthaler R, Phillips T, Castro J. Carcinoma of the extrahepatic bile ducts: UCSF experience. Ann Surg. 1994;219:267–274. doi: 10.1097/00000658-199403000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalser M, Elenberg S. Pancreatic cancer: Adjuvant combined radiation and ChT following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 8.Moertel C, Childs D, Reitmeier R, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 9.Moertel C, Frytak S, Hahn R, et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of highdose (6000 rads) radiation alone, moderate dose radiation (4000 Rads + 5-Fluorouracil) and high-dose radiation plus 5-Fluorouracil. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Minsky B, Wesson M, Armstrong JG, et al. Combined modality therapy of extrahepatic biliary system cancer. Int J Radiat Oncol Biol Phys. 1990;18:1157–1163. doi: 10.1016/0360-3016(90)90453-q. [DOI] [PubMed] [Google Scholar]
- 11.Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28:945–951. doi: 10.1016/0360-3016(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 12.Foo ML, Gunderson LL, Bender CE, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 13.Crane C, McDonald K, Bauthty J, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. doi: 10.1016/s0360-3016(02)02845-6. [DOI] [PubMed] [Google Scholar]
- 14.Mittal M, Romestaing P, Iwatsuki S, et al. Primary cancers of extrahepatic biliary passages. Int J Radiat Oncol Biol Phys. 1985;11:849–854. doi: 10.1016/0360-3016(85)90320-7. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez Gonzalez D, Gouma D, Rauws E, et al. Role of radiotherapy, in particular intraluminal brachytherapy, in the treatment of proximal bile duct carcinoma. Ann Oncol. 1999;10:215–220. [PubMed] [Google Scholar]
- 16.Buskirk SJ, Gunderson LL, Schild S, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg. 1991;215:125–131. doi: 10.1097/00000658-199202000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eschelman D, Shapiro M, Bonn J, et al. Malignant biliary duct obstruction: Long-term experience with Gianturco stents and combined-modality radiation therapy. Radiology. 1996;200:717–724. doi: 10.1148/radiology.200.3.8756921. [DOI] [PubMed] [Google Scholar]
- 18.Fritz P, Branbs H, Schraube P, et al. Combined external beam radiotherapy in intraluminal high-dose-rate brachytherapy on bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1994;29:855–861. doi: 10.1016/0360-3016(94)90576-2. [DOI] [PubMed] [Google Scholar]
- 19.Takamura A, Saito H, Camada T, et al. Intraluminal low-doserate- Ir192 brachytherapy combined with external beam radiotherapy and biliary stenting for unresectable extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:1357–1365. doi: 10.1016/s0360-3016(03)00770-3. [DOI] [PubMed] [Google Scholar]
- 20.Shinchi H, Takao S, Nishida H, et al. Length and quality of survival following external beam radiotherapy combined with expandable metallic stent for unresectable hilar cholangiocarcinoma. J Surg Oncol. 2000;75:89–94. doi: 10.1002/1096-9098(200010)75:2<89::aid-jso3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Baisden JM, Kahaleh M, Weiss GR, et al. Multimodality treatment with helical tomotherapy intensity modulated radiotherapy, capecitabine, and photodynamic therapy is feasible and well tolerated in patients with hilar cholangiocarcinoma. Gastrointest Cancer Res. 2008;2:219–224. [PMC free article] [PubMed] [Google Scholar]
- 22.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Gruenberger B, Schueller J, Tamandl D, et al. K-ras status and response in patients with advanced or metastatic cholangiocarcinoma treated with cetuximab plus gemcitabine-oxaliplatin (GEMOX): A single center phase II study. Proceedings of the 45th Annual American Society of Clinical Oncology Meeting. Orlando, FL. J Clin Oncol. 2009;27 15s (abstract 4586) [Google Scholar]
- 24.Hwang I, Chi K, Do J, et al. Clinical implication of ERCC1 overexpression in advanced biliary tract adenocarcinoma patients treated with platinum-based palliative ChT. Proceedings of the 45th Annual American Society of Clinical Oncology Meeting. Orlando, FL. J Clin Oncol. 2009;27 (abstract e22018) [Google Scholar]
- 25.Valle J, Hs W, Palmer D, et al. Gemcitabine with or without cisplatin in patients (pts) with advanced or metastatic biliary tract cancer (ABC): Results of a multicenter, randomized phase III trial (the UK ABC-02 trial). Proceedings of the XXXX Annual American Society of Clinical Oncology Meeting. Orlando, FL. J Clin Oncol. 2009;27 15s (abstract 4503) [Google Scholar]
- 26.Farley D, Weaver A, Nagorney D. ‘‘Natural history’’ of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 27.Grove M, Hermann R, Vogt D, et al. Role of radiation after operative palliation in cancer of the proximal bile ducts. Am J Surg. 1991;161:454–458. doi: 10.1016/0002-9610(91)91111-u. [DOI] [PubMed] [Google Scholar]
- 28.Veeze-Kuijpers B, Merrwaldt J, Mameris J, et al. The role of radiotherapy in the treatment of bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1990;18:63–67. doi: 10.1016/0360-3016(90)90268-o. [DOI] [PubMed] [Google Scholar]
- 29.Brunner T, Schwab D, Meyer T, et al. Chemoradiation may prolong survival of patients with non-bulky unresectable extrahepatic biliary carcinoma: A retrospective analysis. Strahlenther Onkol. 2004;180:751–757. doi: 10.1007/s00066-004-1315-1. [DOI] [PubMed] [Google Scholar]
- 30.Valek V, Kysela P, Kala Z, et al. Brachytherapy and percutaneous stenting in the treatment of cholangiocarcinoma: a prospective randomised study. Eur J Radiol. 2007;62:175–179. doi: 10.1016/j.ejrad.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Montemaggi O, Costamagna G, Dobelbower R, et al. Intraluminal brachytherapy in the treatment of pancreas and bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1995;32:437–443. doi: 10.1016/0360-3016(95)00518-4. [DOI] [PubMed] [Google Scholar]
- 32.Kamada T, Saitou H, Takamura A, et al. The role of radiotherapy in the management of extrahepatic bile duct cancer: An analysis of 145 consecutive patients treated with intraluminal and/or external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:767–774. doi: 10.1016/0360-3016(95)02132-9. [DOI] [PubMed] [Google Scholar]
- 33.Minsky BD, Kemeny N, Armstrong JG, et al. Extrahepatric biliary system cancer: an update of a combined modality approach. Am J Clin Oncol. 1991;14:433–437. doi: 10.1097/00000421-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Kopelson G, Harisiadis L, Tretter P, et al. The role of radiation therapy in cancer of the extra-hepatic biliary system: An analysis of thirteen patients and a review of the literature of the effectiveness of surgery, chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1977;2:883–894. doi: 10.1016/0360-3016(77)90186-9. [DOI] [PubMed] [Google Scholar]
- 35.Deodata F, Clemente G, Mattiucci G, et al. Chemoradiation and brachytherapy in biliary tract carcinoma: Long-term results. Int J Radiat Oncol Biol Phys. 2006;64:683–688. doi: 10.1016/j.ijrobp.2005.07.977. [DOI] [PubMed] [Google Scholar]