Abstract
Background
Cardiovascular disease in Asia has reached epidemic proportions in recent years. Use of drug eluting stents in Asians has rapidly expanded with varying penetration rates across different countries. The XIENCE V® INDIA Study included ‘real world’ patients who underwent XIENCE V® stent implantation to assess short and intermediate term outcomes in Indian patients with diverse risk factors.
Objective
To evaluate 3-year clinical outcomes in a cohort of ‘real world’ Indian patients with CAD being treated with XIENCE V® Everolimus Eluting Coronary Stent System.
Methods
1000 patients were enrolled from 18 sites in India between June 2008 and March 2009. Patients were included if their index procedures were completed using only XIENCE V®. There were no clinical or angiographic exclusions. An independent Clinical Events Committee adjudicated all endpoint-related events. The primary endpoint was stent thrombosis rate annually through to 3 years as defined by the Academic Research Consortium criteria. The co-primary endpoint was the composite rate of cardiac death and myocardial infarction at 1 year.
Results
At 1-year the primary endpoint of definite/probable stent thrombosis rate was 0.51%. No additional very late stent thrombosis was reported through a 3-year follow up. The composite endpoint of cardiac death and any myocardial infarction was 1.9%, 2.7% and 3.1% at 1, 2 and 3 years respectively.
Conclusion
Despite the high risk population of coronary artery disease, the use of XIENCE V® in 'real world' Indian patients was associated with very low clinical event rates upto three years of follow up.
Keywords: Coronary artery disease, Drug-eluting stent, XIENCE V®, Percutaneous coronary intervention, XIENCE V® INDIA
1. Introduction
India is currently experiencing a rapid economic, demographic, lifestyle transition and non-communicable diseases such as coronary artery disease (CAD) and diabetes mellitus have overtaken infectious diseases as the most important cause of death.1,2 Due to its large population, India currently represents a quarter of the world's chronic heart disease burden.3,4 Cross-sectional studies estimate the prevalence of coronary heart disease in India to be 3−4% in rural and 8−10% in urban areas, representing a two-fold rise in rural areas and a six-fold rise in urban areas, between the years 1960 and 2000.5–8
Traditionally coronary heart disease manifests almost 10 years earlier in the Indian population when compared to the rest of the world suggesting that unique environmental and/or genetic risk factors exist for this population.8,9 There is also a high prevalence of diabetes, hyperlipidemia and glucose intolerance in the CREATE registry of treatment of acute coronary syndrome in India.10 However, there is no data on long term outcome of Indian patient treated by DES, keeping in mind that this population has a unique spectrum of high risk factors for restenosis.
Long term surveillance studies using DES may help elucidate mechanisms correlated to death, myocardial infarction, and late stent thrombosis risks not observed during controlled pre-market trials.11 Finally, adjunctive anti-platelet therapy may be a critical factor in optimizing long term DES safety.12,13 Despite established guidelines that recommend 6–12 months of dual anti-platelet therapy, patients with DES implants occasionally stop taking their medication early.
We report the 3-year clinical follow up of the XIENCE V® INDIA single arm prospective study which evaluated the outcomes following implantation of the XIENCE V® stent in complex lesions in a ‘real world’ 1000 Indian patients when used by a broad group of physicians at 18 centers across the country. Compliance to anti-platelet therapy in an Indian population undergoing PCI treatment has also been documented in this XIENCE V® INDIA single arm study.
This study represents the only large and long term study of its kind, evaluating a second generation DES among high risk Indian population.
2. Methods
2.1. Study design and patients
XIENCE V® INDIA is a prospective, open-label, multi-center, observational, single arm study designed to evaluate the safety of the XIENCE V® everolimus eluting stent during commercial use in real-world settings. Protocol approval was obtained by the medical ethics committee of each participating institution and all patients gave written informed consent. All patients who could provide informed consent and whose procedures were completed with only XIENCE V® during the index procedure were considered enrolled in this study. There were no additional clinical or angiographic exclusion criteria. Enrollment of patients was done via the interactive voice or web response enrollment service (Covance, New Jersey). The study was conducted in compliance with the Central Drugs Standard Control Organization (CDSCO) approved protocol and the Declaration of Helsinki, International Organization for Standardization (ISO) 14155, Guidelines for Clinical Trials on Pharmaceutical Products in India – Good Clinical Practice (GCP), International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, and GCP Guidelines. Between 03 June 2008 and 13 March 2009, 1000 patients were consecutively enrolled at 18 sites in India. Ten patient records were excluded from the analysis population due to enrollment errors, leaving an analysis population of 990.
2.2. The XIENCE V® everolimus eluting stent
A detailed description of the device has been published previously.14 XIENCE V® received CE mark approval on 30 January 2006, DCGI marketing approval on 05 July 2006 and US FDA approval on 02 July 2008. Stents were available in diameter sizes of 2.25–4.0 mm and lengths of 8–28 mm.
2.3. Study procedure
The treatment strategy and post procedural medication regimen was determined by the investigator. It was recommended that each enrolling investigator review the Instructions for use and assess the indications, contraindications, warnings and precaution sections with respect to risks and benefits for treating potential patients. Protocol recommendations for anti-platelet therapy suggested indefinite duration of aspirin, along with at least six months of clopidogrel extended to at least 12 months as recommended by ACC/AHA/SCAI guidelines in patients presenting with acute coronary syndromes.15–17
2.4. Follow up
Clinical follow up was scheduled at 14, 30 and 180 days, and annually through 3 years, either by telephone contact or office visit. Each time point included collection of data regarding adverse events and use and changes in concomitant medications.
2.5. Study endpoints
All clinical endpoint events, including stent thrombosis (ST), death, myocardial infarction (MI), coronary revascularization and major bleeding, were adjudicated by an independent Clinical Events Committee (CEC), Cardiovascular Research Foundation, New York, New York. The Data Safety Monitoring Board (DSMB) (Axio, Seattle, Washington) reviewed the cumulative safety data on a regular basis.
The primary endpoint was the incidence of ST rates annually through to 3 years as defined by the Academic Research Consortium (ARC) definition.18 The co-primary endpoint was the composite rate of cardiac death and any ARC defined MI at one year. Secondary endpoints included the composite rate of all deaths, any MI and any repeat revascularization; the composite of cardiac death and any MI; composite rate of all death and any MI; target lesion failure (TLF), defined as the composite rate of cardiac death, any MI attributed to the target vessel, and clinically indicated target lesion revascularization (CI-TLR); all death; any MI; revascularization (target lesion, target vessel [TVR], and non-target vessel) (PCI and coronary artery bypass graft surgery [CABG]); major bleeding complications (by TIMI definition); and clinical device and procedural success.
2.6. Source document verification
Source document verification was routinely performed in 30% of random cases and 100% of all reported adverse events at the time of the study conduct. For sites with low rates of adverse event reporting, additional monitoring visits and source document verification checks were performed.
2.7. Definitions
2.7.1. Death
All deaths were considered cardiac unless an unequivocal non-cardiac cause could be established. Specifically, any unexpected death, even in patients with coexisting potentially fatal non-cardiac disease (e.g. cancer, infection), were classified as cardiac.
2.7.2. Cardiac death
Any death due to immediate cardiac cause (e.g. MI, low-output failure, fatal arrhythmia). Unwitnessed death and death of unknown cause were classified as cardiac death. This included all procedure-related deaths including those related to concomitant treatment.
2.7.3. Myocardial infarction
MI classification and criteria for diagnosis were defined according to the ARC18 All late events that were not associated with a revascularization procedure were considered spontaneous. One blood sample was taken from each patient within the post-procedure hospitalization period for the analysis of CK-MB or troponin levels.
2.7.4. Target lesion revascularization
TLR was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion. The target lesion is defined as the treated segment from 5 mm proximal and 5 mm distal to the stent.
2.7.5. Target vessel revascularization (TVR)
TVR is defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel. The latter is defined as the entire major coronary vessel proximal and distal to the target lesion including upstream and downstream branches and the target lesion itself.
2.7.6. Stent thrombosis
Was categorized as acute (<1 day), subacute (1–30 days), late (>30 days) and very late (>1 year) and was defined according to the ARC guidelines as follows: definite: acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis; probable: unexplained death ≤ 30 days or TV-MI without angiographic information; and possible: unexplained death > 30 days after stent placement.
2.7.7. Clinical device success
Successful delivery and deployment of the study stent (in an overlapping stent setting a successful delivery and deployment of the first and following study stent) at the intended target lesion and successful withdrawal of the stent delivery system with attainment of final residual stenosis of less than 50% of the target lesion by QCA (by visual estimation if QCA unavailable), without use of a device outside the assigned treatment strategy. Bailout patients were included as clinical device success only if the above criteria were met.
2.7.8. Clinical procedure success
Clinical device success without the occurrence of cardiac death, MI not clearly attributed to a non-target vessel and/or CI-TLR during the hospital stay with a maximum of first seven days post index procedure. In multiple lesions setting each lesion must have met clinical procedure success criteria.
2.7.9. Thrombosis in myocardial infarction (TIMI) bleeding classification
Major bleeding per TIMI classification included intracranial hemorrhage, or a ≥5 g/dL decrease in the hemoglobin concentration, or a ≥15% absolute decrease in the hematocrit.
2.8. Statistical analysis
The sample size for the study was determined for descriptive purposes only. Descriptive analyses were performed on the intent-to-treat population.
3. Results
A total of 1000 patients were enrolled in the study. As defined by the protocol, all results are presented for the intent-to-treat population. Ten patient records were excluded from the intent-to-treat (ITT) population (analytical population) due to enrollment errors, leaving an ITT population of 990. Patient follow up is presented in Fig. 1. 938 of 990 patients (94.7%) continued in the study at 3 years.
Fig. 1.
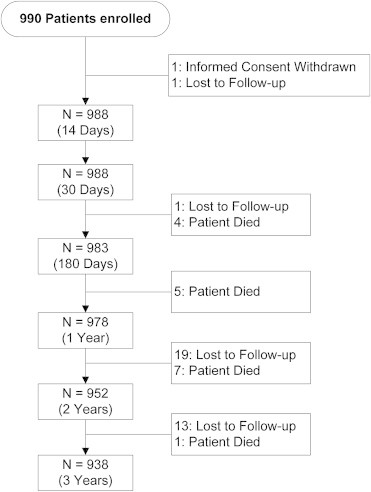
Patient flow and follow up in XIENCE V® INDIA study through 3 years.
Baseline demographics and risk factors are summarized in Table 1. Male patients accounted for 84% of the study population and the mean age was 58 ± 10 years. 44% of patients were diabetic, with 9% requiring insulin. The percentage of patients presented with hypertension was 62% and with dyslipidemia requiring medication was 33%. In addition, 21% of patients had experienced a prior myocardial infarction, with 9% of those within the 2 months prior to the index procedure. The percentage of patients presented with an acute coronary syndrome (ACS) was 19%. In the ITT population 57% of patients presented with Unstable Angina of which 19% were Braunwald Class III. There were 14.4% of patients with BMI ≥30 kg/m2.
Table 1.
Baseline patient characteristics and risk factors of subject enrolled in XIENCE V® INDIA study.
| Baseline characteristics and risk factors | ITT population (n = 990) |
|---|---|
| Age (mean ± SD, years) | 58.23 ± 10.16 |
| Male (%) | 84 |
| Body mass index ≥30 kg/m2 (%) | 14.4 |
| Current smoker (%) | 10 |
| Family history of premature CAD (%) | 24 |
| Hypertensiona (%) | 62 |
| Dyslipidemiaa (%) | 33 |
| Diabetes (%) | 44 |
| Treated with Insulin (%) | 9 |
| Prior cardiac intervention (%) | 13 |
| Prior myocardial infarction (%) | 21 |
| Within 2 months prior to index procedure | 9 |
| Acute coronary syndrome (%) | 19 |
| Current angina status: unstable angina (%) | 57 |
| Braunwald class III (%) | 19 |
Treated with medication.
Lesion and procedural characteristics are summarized in Table 2. As assessed by the treating physician, the mean lesion length was 16.9 mm (40% of lesions were ≥20 mm in length) and the mean reference vessel diameter (RVD) was 2.9 mm. In the ITT population, 47% of the lesions were of either AHA/ACC lesion Type B2 or C. The majority of treated lesions were in the left anterior descending artery (52%) and LMCA constituted 0.7%. The percentage of bifurcated lesions were 4% and 6.4% were ostial lesions. The percentage of lesions that had a TIMI flow of 0 was 10.4% with 2.4% of these being present for ≥3 months. Patients were treated according to standard interventional techniques with high acute device and procedure success rates of 100% and 99.6% respectively.
Table 2.
Baseline coronary lesion characteristics of subjects enrolled in XIENCE V® INDIA study.
| Baseline lesion characteristicsa | ITT population (nL = 1324b) |
|---|---|
| Lesion length (mean ± SD, mm) | 16.9 ± 6.4 |
| Longer lesions ≥20 mm (%) | 39.6 |
| Pre-procedure RVD (mean ± SD, mm) | 2.89 ± 0.4 |
| AHA/ACC lesion type B2 or C (%) | 46.8 |
| Bifurcation lesion (%) | 4.4 |
| Left main (%) | 0.7 |
| LAD (%) | 52.3 |
| Ostial location (%) | 6.4 |
| Pre-procedure % diameter stenosis (mean ± SD) | 87.7 ± 9.7 |
| TIMI flow of 0 (%) | 10.4 |
| Chronic total occlusion (TIMI 0 for ≥3 months) (%) | 2.4 |
Visual assessment by the investigator.
nL = number of lesions.
The primary endpoint, definite/probable stent thrombosis rate through 3 years, was 0.52%. At one year the ST rate was 0.51%, with no further events up to completion of 3-year follow up. The definite/probable early (30 days) and late (one year) ST rates were 0.3% and 0.2%, respectively (Fig. 2).
Fig. 2.
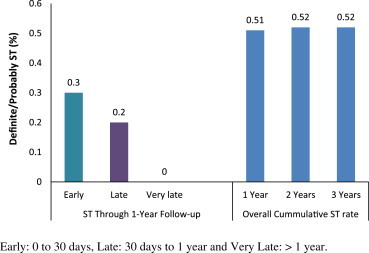
Definite/probable stent thrombosis rates in XIENCE V® INDIA study through 3-year follow up.
The percentage of patients who remained on dual anti-platelet therapy at 1 2, and 3 years were 96.9%, 93.1% and 91.5% respectively. Major bleeding by TIMI classification was noted in 0.1% of patients through to 3 years.
The co-primary endpoint rate of the composite of cardiac death and any myocardial infarction (MI) at 1 year was low at 1.9% (see Table 3). The rate of ARC defined patient-oriented (hierarchical) composite endpoint (all death, all MI and all revascularization) was 2.9%, 4.3% and 5.1% at 1, 2 and 3 years respectively (see Table 3). The overall death rate was 0.9%, 1.6% and 2% at 1, 2 and 3 years respectively. The MI rate was 1.3%, 1.5% and 1.7% at 1, 2 and 3 years respectively. The Q-wave MI contributed to only 0.5% of the total MI rate of 1.7% at 3 years (see Table 4).
Table 3.
Adjudicated hierarchical major adverse cardiac events at 1, 2 and 3 years in XIENCE V® INDIA study.
| 1-year ITT population (n = 986) | 2-year ITT population (n = 970) | 3-year ITT population (n = 957) | |
|---|---|---|---|
| Cardiac death and MI (%) | 1.9 | 2.7 | 3.1 |
| All death and MI (%) | 1.9 | 2.7 | 3.1 |
| All death, all MI and all revascularization (%) | 2.9 | 4.3 | 5.1 |
| Target lesion failurea (%) | 2.4 | 3.3 | 3.9 |
Cardiac death, MI attributed to target vessel and clinically indicated target lesion revascularization.
Table 4.
Non-hierarchical major adverse cardiac events at 1, 2 and 3 years in XIENCE V® INDIA study.
| 1 year ITT population (n = 986) | 2 year ITT population (n = 970) | 3 year ITT population (n = 957) | |
|---|---|---|---|
| All death (%) | 0.9 | 1.6 | 2.0 |
| Cardiac death (%) | 0.9 | 1.6 | 2.0 |
| All MI | 1.3 | 1.5 | 1.7 |
| Q-wave MI (%) | 0.4 | 0.5 | 0.5 |
| Non Q-wave MI (%) | 0.9 | 1.0 | 1.1 |
| All Revascularization (%) | 1.6 | 2.4 | 2.7 |
| TLR (%) | 1.2a | 1.4 | 1.6 |
Not clearly attributed to a non-target vessel.
At 1, 2 and 3 years, the composite rate of target lesion failure (TLF; defined as composite of cardiac death, MI related to the target vessel and TLR) was 2.4%, 3.3% and 3.9% respectively (see Table 3). All revascularizations were considered clinically indicated and the TLR rate was 1.2%, 1.4% and 1.6% at 1, 2 and 3 years respectively (see Table 4).
4. Discussion
The data presented shows that the XIENCE V® everolimus eluting coronary stent is safe and efficacious when used in a real world patient population in India. The composite endpoint of cardiac death and any myocardial infarction (MI) was low at 3.1% in this ‘real world’ Indian population with CAD. The primary endpoint of stent thrombosis was low at 0.51% at one year which did not increase beyond this upto 3 years (0.52%). The prevalence of diabetes, a known CAD risk factor in the Asian population,19 is higher in this study when compared to other published studies.20–22 Forty four percent of patients in XIENCE V® INDIA had diabetes mellitus compared to 26% of patients in the SPIRIT V study. Despite this, the clinical outcomes in this study were comparable to those of other studies with lower risk populations. When compared to patients from SPIRIT V, XIENCE V® INDIA patients respectively were younger (mean age of 63 years vs. 58 years), presented with less incidence of smoking (24% vs. 10%), and less family history of premature coronary artery disease (34% vs. 24%). They also presented with less dyslipidemia (33% vs. 59%). Of note, the diagnosis of dyslipidemia was made by the investigator and was based on whether or not the patient was treated with lipid-lowering medication. It has previously been shown in India that patients from lower socio-economic backgrounds were less likely to receive lipid-lowering drugs and coronary revascularization than their wealthier counterparts.10 In these cases, lifestyle changes might be the preferred treatment for dyslipidemia. However, as this study did not collect patient socio-economic data, this hypothesis remains unconfirmed.
Despite the fact that, in general, the Indian population is considered more likely to present with known predictors of restenosis and TLR, the 2-year and 3-year non-hierarchical TLR rate was appreciably lower at 1.4% and 1.6% respectively in this study than the TLR rates in other reports with similar patient populations.23 This finding may be in part attributed to the higher threshold for repeat revascularization (TLR/TVR) for minimally symptomatic patients in the Indian cardiology practice. This may be attributed to both socio-economic and cultural issues. Barely 10% of Indian households (with at least one member of the family covered) are covered by medical insurance24 thus leading to possible caution for subjecting to repeat revascularization at low symptom level. As shown in the CREATE registry, approximately 75% of patients in India pay directly for their own treatment.10 In addition, diagnostic tests such as EKG, CK-MB and troponin measurements may not have been routinely performed in patients seeking care during follow up.
Late stent thrombosis is a major concern following drug-eluting stent implantation in interventional cardiology and non-compliance to anti-platelet therapy is an important predictor of stent thrombosis.25 At 3 years, the overall rate of ARC defined definite and probable stent thrombosis in this study was 0.52%. It is possible that there might have been some under-reporting of adverse events in this study; every effort was made to obtain all adverse events through diligent monitoring. The low rates of stent thrombosis in XIENCE V® INDIA are consistent with numerous other trials, and are likely to be due to an optimal combination of thin fracture-resistant cobalt-chromium struts, low dose of everolimus elution, and well-known thromboresistant non-inflammatory proprieties of the fluorinated polymer.26 In addition, there was a high rate of compliance to aspirin and clopidogrel dual anti-platelet therapy. At 3 years 93.3% of patients were still taking aspirin, 92.8% clopidogrel and 91.5% remained on dual anti-platelet therapy. Low event rates seen at one year from the XIENCE V® INDIA study were maintained through 3 years which further demonstrate the long term safety and efficacy of XIENCE V® in this real-world population.
4.1. Limitations of the study
This study was limited by the single arm nature of the design and thus the inherent lack of a control arm for direct comparison. Lesion characteristics were assessed and reported by the investigator at the time of the procedure. No conclusion can be drawn from this study regarding outcomes in specific patient subsets. For example, 2.4% (24 of the 990) patients had a diagnosis of chronic total occlusion (CTO). Thus despite the high procedural success in this study in general, procedural outcomes in subgroups need to be examined in larger numbers of patients.
5. Conclusion
In conclusion, in these real world Indian patients undergoing PCI, despite a high prevalence of cardiac risk factors, low 3-year rates of stent thrombosis, cardiac death and myocardial infarction demonstrate long term safety and efficacy of XIENCE V® stent.
Funding
Abbott Cardiovascular Systems, Inc, A subsidiary of Abbott Vascular, 3200 Lakeside Drive, Santa Clara, CA 95045 USA.
M Stuteville, R Kumar, S Ying and K Sudhir are employees of Abbott Vascular.
Conflicts of interest
All authors have none to declare.
Appendix A. Supplementary data
References
- 1.Misra A., Nigam P., Hills A.P. Consensus physical activity guidelines for Asian Indians. Diabetes Technol Ther. 2012;14:83–98. doi: 10.1089/dia.2011.0111. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V., Venkatraman J.V., Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J Diabetes Sci Technol. 2010;4:158–170. doi: 10.1177/193229681000400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy K.S., Shah B., Varghese C. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1744–1749. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi V., Bhargava B. Health care delivery for coronary heart disease in India – where are we headed? Am Heart Hosp J. 2007;5:32–37. doi: 10.1111/j.1541-9215.2007.06015.x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57:632–638. [PubMed] [Google Scholar]
- 6.Gupta R., Joshi P., Mohan V., Reddy K.S., Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 7.Ajay V.S., Prabhakaran D. Coronary heart disease in Indians: implications of the INTERHEART study. Indian J Med Res. 2010;132:561–566. doi: 10.4103/0971-5916.73396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhakaran D., Singh K. Premature coronary heart disease risk factors & reducing the CHD burden in India. Indian J Med Res. 2011;134:8–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 10.Xavier D., Pais P., Devereaux P.J. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 11.Farb A., Boam A.B. Stent thrombosis redux – the FDA perspective. N Engl J Med. 2007;356:984–987. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 12.Grines C.L., Bonow R.O., Casey D.E. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813–818. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- 13.Spertus J.A., Kettelkamp R., Vance C. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 14.Stone G.W., Midei M., Newman W. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903–1913. doi: 10.1001/jama.299.16.1903. [DOI] [PubMed] [Google Scholar]
- 15.Grines C.L., Bonow R.O., Casey D.E. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Dent Assoc. 2007;138:652–655. doi: 10.14219/jada.archive.2007.0237. [DOI] [PubMed] [Google Scholar]
- 16.Smith S.C., Feldman T.E., Hirshfeld J.W. ACC/AHA/SCAI 2005 Guideline update for percutaneous coronary intervention–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 17.King S.B., Smith S.C., Hirshfeld J.W. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip D.E., Windecker S., Mehran R. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 19.Ueshima H., Sekikawa A., Miura K. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul U., Gupta R.K., Mathur A. Cobalt chromium stent with antiproliferative for restenosis trial in India (COSTAR I) Indian Heart J. 2007;59:165–172. [PubMed] [Google Scholar]
- 21.Agarwal P., Bhandari S., Subramanyam K. EMPIRE (Escorts Multiple ProNova Implantation Registry) Study: evaluating the ProNova SES in De Novo coronary artery lesions. Indian Heart J. 2006;58:230–233. [PubMed] [Google Scholar]
- 22.Gao Z., Yang Y., Xu B. Three year follow-up of the sirolimus-eluting stent and the paclitaxel-eluting stent in daily practice. Clin Cardiol. 2009;32:E63–E67. doi: 10.1002/clc.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul U., Patel T.M., Zambahari R. Evaluation of the XIENCE V® everolimus eluting coronary stent system in the Asian population of the SPIRIT V single arm study. 2-year clinical follow-up data. Indian Heart J. 2011;63:402–408. [PubMed] [Google Scholar]
- 24.Shiva K.A.K., Chen L.C., Choudhury M. Financing health care for all: challenges and opportunities. Lancet. 2011;377:668–679. doi: 10.1016/S0140-6736(10)61884-3. [DOI] [PubMed] [Google Scholar]
- 25.Gao R.L., Xu B., Lu S.Z. Safety and efficacy of the CYPHER Select Sirolimus-eluting stent in the “Real World”–clinical and angiographic results from the China CYPHER Select registry. Int J Cardiol. 2008;125:339–346. doi: 10.1016/j.ijcard.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Sudhir K., Hermiller J.B., Ferguson J.M. Risk factors for coronary drug-eluting stent thrombosis: influence of procedural, patient, lesion, and stent related factors and dual antiplatelet therapy. ISRN Cardiol. 2013:736–748. doi: 10.1155/2013/748736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


