Abstract
Background
There is limited available information for treatment of acute coronary syndrome (ACS) with respect to outcomes, therapeutic agents and treatment practices. Our retrospective registry study collected and evaluated varying anti-platelet treatment strategies and outcomes of ACS patients who were admitted to 9 different tertiary care hospitals in India. This study was carried out to provide an insight to anti-platelet treatment patterns and analyze outcomes of ACS patients in India.
Methods
All the relevant data, including anti-platelet treatment strategies, outcomes and patient treatment compliance were collected from 500 ACS (defined as STEMI, NSTEMI and unstable angina [UA]) cases from January 2007 to December 2009. These ACS cases were randomly collected from the hospital records and included in the analysis. The patient follow up data was acquired either from the hospital records or via telephonic contact for a period of one year following the event.
Results
Out of 500 ACS patients, 59.8% had UA/NSTEMI and 40.2% had STEMI. On hospital admission, aspirin, clopidogrel, statins, beta-blockers and angiotensin converting enzyme inhibitors (ACE-Is) were used by 83%, 83%, 68%, 43.2% and 31.6% patients, respectively. On discharge, aspirin, clopidogrel, statins and beta-blockers were used by 90.2%, 88%, 80.6%, and 59% patients, respectively. The average patient compliance to statins, clopidogrel and aspirin was recorded as 74.28%, 69.7% and 68.66%, respectively during discharge and follow-up visits. Greater than 50% of ACS patients after discharge were lost to follow-up and as a result there was significant drop in the number of clinical events reported.
Conclusion
This pilot study conducted in tertiary care centers in India showed that patients with ACS were more often diagnosed with UA/NSTEMI as compared to STEMI and reported maximum compliance to statins, clopidogrel and aspirin after discharge over 1 year follow-up. More ACS patients were lost to follow up that resulted in low reporting of clinical outcomes, following discharge upto 1 year.
Keywords: Acute coronary syndrome, ST-elevation myocardial infarction, Non-ST elevation myocardial infarction, Unstable angina
1. Introduction
Acute coronary syndrome (ACS) represents a continuum of acute myocardial ischemia, spanning from acute transmural infarction with ST-segment elevation to unstable angina (UA) characterized by ischemia without ST-elevation.1 Ischemic heart disease is the leading cause of death globally. In 2001, ischemic heart disease accounted for 7.1 million deaths worldwide.2 More than 3 million people each year are estimated to have an acute ST-elevation myocardial infarction (STEMI) and approximately 4 million people suffer from non-ST-elevation myocardial infarction (NSTEMI) per year.3 India has the highest burden of coronary artery disease (CAD). The CAD has resulted in 3 million deaths annually, accounting for 25% of all mortality in India.4 Hospitals statistics revealed that 20–25% of all medical admissions were due to CAD. According to the National Commission on Macroeconomics and Health, approximately 62 million patients with CAD are expected by 2015 in India, of which 23 million people are assumed to have age lower than 40 years.
Anti-thrombotic therapy is the cornerstone of treatment for patients with ACS. It has 3 components: (1) Anti-platelet therapy – therapy which reduces platelet activation and aggregation and integral steps in the formation of a thrombus after plaque disruption. It includes oral anti-platelet agents like aspirin, clopidogrel or prasugrel, intravenous anti-platelet drugs (glycoprotein IIb/IIIa inhibitors). (2) Anticoagulant therapy – therapy which targets the clotting cascade to prevent the deposition of fibrin strands in the clot. It includes unfractionated heparin, low molecular weight heparins, fondaparinux and bivalirudin. (3) Fibrinolytic drugs – drugs which are used to lyse thrombus/clot and include streptokinase, t-PA or tenecteplase. The guidelines recommend tailoring the specific anti-thrombotic agents to the treatment strategy selected.5
There has been a constant change in the international guidelines on the choice of anti-thrombotic therapies and strategies for their use in ACS spectrum. Most data for patients with ACS are from several large registries with data on demography, treatments, and outcomes of patients in middle-income and high-income countries.6–10
From the Indian perspective, there is an inherent dearth of data regarding anti-thrombotic treatment strategies used and related outcomes in ACS patients due to variation in anti-thrombotic treatment patterns and outcomes across different centers. However, very few studies have been conducted in India to understand the patient profile, management patterns and outcomes of ACS patients. Data from these registries concluded that ACS patients were young, belong to low socio-economic groups and have a higher rate of STEMI than patients in other developed countries. In addition, the patients in these low socio-economic groups did not receive medical attention at the right time and had poor access to proven therapies which resulted in higher 30-day mortality rate than patients belonging to high socio-economic groups.11 However, the available data is generally available for upto 30 days in ACS patients in India and often lacks long-term follow up. In spite of many different anti-thrombotic options used in every day clinical practice, there remains a paucity of data on its usage in CAD patients in India.
The present study was a multicentric retrospective pilot study which gathered follow-up data of 500 patients at 9 centers across India for a period of 1 year to understand anti-thrombotic management strategies (short and long-term), ACS outcomes and patient compliance in institutes where there were no limitation of cardiac intervention facilities and were independent of patient affordability for optimal treatment (like PCI or CABG, besides medical management). These anti-thrombotic treatment strategies reflected ‘real-life’ observational setting through different sites across India. Our study used available registry data from January 2007 to December 2009.
2. Methods
This retrospective pilot study was conducted at 9 centers to understand anti-thrombotic treatment trends/patterns and outcomes of 500 ACS patients in India. The ethics committee approval was obtained prior to study initiation and a telephonic consent was sought for patients whose follow up data with respect to medications and outcomes were not available in the source documents/medical records. The data were collected from patients who were 18 years or older and were hospitalized for ACS from January 2007 to December 2009.
2.1. Inclusion criteria
Patients with diagnosis of STEMI, NSTEMI or UA were included in the study. Patients were said to have STEMI diagnosis if had a history of chest pain/discomfort, persistent ST-segment elevation (>30 min) of ≥0.1 mV in 2 or more contiguous ECG leads or presumed new left bundle branch block (LBBB) on admission and elevation of cardiac biomarkers (CK-MB, troponins): at least one value above the 99th percentile of the upper reference limit. Patients were said to have NSTEM1 diagnosis if possessed a history of chest pain/discomfort, lack of persistent ST-segment elevation, LBBB or intraventricular conduction disturbances and elevation of cardiac biomarkers (CK-MB, troponins): at least one value above the 99th percentile of the upper reference limit. Patients had diagnosis of UA if had the symptoms of angina at rest or on minimal exercise, (transient) ST-T changes and no significant increase in biomarkers of necrosis but objective evidence of ischemia by non-invasive imaging or significant coronary stenosis (at angiography).
2.2. Exclusion criteria
Patients were excluded from the study if UA, STEMI and NSTEMI, 1) precipitated by or as a complication of surgery, trauma, or GI bleeding or post-PCI and 2) occurred in patients already hospitalized for other reasons. Active pathological bleeding (Ex. Peptic ulcers, Severe hepatic failure), Simultaneous use of fibrinolytics, Allergic reaction to active compounds.
Records of all patients satisfying inclusion and exclusion criteria over the stipulated time period were retrieved from the database and records of 500 patients were randomly chosen irrespective of the treatment administered, outcome or availability of the follow up data. In addition to data pertaining to the pre-hospital and in-hospital course, follow up data were collected at 30 days, 6 months and 1 year to ascertain the occurrence of selected long-term study outcomes like revascularization, angina/reinfarction, cardiogenic shock, heart failure, cardiac arrest, atrial fibrillation, flutter, ventricular fibrillation/sustained ventricular tachycardia, stroke, mortality and bleeding. Demographic characteristics, medical history, presenting symptoms, biochemical and electrocardiographic findings, treatment practices (use of interventions and procedures, medical treatments) and outcome data were collected.
Statistical analyses were performed with the use of SAS software (version 9.2, SAS institute, Cary, NC). For continuous variables, descriptive summary statistics was used which included number of observations, mean, standard deviation, coefficient of variance, median, minimum and maximum. The categorical data, including diagnosis, co-morbidity and coronary procedures were presented by frequency and percentage of patients. Deaths were categorized as cardiovascular and non-cardiovascular. No imputation was conducted for the missing or inconsistent data.
3. Results
3.1. Demographic information
Out of 500 case records, randomly chosen from 9 centers, 201 (40.2%) patients had STEMI, 118 (23.6%) had NSTEMI and 181 (36.2%) patients had UA. Three hundred and ninety four (78.8%) patients were males. Higher proportion (78.40%) of patients had age ≥50 years followed by 20.80% patients with age between ≥30 and <50 years. Patients with STEMI were older (≥50 years) than patients with NSTEMI or UA (Table 1).
Table 1.
Demographic characteristics of ACS patients.
| Parameters | Diagnosis |
Total n (%) |
||
|---|---|---|---|---|
| STEM1 (n = 201) | NSTEM1 (n = 118) | UA (n = 181) | 500 | |
| Gender (Total) | ||||
| Male | 175 | 90 | 129 | 394 (78.80) |
| Female | 26 | 28 | 52 | 106 (21.20) |
| <30 years | 2 | 1 | 1 | 4 (0.80) |
| ≥30 and <50 years | 50 | 15 | 39 | 104 (20.80) |
| ≥50 years | 149 | 102 | 141 | 392 (78.40) |
3.2. Treatment characteristics
3.2.1. Pre-hospitalization data
Out of 500 ACS patients, the history of hospitalization was available for only 364 patients. A total of 158 patients received pre-hospital care where 77 (48.73%) were treated at primary care/clinic and 65 (41.14%) patients were treated at secondary care/nursing home (without CCU). High proportion (98.7%) of patients receiving pre-hospital care received medications prior to hospital admission. However, the details of these medications were not available in hospital records.
3.2.2. Hospital anti-thrombotic treatment and procedure
Different anti-thrombotic therapies were used at 9 centers. PCI was done in 350 patients where 161 (80.10%) patients had STEM1, 66 (55.93%) patients had NSTEM1 and 123 (67.96%) patients had UA. Twenty nine (STEMI-14; NSTEMI-5; UA-10) patients received facilitated PCI where 21 (STEM1-19; NSTEMI-2; UA-0) received streptokinase as a fibrinolytic agent. Coronary artery bypass graft (CABG) was performed in 3.48%, 6.78% and 6.63% of patients with STEMI, NSTEMI and UA, respectively. Cardiac catheterization was performed in 87.06%, 76.27% and 89.50% of patients with STEMI, NSTEMI and UA respectively.
On hospital admission, higher proportion (83%) of patients received both aspirin and clopidogrel, 68% had statins, 0.6% had unfractionated heparin and warfarin was received by 0.8% patients. At the time of discharge from the hospital, aspirin was the most prescribed medication (90.2% patients) followed by clopidogrel (88% patients), statins (80.6% patients) and beta-blockers (∼60% patients) (Fig. 1).
Fig. 1.
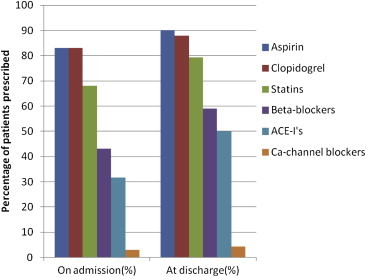
Drugs prescribed on hospital admission and at time of discharge.
3.3. Primary variables
For analysis of key primary variables, including compliance to medication during follow-ups, clinical events and outcome, only 480 records were analyzed. The remaining 20 records were not considered for analyses as the data pertaining to these variables were found missing. The follow up records of only 270 patients were available.
3.3.1. Compliance
During the study, low treatment compliance was reported across follow-up visits (30 days, 6 months and 1 year) for unknown reasons (Fig. 2). p-Values are non-significant (Table 2).
Fig. 2.
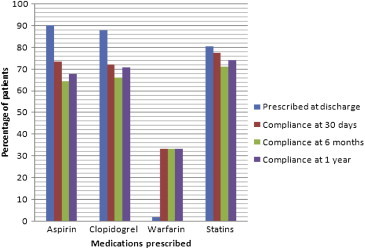
Prescription at discharge versus compliance at 30 days, 6 months and one year.
Table 2.
Significance of compliance at 30 days, 6 months and 1 year versus at discharge.
| Prescription at discharge vs compliance | p-Value |
|---|---|
| At 30 days | 0.2694 |
| At 6 months | 0.1552 |
| At 1 year | 0.2131 |
3.3.2. Clinical events
Sixty two in-hospital clinical events were reported in the study (Table 3). The in-hospital clinical events which had >1% distribution were death (4.2%) followed by revascularization (3.4%) and cardiogenic shock (1.6%).
Table 3.
Distribution of in-hospital clinical events.
| Clinical event | No of cases (n = 500) | (%) |
|---|---|---|
| Angina | 3 | 0.6 |
| Atrial fibrillation/flutter | 1 | 0.2 |
| Bleeding event | 2 | 0.4 |
| Cardiac arrest | 3 | 0.6 |
| Cardiogenic shock | 8 | 1.6 |
| Death | 21 | 4.2 |
| Hypokalemia | 1 | 0.2 |
| Hypotension | 1 | 0.2 |
| Mild renal failure | 2 | 0.4 |
| Recurrent MI | 1 | 0.2 |
| Revascularization | 17 | 3.4 |
| Stent thrombosis | 1 | 0.2 |
| Stroke | 1 | 0.2 |
Out of 480 ACS cases analyzed, 1 month follow-up data following an index event was available for 208 patients, 6 months follow-up data for 181 patients and 12 months follow-up data was available for 218 patients. Data was unavailable for 57.8% of patients at different follow up periods. Follow up data from hospitals were incomplete as many of patients did not comply with the follow up visits (Table 4). Higher proportions of patients were observed asymptomatic during different follow up periods (1st month, 169 [81.25%] patients; 6th month, 151 [83.43%] patients; 12th month, 176 [80.73%] patients). At 1st month follow up visit, 39 (18.75%) patients were observed symptomatic where 10 (25.64%) patients reported angina and 1 (2.56%) patient each reported heart failure, revascularization, stent thrombosis and death. At 6 months follow up visit, 30 (16.57%) patients were observed symptomatic where 7 (23.33%) reported angina and 1 (3.33%) patient each reported stent thrombosis and bleeding. No deaths were reported at this follow up period. At 12th month post index event, 42 (19.27%) patients were symptomatic where 5 (11.90%) patients each reported angina and death (Table 5).
Table 4.
Follow-up status at different follow-up periods.
| Follow-up | 1st month |
6th month |
12th month |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| No | 272 | 56.67 | 299 | 62.29 | 262 | 54.58 |
| Yes | 208 | 43.33% | 181 | 37.70% | 218 | 45.42% |
| Yes – Asymptomatic | 169 | 81.25 | 151 | 83.43 | 176 | 80.73 |
| Yes – Symptomatic | 39 | 18.75 | 30 | 16.57 | 42 | 19.27 |
| Total | 480 | 100.00 | 480 | 100.00 | 480 | 100.00 |
Table 5.
Distribution of clinical events for follow up visits (30 days, 6 months and 1 year).
| Follow-up | 1st month (n = 39) |
6th month (n = 30) |
12th month (n = 42) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Angina | 10 | 25.64 | 7 | 23.33 | 5 | 11.90 |
| Heart failure | 1 | 2.56 | 0 | 0.00 | 0 | 0.00 |
| Revascularization | 1 | 2.56 | 0 | 0.00 | 0 | 0.00 |
| Stent thrombosis | 1 | 2.56 | 1 | 3.33 | 0 | 0.00 |
| Bleeding event | 0 | 0.00 | 1 | 3.33 | 0 | 0.00 |
| Death | 1 | 2.56 | 0 | 0.00 | 5 | 11.90 |
| Othersa + NA | 25 | 64.10 | 21 | 70 | 32 | 76.19 |
Hypokalemia, hypotension.
3.3.3. Outcome
Out of 480 analyzed ACS cases, 39 deaths were reported where 21 were in-hospital deaths and remaining 18 deaths were reported during different follow up periods. Of 18 deaths, 1 was reported at month 1 and 5 deaths at month 12 (Table 5). The exact follow up visit was not traceable for 12/18 deaths due to unavailable data. Higher proportion (6%) of deaths were due to thrombotic events followed by cardiac reasons (1.4% deaths) (Fig. 3). Higher percentage of death was recorded in patients who were non-compliant to aspirin (33.3%) followed by clopidogrel (30.8%) and statins (30.8%) at 12 month follow up period (Table 6).
Fig. 3.
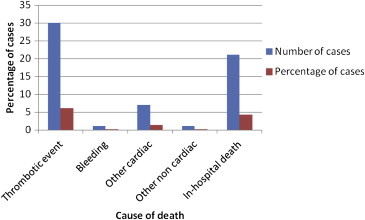
Distribution of in-hospital deaths and primary cause of death across all follow-up visits.
Table 6.
Frequency distribution of mortality versus compliance to medication at one year.
| Medication | Compliance n (%) | Non-compliance n (%) |
|---|---|---|
| Aspirin | 4 (10.25) | 13 (33.3) |
| Clopidogrel | 4 (10.25) | 12 (30.76) |
| Beta-blockers | 4 (10.25) | 8 (20.51) |
| ACE'Is | 9 (23.07) | 6 (15.38) |
| Statins | 1 (2.56) | 12 (30.76) |
4. Discussion
We analyzed data from a registry of patients with ACS from 9 different hospitals across India. We assessed patient's characteristics, treatment patterns, compliance to medications and major outcomes. We collected data pertaining to pre-hospital care and analyzed the medications that were administered in the pre-hospital setting and the procedures performed in the primary care centers. The data following hospitalization, including diagnostic procedures to diagnose STEMI, NSTEMI and UA, types of PCI performed and other coronary procedures were collected. The follow up data relating to medications prescribed, compliance to the medications and outcomes were collected at discharge, 30 days, 6 months and 1 year from the index event. This study did not analyze outcomes with respect to the diagnosis of STEMI, NSTEMI or UA, but looked at ACS in general. It aimed to collect data pertaining to treatment practices across various hospitals which were consistent with the treatment guidelines for ACS.
In 2008, Denis et al suggested that approximately 7.68 million out of 64 million CVD patients in India suffer from ACS, of which 60% patients had STEM1.11 Nearly 3.5–4.6 million patients with STEMI were diagnosed yearly. Approximately 12% of the 13 million patients in United States present with ACS (1.57 million) where 30% patients present with STEMI (0.33 million). In our study, 40.2% of the ACS patients had STEMI. In addition, patients with STEMI were significantly older (≥50 years) than patients with NSTEMI or UA. According to the hospital records, patients with previous history of myocardial infarction, hypertension, diabetes and dyslipidemia were more predisposed to ACS. In a recently published Kerala registry the presentation with NSTEMI and UA constituted 62% of the cases.12 These figures are in agreement with our data as well as the previously published data from Europe and North America.8,9
The use of key medical treatments, including anti-platelet drugs, beta-blockers, ACE-inhibitors and statins were similar to other registries.11 In our study, average compliance recorded was highest in case of aspirin and clopidogrel across all follow up visits. The higher percentage of death was reported in patients who showed non-compliance to aspirin, clopidogrel, ACE-inhibitors, statins and beta-blockers. More than 50% data of ACS patients, post discharge, were not available pertaining to follow up medications and clinical outcomes. The low rate of clinical events at follow up intervals (30 days, 6 months, and 1 year) may be attributed to >50% of ACS patients, post discharge, who were lost to follow-up during the course of the study.
This study had few limitations. Firstly, the information from medical records was inadequate to analyze key variables due to retrospective nature of this study. Secondly, the follow-up data was not available for all the patients due to inadequate information in the hospital records. Thirdly, attempts made to contact patients telephonically for follow up information was also not always successful. Lastly, the practice patterns at all participating centers in this study might not necessarily represent practice patterns at all hospitals of India.
5. Conclusion
This retrospective pilot registry evaluated anti-thrombotic treatment strategies in ‘real-life’ observational setting in patients with ACS (STEMI, NSTEMI and UA) across 9 PCI capable centers in India.
Patients with ACS were often diagnosed with UA/NSTEMI than with STEM1 and also reported maximum compliance to statins, clopidogrel and aspirin after discharge over 1 year follow up. Out of 480 analyzed cases 39 deaths were reported out of which for 12 deaths no data is available. Taking into account India's vast geographic diversity and high drop-out rate (>50%) in follow-up for many ACS patients, post discharge, which leads to unavailability of data, resulting in low reporting of ACS clinical outcomes. Also, results obtained from this study cannot be extrapolated to Indian settings as a whole as the settings vary from a primary care to multi specialty centers. To overcome these limitations, a prospective pivotal study is needed to analyze and evaluate anti-thrombotic treatment patterns and ACS outcomes over a longer duration of period.
Conflicts of interest
All authors have none to declare.
Acknowledgment
We acknowledge the help of the following persons who were an integral part of the study (S S Ramesh, Santosh Kumar, Alap Gandhi, and Ammar Raza) and Astra Zenica for all the help and cooperation extended.
References
- 1.Fuster V., Badimon L., Cohen M., Ambrose J.A., Badimon J., Chesebro J.H. Insights into the pathogenesis of acute ischemic syndromes. Circulation. 1988;77:1213–1220. doi: 10.1161/01.cir.77.6.1213. [DOI] [PubMed] [Google Scholar]
- 2.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Bassand J.P., Hamm C.W., Ardissino D. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: the task force for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 4.Reddy K.S., Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–810. doi: 10.1136/bmj.328.7443.807. Gupta R. Rapid response to Ghaffar A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaffar A., Reddy K.S., Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–810. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar Amit, Cannon Christopher P. Mayo Clin Proc. October 2009;84:917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budaj A., Brieger D., Steg P.G. Global patterns of use of antithrombotic and antiplatelet therapies in patients with acute coronary syndromes: insights from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2003;146:999–1006. doi: 10.1016/S0002-8703(03)00509-X. [DOI] [PubMed] [Google Scholar]
- 8.Fox K.A., Goodman S.G., Klein W. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2002;23:1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 9.Steg P.G., Goldberg R.J., Gore J.M. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–363. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 10.Hasdai D., Behar S., Wallentin L. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS) Eur Heart J. 2002;23:1190–1201. doi: 10.1053/euhj.2002.3193. [DOI] [PubMed] [Google Scholar]
- 11.Xavier Denis, Pais Prem, Yusuf Salim, on behalf of the CREATE registry investigators* Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 12.Mohanan P.P., Mathews R., Harikrishnan S. Presentation management, and outcomes of 25748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]


