Abstract
Objective
To determine levels of cholesterol lipoproteins and prevalence of dyslipidemias in urban Asian Indians.
Methods
Population based 6123 subjects (men 3388) were evaluated. Mean±1SD of various cholesterol lipoproteins (total, HDL, LDL and non-HDL cholesterol) and triglycerides were reported. Subjects were classified according to US National Cholesterol Education Program.
Results
Age-adjusted levels in men and women were cholesterol total 178.4 ± 39 and 184.6 ± 39, HDL 44.9 ± 11 and 51.1 ± 11, LDL 102.5 ± 33 and 106.2 ± 33, total:HDL 4.15 ± 1.2 and 3.79 ± 1.0 and triglycerides 162.5 ± 83 and 143.7 ± 83 mg/dl. Age-adjusted prevalence (%) in men and women, respectively were, total cholesterol ≥200 mg/dl 25.1 and 24.9, LDL cholesterol ≥130 mg/dl 16.3 and 15.1 and ≥100 mg/dl 49.5 and 49.7, HDL cholesterol <40/<50 mg/dl 33.6 and 52.8, total:HDL cholesterol ≥4.5 29.4 and 16.8, and triglycerides ≥150 mg/dl 42.1 and 32.9%. Cholesterol level was significantly greater in subjects with better socioeconomic status, body mass index and waist circumference while triglycerides were more among those with high socioeconomic status, fat intake, body mass index and waist circumference (p < 0.05). Hypercholesterolemia awareness (15.6%), treatment (7.2%) and control (4.1%) were low.
Conclusions
Mean cholesterol and LDL cholesterol are low and triglycerides were high in urban Asian Indians. Most prevalent dyslipidemias are borderline high LDL, low HDL and high triglycerides. Subjects with high socioeconomic status, high fat intake and greater adiposity have higher total and LDL cholesterol and triglyceride and lower HDL cholesterol.
Keywords: Coronary heart disease, Lipids, Cholesterol, Atherogenic dyslipidemia, Epidemiology
1. Introduction
Lipid abnormalities, such as high total and low density lipoprotein (LDL) cholesterol and low high density lipoprotein (HDL) cholesterol, are the most important cardiovascular risk factors.1 Prospective epidemiological studies in high and middle income countries in Europe and North America have consistently reported a direct and continuous association of total and LDL cholesterol and inverse association of low HDL cholesterol with coronary heart disease (CHD) morbidity and mortality.1–3 Similar results have been reported in prospective studies from Australasia and East Asia.4 No prospective studies exist in low-income countries including India.5,6 INTERHEART case–control study7 in 52 countries, including many low income countries in Asia, Africa and South America, reported significant association of high apolipoprotein B (apoB), total and LDL cholesterol, and total:HDL cholesterol ratio and low apoA1 and HDL cholesterol with incident acute myocardial infarction.8
Population-wide levels of various cholesterol lipoproteins (total, LDL and HDL) and triglycerides as well as prevalence of various dyslipidemias have been well reported from high and middle-income countries.1,9 The US National Health and Nutrition Evaluation Surveys periodically report population-wide lipid levels.10,11 Mean population cholesterol levels were 240 mg/dl in 1960's and have declined to 202 mg/dl by early 2010.10,11 Similar data have been reported from many countries in Western Europe and Australasia.12 The Global Burden of Chronic Diseases Risk Factor study reported mean levels of total cholesterol and determined trends in 180 countries over a 35-year period from 1980 to 2005.13 It was observed that total cholesterol levels were high in high and middle income countries at baseline and declined significantly over this period in high income countries and remained unchanged in upper middle income countries. In lower middle and low income countries total cholesterol levels were low and there was an increase over this 35-year period.13 Only a few large population based studies to determine mean population cholesterol and cholesterol lipoprotein levels and prevalence of hypercholesterolemia or other lipid abnormalities have been performed in India. Previous studies have been limited to local (a city) or regional (a particular state) levels. The only multisite studies that reported lipid levels and prevalence of various dyslipidemias were Indian Industrial Population Study,14 and India Migration Study.15 Multisite Indian Council of Medical Research Integrated Disease Surveillance Project16 and Indian Women's Health Study17 reported prevalence of hypercholesterolemia only. We designed the India Heart Watch to evaluate multiple cardiometabolic risk factors in urban populations in India.6 Details of rationale and methodology have been reported.6,18 The present study was performed to assess population levels of total cholesterol, LDL cholesterol, non-HDL cholesterol, HDL cholesterol and triglycerides in urban men and women and to assess prevalence of various dyslipidemias. We evaluated lipid abnormalities among the urban middle class because it is the biggest subset of Indian population.19,20 This group of apparently homogenous subjects provides unique opportunity to identify influence of lifestyles on cardiometabolic risk factors,19,21 including dyslipidemias. Study among this subset of Indian population is also important because this segment of more than 350 million subjects is poorly represented in previous national studies and majority of the Indians shall reside in urban locations by middle of this century.22
2. Methods
A multisite study to identify prevalence of cardiovascular risk factors in urban populations in India was organized.6 Protocol was approved by the institutional ethics committee of the national coordinating center at Jaipur, India. Written informed consent was obtained from all participants. Details of methodology have been reported earlier.18
2.1. Sampling
Medium sized cities were identified in each of the large states of India and investigators who had a track record of research in cardiovascular or diabetes epidemiology were invited for participation. 20 investigators were invited 15 agreed to participate. The cities were in northern (Jammu, Chandigarh, Karnal, Bikaner), western (Ahmadabad, Jaipur), eastern (Lucknow, Patna, Dibrugarh), southern (Madurai, Hyderabad, Belgaum) and central (Indore, Nagpur) regions of India. Four investigators dropped out due to non-availability of technical support and motivation and 11 investigators and their teams finally performed surveys. An accredited national laboratory (www.thyrocare.com) was identified and contracted for collection and processing the blood samples at their national center. Simple cluster sampling was performed at each site. A middle-class location was identified at each city. This depended upon the municipal classification which is based on reserve land price and is periodically developed by local government for taxation purposes. A sample size of about 250 men and 250 women (n = 500) at each site is considered adequate by World Health Organization to identify 20% difference in mean level of biophysical and biochemical risk factors.21 We invited 800-1000 subjects in each location to ensure participation of at least 500 subjects at each site estimating a response of 70% as reported in previous studies.23 At each site a uniform procedure for recruitment was followed. In short, a locality within the urban boundaries of a city was identified, houses were enumerated, number of subjects 20–75 years living in each house was determined, and all of these individuals were invited to a local community center of healthcare facility (clinic, dispensary) for examination and blood collection. A reference home within each locality was identified and every subsequent household was contacted until the sample size was reached. This procedure ensured participation of consecutive members of the locality and was representative even if the survey was prematurely abandoned at a particular location (e.g., Belgaum, Nagpur). This method also ensured representativeness at sites where oversampling was performed (e.g., Jaipur, Madurai). The surveys were preceded by meetings with community leaders to ensure good participation. Subjects were invited in fasting state to a community/medical center within each locality either twice or thrice a week depending upon the investigator's schedule.
2.2. Data collection and measurements
The study case report form was filled by a research worker from local investigators' team after details were inquired from the subject. Apart from demographic history, details of socioeconomic status based on self-assessment, educational status and occupational class were recorded. Smoking details were inquired for type of smoking or non-smoked tobacco use, number of cigarettes/bidis smoked and years of smoking or tobacco use. Intake of alcohol was assessed as drink per week. Qualitative method was used to assess dietary fat intake with questions about type of cooking oil used and self-estimated visible fat intake in g/day. Fruits and vegetables intake was also assessed by a question that inquired number of helpings (medium portions) of either fruits or green leafy vegetables. Details of physical activity were assessed by questions for exact daily duration (minutes) of work related, commute related, or leisure time physical activity.
All the equipments for measurements of height, weight, waist and hip size and blood pressure (BP) were similar at all centers for ensuring uniformity. Physical examination emphasized measurement of height using stadiometer, weight using calibrated spring weighing machines, waist and hip were measured using tapes and sitting BP measured after at least 5 min rest using electronic instruments.21 Three readings were obtained and averaged for analysis. Fasting blood sample was obtained from all individuals after 8–10 h fasting. Samples were collected at community centers by technicians from the local unit of the accredited national laboratory, Thyrocare Technologies Ltd (www.thyrocare.com).24 Blood glucose was measured at the local biochemistry facility of these laboratories and serum transported in dry-ice to the national referral laboratory at Mumbai (India) where a uniform protocol was used for measurements. Total cholesterol, HDL cholesterol and triglyceride levels were measured using enzyme-based assays with internal and external quality control as reported earlier.18 Values of LDL cholesterol and ratio of total:HDL cholesterol were calculated.18
2.3. Diagnostic criteria
High total cholesterol was defined as ≥200 mg/dl, high LDL cholesterol as ≥130 mg/dl, high non-HDL cholesterol as ≥160 mg/dl, low HDL cholesterol <40 mg/dl in men and <50 mg/dl in women and high triglycerides ≥150 mg/dl.25 Ideal LDL cholesterol was defined as <100 mg/dl.26 High total:HDL cholesterol was defined as ≥4.5 according to a previous Indian study.14 Overweight or obesity was defined as body mass index (BMI) ≥25 kg/m2 and truncal obesity was diagnosed when waist:hip ratio was >0.9 in men and >0.8 in women.27 It was also defined as waist circumference was >90 cm in men and >80 cm in women according to the harmonized guidelines of NCEP.28 Hypertension was diagnosed when systolic BP was ≥140 mm Hg and/or diastolic BP ≥ 90 mm Hg or a person was a known hypertensive. Diabetes was diagnosed on the basis of either history of known diabetes or fasting glucose ≥126 mg/dl. Metabolic syndrome was diagnosed according to the harmonized NCEP definition for Asian Indians.28 Socioeconomic status was categorized according to education, occupation and socioeconomic scale.18 Smokers included subjects who smoked cigarettes, bidis, or other smoked forms of tobacco daily, past smokers were subjects who had smoked for at least 1 year and had stopped more than a year ago. Users of other forms of tobacco (nasal, oral, etc) were classified as non-smoked tobacco use. The diagnostic criteria for tobacco use as well as other coronary risk factors have been advised by the World Health Organization.21 Subjects consuming more than 20 g visible fat daily were categorized as high fat intake and those consuming ≤2 servings of fruits or vegetables daily as low intake.18 Those involved in moderate degree of work or leisure time related physical activity were classified as active and others as not active, as in earlier studies.18
2.4. Statistical analyses
All the case-report form data in the study were entered into a database (SPSS V13.0, SPSS Inc, Chicago). Values for men and women have been analyzed separately. Numerical variables are reported as mean ± 1 SD and categorical variables as percent. Age-adjustment was performed using direct method with 2001 Indian census population as standard. In subjects with various cholesterol lipoprotein abnormalities, intergroup comparisons in risk factors were performed using X2 test and means compared using unpaired t-test or ANOVA as appropriate. Intergroup trends were calculated using Mantel-Haenszel X2 test for trend for categorical variables. p < 0.05 was considered significant.
3. Results
The study was performed at eleven cities located in all regions of India. 6198 subjects of the targeted 9900 subjects were recruited (response 62%). Details of lipid levels were available in 6123 subjects (men 3388, women 2735). Social and demographic characteristics in men and women have been previously reported.18 Men were slightly older than women. Low educational status (illiteracy and <10 years of formal education) was more among women (47.6%) as compared to men (22.3%). Majority of subjects belonged to middle socioeconomic status. More than half of all men and women lived in joint families and 85.6% were married. 17% subjects had migrated from rural-to-urban locations. Prevalence of various cardiovascular risk factors has been reported.18
Mean cholesterol lipoproteins and triglycerides levels in different age-groups are shown in Table 1. Age-adjusted levels, in men and women respectively, were total cholesterol 178.4 ± 39 and 184.6 ± 39 mg/dl, LDL cholesterol 102.5 ± 33 and 106.2 ± 33 mg/dl, non-HDL cholesterol 134.7 ± 37 and 135.0 ± 37 mg/dl, HDL cholesterol 44.9 ± 11 and 51.1 ± 11 mg/dl, total:HDL cholesterol 4.15 ± 1.22 and 3.79 ± 1.04 and triglycerides 162.5 ± 83 and 143.7 ± 83 mg/dl. Age-group associated trends in mean levels of total, LDL and HDL cholesterol and triglycerides are shown in Fig. 1. There is significant increase in total and LDL cholesterol (ANOVA trend, p < 0.001), more in women than men. Triglyceride levels are greater in younger men as compared to women with a significant age-associated increase. Mean HDL cholesterol levels are greater in women as compared to men but no significant age-related trend is observed.
Table 1.
Age-specific mean levels of various cholesterol lipoproteins in men and women.
| Cholesterol lipoproteins, triglycerides (mg/dl) | Age-group | Men | Women |
|---|---|---|---|
| Total cholesterol (Men 3388, women 2735) | <30 | 163.7 ± 40.3 | 160.5 ± 31.4 |
| 30–39 | 177.3 ± 38.3 | 174.3 ± 32.9 | |
| 40–49 | 184.0 ± 36.4 | 185.7 ± 36.0 | |
| 50–59 | 183.8 ± 40.9 | 192.4 ± 39.0 | |
| 60–69 | 178.6 ± 42.1 | 197.5 ± 43.4 | |
| 70+ | 179.2 ± 43.3 | 199.3 ± 42.8 | |
| HDL cholesterol (Men 3385, women 2735) | <30 | 43.7 ± 10.2 | 47.9 ± 11.3 |
| 30–39 | 43.0 ± 9.5 | 48.9 ± 11.1 | |
| 40–49 | 44.4 ± 9.7 | 50.6 ± 11.4 | |
| 50–59 | 45.5 ± 10.5 | 51.1 ± 12.0 | |
| 60–69 | 46.9 ± 11.0 | 53.5 ± 13.2 | |
| 70+ | 47.8 ± 9.6 | 57.0 ± 13.0 | |
| LDL cholesterol (Men 3378, women 2733) | <30 | 93.8 ± 33.6 | 89.7 ± 26.0 |
| 30–39 | 100.9 ± 31.0 | 99.2 ± 27.4 | |
| 40–49 | 104.6 ± 31.0 | 106.3 ± 29.9 | |
| 50–59 | 106.3 ± 34.8 | 110.0 ± 33.4 | |
| 60–69 | 100.6 ± 34.9 | 113.5 ± 36.9 | |
| 70+ | 102.0 ± 35.6 | 113.3 ± 37.8 | |
| Non-HDL cholesterol (Men 3385, women 2735) | <30 | 120.0 ± 37.0 | 112.6 ± 30.2 |
| 30–39 | 134.2 ± 36.4 | 125.4 ± 31.6 | |
| 40–49 | 139.7 ± 34.2 | 135.4 ± 33.9 | |
| 50–59 | 138.3 ± 39.0 | 141.3 ± 37.1 | |
| 60-69 | 131.5 ± 39.9 | 144.0 ± 40.5 | |
| 70+ | 131.3 ± 42.0 | 142.3 ± 40.3 | |
| Triglycerides (Men 3382, women 2733) | <30 | 130.5 ± 89.9 | 114.3 ± 57.7 |
| 30–39 | 167.5 ± 93.7 | 130.7 ± 68.5 | |
| 40–49 | 178.1 ± 93.9 | 144.7 ± 67.2 | |
| 50–59 | 162.4 ± 88.4 | 156.0 ± 86.1 | |
| 60–69 | 155.6 ± 89.6 | 152.6 ± 64.7 | |
| 70+ | 146.5 ± 78.5 | 145.2 ± 53.7 | |
| Total:HDL cholesterol (Men 3368, women 2731) | <30 | 3.9 ± 1.2 | 3.5 ± 0.9 |
| 30–39 | 4.2 ± 1.1 | 3.7 ± 0.9 | |
| 40–49 | 4.3 ± 1.2 | 3.8 ± 1.0 | |
| 50–59 | 4.2 ± 1.2 | 3.9 ± 1.1 | |
| 60–69 | 4.0 ± 1.2 | 3.8 ± 1.1 | |
| 70+ | 3.9 ± 1.2 | 3.6 ± 0.9 |
Fig. 1.
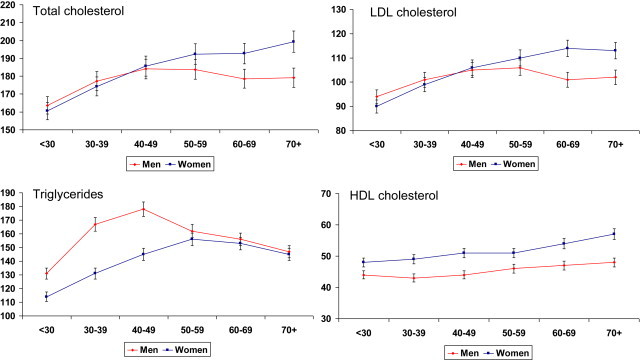
Age-group specific levels of total cholesterol, LDL cholesterol, triglycerides and HDL cholesterol (mg/dl). There is significant age-associated increase in total and LDL cholesterol (ANOVA trend, p < 0.001), more in women than men. Triglyceride levels are greater in younger men as compared to women with a significant age-associated increase. No significant trend is observed in HDL cholesterol levels.
Age-specific prevalence of various dyslipidemias is shown in Table 2. There is age-associated increase in prevalence of high total, LDL and non-HDL cholesterol (Mantel-Haenszl X2, ptrend < 0.05). Age-adjusted prevalence of high cholesterol (≥200 mg/dl) was 25.0% (men 24.8%, women 25.3%), high LDL cholesterol (≥130 mg/dl) in 15.8% (men 16.3%, women 15.1%), high non-HDL cholesterol (≥160 mg/dl) in 18.8% (men 20.9%, women 16.2%), high total:HDL cholesterol ratio (>4.5) in 23.8% (men 29.4%, women 16.8%), high triglycerides (≥150 mg/dl) in 36.9% (men 41.2%, women 31.5%), and low HDL cholesterol in 42.5% (men 34.1%, women 53.0%). Age adjusted prevalence of atherogenic dyslipidemia29 defined by LDL cholesterol ≥100 mg/dl, triglycerides ≥150 mg/dl and HDL cholesterol <40 mg/dl men and <50 mg/dl women was in 7.8% (men 7.0%, women 8.9%).
Table 2.
Age-specific prevalence of dyslipidemias.
| Age-group | Number of men/women | Men (n = 3388) | Women (n = 2735) | |
|---|---|---|---|---|
| Total cholesterol: ≥200 mg/dl | <30 | 253/204 | 47 (18.6) | 20 (9.8) |
| 30–39 | 570/565 | 136 (23.8) | 113 (20.2) | |
| 40–49 | 899/777 | 279 (31.0) | 264 (33.9) | |
| 50–59 | 869/626 | 286 (32.9) | 255 (40.7) | |
| 60–69 | 532/443 | 167 (31.4) | 207(46.7) | |
| 70+ | 265/122 | 73 (27.5) | 61 (50.0) | |
| X2 for trend (p value) | <0.001 | <0.001 | ||
| HDL cholesterol: men<40 mg/dl; women<50 mg/dl | <30 | 253/204 | 88 (34.8) | 127 (62.2) |
| 30–39 | 569/565 | 230 (40.4) | 325 (57.5) | |
| 40–49 | 899/776 | 298 (33.1) | 380 (48.9) | |
| 50–59 | 868/626 | 268 (30.9) | 291 (46.5) | |
| 60–69 | 531/443 | 131 (24.7) | 176 (39.7) | |
| 70+ | 265/122 | 50 (18.8) | 35 (28.7) | |
| X2 for trend (p value) | <0.001 | <0.001 | ||
| LDL cholesterol ≥130 mg/dl | <30 | 253/204 | 32 (12.6) | 12 (5.9) |
| 30–39 | 568/564 | 93 (16.4) | 62 (11.0) | |
| 40–49 | 899/776 | 175 (19.5) | 149 (19.2) | |
| 50–59 | 868/626 | 196 (22.6) | 156 (24.9) | |
| 60–69 | 531/443 | 93 (17.6) | 143 (32.3) | |
| 70+ | 265/122 | 48 (18.1) | 43 (35.2) | |
| X2 for trend (p value) | 0.054 | <0.001 | ||
| Non-HDL cholesterol ≥160 mg/dl | <30 | 253/204 | 41 (16.2) | 11 (5.4) |
| 30–39 | 569/565 | 124 (21.8) | 64 (11.3) | |
| 40–49 | 899/775 | 232 (25.8) | 163 (21.0) | |
| 50–59 | 868/626 | 232 (26.7) | 181 (28.9) | |
| 60–69 | 531/443 | 113 (21.3) | 146 (32.9) | |
| 70+ | 265/122 | 58 (21.9) | 42 (34.4) | |
| X2 for trend (p value) | 0.280 | <0.001 | ||
| Triglycerides ≥150 mg/dl | <30 | 253/204 | 77 (30.4) | 46 (22.5) |
| 30–39 | 569/564 | 260 (45.7) | 160 (28.3) | |
| 40–49 | 899/774 | 480 (53.4) | 307 (39.7) | |
| 50–59 | 867/626 | 391 (45.1) | 275 (43.9) | |
| 60–69 | 529/443 | 211 (39.9) | 194 (43.8) | |
| 70+ | 265/122 | 98 (37.0) | 46 (37.7) | |
| X2 for trend (p value) | 0.250 | <0.001 | ||
| Total:HDL cholesterol ≥4.5 | <30 | 253/204 | 58 (22.9) | 26 (12.7) |
| 30–39 | 568/564 | 194 (34.1) | 98 (17.4) | |
| 40–49 | 899/774 | 307 (34.1) | 142 (18.3) | |
| 50–59 | 865/626 | 294 (34.0) | 137 (21.9) | |
| 60–69 | 529/443 | 136 (25.7) | 95 (21.4) | |
| 70+ | 265/122 | 59 (22.2) | 16 (13.1) | |
| X2 for trend (p value) | 0.033 | 0.036 |
We also determined mean levels of various cholesterol lipoproteins and triglycerides in various sociodemographic and anthropometric groups (Table 3). Total cholesterol levels were greater in high (compared to low) socioeconomic status (men 4.8%, women 3.3%), educational status (men 2.5%, women 1.2%), body mass index (men 7.5%, women 2.6%) and waist size (men 5.5%, women 3.4%) subjects (p < 0.05). Similar associations were observed for LDL and non-HDL cholesterol (Table 3). Triglyceride levels were more in subjects with smoking/tobacco use (men 7.3%, women 6.3%), high fat intake (men 9.4%, women 1.3%) and high waist size (men 10.1%, women 15.8%); while significantly lower HDL cholesterol levels were observed in subjects with high body mass index (men 4.1%, women 3.4%) and waist circumference (men 3.9%, women 2.6%) (p < 0.05).
Table 3.
Age-adjusted mean and 95% confidence intervals (mg/dl) of cholesterol lipoproteins and triglycerides in various socio-demographic and anthropometric groups.
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total cholesterol | HDL cholesterol | LDL cholesterol | Triglyceride | Total cholesterol | HDL cholesterol | LDL cholesterol | Triglyceride | |
| Socioeconomic status | ||||||||
| Low | 174.0 (168.6–179.6) | 44.2 (42.8–45.5) | 99.7 (95.0–104.2) | 151.3 (139.2–163.4) | 179.0 (173.8–186.0) | 50.0 (48.1–51.9) | 103.7 (98.6–108.9) | 130.7 (119.5–141.1) |
| Medium | 178.7 (176.9–180.5) | 45.1 (44.6–45.5) | 101.3 (99.8–102.8) | 164.3 (160.4–168.2) | 186.5 (184.7–188.3) | 50.7 (50.2–51.3) | 106.4 (104.9–107.9) | 147.0 (143.7–150.3) |
| High | 182.4 (179.3–185.4) | 44.7 (44.0–45.5) | 106.2 (103.6–108.7) | 157.3 (150.6–163.9) | 184.9 (181.3–188.5) | 51.4 (50.3–52.5) | 105.7 (102.7–108.7) | 137.9 (131.4–144.5) |
| p value (ANOVA) | 0.016 | 0.316 | 0.002 | 0.046 | 0.137 | 0.550 | 0.606 | 0.002 |
| Education level | ||||||||
| 0-10 years | 176.7 (173.0–180.5) | 43.9 (42.9–44.8) | 101.3 (98.2–104.5) | 158.6 (150.1–167.0) | 184.1 (181.5–186.7) | 49.1 (48.2–49.9) | 105.9 (103.7–108.1) | 145.4 (140.8–150.0) |
| 11-15 years | 180.0 (178.2–181.9) | 45.3 (44.8–45.8) | 102.9 (101.4–104.5) | 161.8 (157.6–166.0) | 186.0 (183.9–188.1) | 52.5 (51.8–53.2) | 105.1 (103.3–106.9) | 142.3 (138.5–146.0) |
| >15 years | 181.1(178.6–183.6) | 45.7(45.0–46.3) | 102.8(100.7–104.8) | 163.5(157.8–169.1) | 186.4(182.6–190.1) | 52.7(51.5–53.9) | 107.3(104.1–110.4) | 131.2(124.5–137.9) |
| p value (ANOVA) | 0.232 | 0.031 | 0.772 | 0.595 | 0.576 | <0.001 | 0.185 | <0.001 |
| Visible fat intake | ||||||||
| <20 g/day | 178.1 (175.1–181.0) | 44.4 (43.6–45.1) | 103.5(101.2–105.9) | 154.0 (147.7–160.3) | 184.2 (181.5–186.8) | 49.4 (48.6–50.3) | 106.8 (104.6–109.0) | 140.6 (135.6–145.6) |
| 20–40 g/day | 181.7 (179.6–183.8) | 44.9 (44.3–45.4) | 103.6 (101.8–105.8) | 167.0 (162.2–171.6) | 186.4 (184.1–188.6) | 50.7 (50.0–51.4) | 105.9 (104.0–107.8) | 148.2 (143.9–152.4) |
| >40 g/day | 181.1 (177.6–184.8) | 44.1 (43.1–45.0) | 103.6 (100.6–106.6) | 168.4 (160.4–176.4) | 187.4 (183.3–191.3) | 50.1 (48.9–51.4) | 108.7 (105.3–112.1) | 142.4 (134.8–150.0) |
| p value (ANOVA) | 0.105 | 0.116 | 0.985 | 0.003 | 0.103 | 0.017 | 0.535 | 0.024 |
| Fruit/Vegetable intake | ||||||||
| ≤2 servings/day | 179.8 (178.1–181.5) | 44.7 (44.3–45.1) | 102.8 (101.4–104.2) | 163.1 (159.2–166.9) | 185.3 (183.4–187.0) | 50.4 (49.8–50.9) | 106.7 (105.3–108.2) | 141.0 (137.8–144.3) |
| 3–4 servings/day | 180.4 (177.4–183.5) | 46.1 (45.3–46.8) | 102.8 (100.2–105.3) | 158.8 (151.9–165.6) | 184.3 (180.9–187.7) | 51.3 (50.2–52.3) | 104.3 (101.5107.1) | 143.5 (137.2–149.8) |
| >5 servings/day | 179.7 (173.8–185.7) | 44.5 (43.0–46.0) | 101.7 (96.7–106.6) | 171.2 (157.7–184.6) | 189.0 (181.5–196.7) | 49.8 (47.5–52.5) | 104.4 (98.0–110.9) | 173.8 (159.6–187.9) |
| p value (ANOVA) | 0.959 | 0.012 | 0.892 | 0.248 | 0.331 | 0.398 | 0.203 | <0.001 |
| Smoking/tobacco use | ||||||||
| Non user | 179.9 (178.3–181.5) | 45.2 (44.8–45.6) | 103.1 (101.7–104.4) | 158.8 (155.2–162.4) | 185.3 (183.8–186.7) | 51.2 (50.7–51.6) | 105.5 (104.3–106.8) | 143.0 (140.1–145.8) |
| User | 179.5 (177.0–182.1) | 44.7 (44.0–45.3) | 101.6 (99.5–103.8) | 170.4 (164.7–176.1) | 185.2 (180.6–189.8) | 48.0 (46.6–49.5) | 106.8 (102.9–110.6) | 152.0 (143.2–160.8) |
| p value (ANOVA) | 0.835 | 0.126 | 0.275 | 0.001 | 0.679 | <0.001 | 0.368 | 0.035 |
| Body mass index categories | ||||||||
| <23 kg/m2 | 170.9 (168.5–173.5) | 45.9 (45.2–46.5) | 96.8 (94.7–98.9) | 141.6 (138.0–147.2) | 181.6 (178.9–184.2) | 51.4 (50.6–52.3) | 103.8 (101.5–106.0) | 132.2 (127.2–137.2) |
| 23–24.9 kg/m2 | 183.3 (180.5–186.2) | 45.1 (44.4–45.8) | 105.1 (102.7–107.5) | 167.1 (160.8–173.4) | 185.2 (181.8–188.5) | 51.5 (50.5–52.6) | 105.6 (102.8–108.5) | 139.8 (133.4–146.1) |
| 25–29.9 kg/m2 | 184.3 (182.2–186.5) | 44.6 (44.0–45)0.1 | 106.0 (104.2–107.8) | 171.8 (166.9–176.6) | 188.4 (186.0–190.7) | 50.7(49.9–51.4) | 108.3 (106.3–110.3) | 147.3 (142.8–151.7) |
| ≥30 kg/m2 | 183.8(179.5–188.1) | 44.1(43.0–45.2) | 104.7(101.0–108.2) | 173.3(163.7–182.9) | 186.3 (183.0–189.6) | 49.7 (48.6–50.7) | 106.0 (103.3–108.8) | 152.8 (146.6–159.0) |
| p value (ANOVA) | <0.001 | 0.035 | <0.001 | <0.001 | <0.001 | 0.312 | <0.001 | <0.001 |
| Waist circumference categories | ||||||||
| <90/80 cm | 175.8 (173.9–177.8) | 45.5 (45.0–46.0) | 99.9 (98.3–101.4) | 152.8 (148.5–157.2) | 182.2 (179.6–184.9) | 51.3 (50.5–52.2) | 104.6 (102.4–106.8) | 132.0 (127.1–136.9) |
| 90–100/80–90 cm | 184.5 (182.3–186.8) | 44.9 (44.3–45.5) | 105.8 (103.9–107.6) | 172.2 (167.1–177.2) | 185.7 (183.5–187.9) | 51.2 (50.5–51.9) | 106.2 (104.3–108.1) | 142.3 (138.2–146.5) |
| >100/90 cm | 185.5 (181.8–189.1) | 43.8 (42.9–44.8) | 108.0 (105.0–111.0) | 168.2 (160.1–176.3) | 188.4 (185.8–191.0) | 50.0 (49.2–50.8) | 107.7 (105.5–109.9) | 152.8 (148.0–157.6) |
| p value (ANOVA) | <0.001 | 0.126 | 0.001 | <0.001 | <0.001 | 0.155 | 0.005 | <0.001 |
There was a low awareness and control of borderline and high total cholesterol among the study subjects (Figs. 1 and 2). Awareness was in 17.5% men and 13.2% women with high cholesterol, treatment with statins was in 7.5% men and 6.7% women, while control to targets of total cholesterol <200 mg/dl was in 4.5% men and 3.7% women.
Fig. 2.
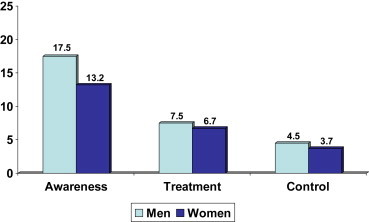
Prevalence, awareness, treatment and control of hypercholesterolemia in the study subjects (age-adjusted percent values among as subjects with total cholesterol ≥200 mg/dl).
4. Discussion
There are only a few studies that evaluated mean levels of various cholesterol lipoproteins and prevalence of various dyslipidemias in India.6 A review reported that among adults, in urban and rural areas respectively, the total cholesterol levels varied from 158 to 248 mg/dl and 166–182 mg/dl, LDL cholesterol 70–120 mg/dl and 97–120 mg/dl, HDL cholesterol 35–54 mg/dl and 39–50 mg/dl, and triglycerides from 80 to 168 and 124–151 mg/dl.30 Prevalence of various types of dyslipidemias ranged from 10 to 73%. However, these studies were confined to one or two centers and not comparable to the present study. There are only a few multicentric studies in India which used common protocol for laboratory assessment similar to the present study. The Indian Industrial Surveillance Study evaluated various lipid abnormalities among male industrial workers in different regions of the country.14 India Migration Study15 evaluated lipid levels only among the rural subjects while in the multisite Integrated Disease Surveillance Project of Indian Council of Medical Research16 only total cholesterol levels were measured. Multisite Indian Women's Health Study17 also evaluated total cholesterol levels only. All these studies reported a low prevalence of hypercholesterolemia, which is similar to the present study. The Indian Industrial Surveillance Study13 and India Migration Study15 reported that prevalence of low HDL cholesterol, hypertriglyceridemia and total:HDL cholesterol was higher than hypercholesterolemia. These findings are similar to the present study and show that various components of atherogenic dyslipidemia may be the more important lipid phenotype in Asian Indians. These risk factors are important components of the metabolic syndrome, which is highly prevalent in India.31
This is the first study, which has collected cross-sectional data across the Indian urban population. It shows that mean levels of total cholesterol, LDL cholesterol and non-HDL cholesterol are lower in urban Asian Indian middle-class subjects than in populations in high income countries as reported in the Global Burden of Diseases Study.13 Triglycerides levels in these subjects are greater and HDL cholesterol levels lower. Components of atherogenic dyslipidemia (borderline high LDL cholesterol, low HDL cholesterol, hypertriglyceridemia)29 are the most prevalent lipid abnormalities. Levels of lipoprotein lipids are greater in subjects with high socioeconomic status and education, high BMI and high waist size. There is a low status of awareness, treatment and control of hypercholesterolemia. We also studied levels of cholesterol lipoproteins in various subgroups (Table 3). Cholesterol, LDL cholesterol and triglyceride levels were greater in subjects with higher socioeconomic status and more education. These findings are similar to previous studies from India.32 We could not find any significant difference in various cholesterol lipoprotein levels with dietary intake of visible fats, fruits, vegetables, physical activity or smoking/tobacco use. This could be due to limitations of the qualitative questionnaire that we used. On the other hand, cholesterol, its lipoproteins and triglycerides were more and HDL cholesterol lower among men and women with greater obesity and central obesity, similar to previous Indian and international studies.9
In contrast to India, population based studies of mean levels of cholesterol and other lipoproteins are available from USA and most high and upper-middle income countries. The US National Health Evaluation Studies (NHES) and National Health and Nutritional Evaluation Studies (NHANES) have periodically reported mean levels of total cholesterol and other cholesterol lipoproteins from mid 20th century.10 Mean cholesterol levels were 222 mg/dl in early 1960's, which decreased to 203 mg/dl in the year 2002.10 Mean LDL cholesterol levels were 129 mg/dl in 1988–1994 and 123 mg/dl in 2002. The mean HDL cholesterol levels in 1976 and 2002 were 45 and 46 mg/dl in men and 54 and 56 mg/dl in women while mean triglyceride levels were 114 mg/dl in 1976 and 122 mg/dl in 2002.10 Total cholesterol levels have further declined to 196 mg/dl in 2010.11 In the international Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) study in 21 countries on 4 continents, the mean total cholesterol levels of adults (35–64 years) were similar to the US populations and significantly greater than in the present study.33 Global Burden of Chronic Diseases Risk Factors study has reported mean level of total cholesterol in 24 high income and 66 middle and low-income countries.13 Fasting total cholesterol concentrations were the highest in high-income countries in years 1980 and 2008 (217 and 202 mg/dl respectively), intermediate in middle income countries (190 and 182 mg/dl) and the lowest in low-income countries (172 and 162 mg/dl). In the present study the age-adjusted total cholesterol levels in men and women are 178 ± 39 and 185 ± 39 mg/dl. These values are similar to those in middle income countries.
In China, He et al evaluated 15,540 men and women 35–74 years old in a nationally representative study,34 24% subjects had borderline high total cholesterol and 9% had high total cholesterol. Among those with borderline and high cholesterol the proportion of men and women, respectively, who were aware, treated, and controlled, was 8.8% and 7.5%, 3.5% and 3.4%, and 1.9% and 1.5%. Another study in China35 which evaluated 46,239 adults, the prevalence of borderline and high total cholesterol was 32% and awareness, treatment, and control were 11.0%, 5.1%, and 2.8%, respectively. These rates are similar to the present study, which shows a low status of awareness, treatment and control of hypercholesterolemia among the urban Asian Indian subjects. On the other hand, in USA, the 1999–2000 NHANES reported that in men and women with borderline high cholesterol (≥200 mg/dl), 34.8% and 35.4% were aware that they had hypercholesterolemia, 14.0% and 10.2% were on treatment, and 7.5% and 3.7% had cholesterol >200 mg/dl.10 Another study reported that in USA between 1988–1994 and 1999–2004 the awareness of hypercholesterolemia increased from 39.2% to 63.0%, use of pharmacologic lipid-lowering treatment increased from 11.7% to 40.8% and LDL cholesterol control increased from 4.0% to 25.1% in those with high LDL cholesterol.36 The present study, thus, indicates a substantial gap in awareness, treatment and control for hypercholesterolemia in India.
This study has a few limitations. We did not study populations in all the Indian states, however, inclusion of all the regions of India is unique. Sampling confined to urban locations in middle-level cities could be criticized for selection bias, however, such urban locations now represent the heart of India19 and is a fertile ground for cardiovascular epidemic. Moreover, rapidly increasing urbanization in the country shall lead to more than 60% of the population shall be in similar locations within the next 20–30 years.22 The study, therefore, illustrates the need to create more healthy cities for control of atherogenic lipid abnormalities.37 Thirdly, the data from middle-class locations in urban areas may not be generalizable to the whole country. Almost 30% of Indian population live in urban slums and more than 65% dwell in rural locations. However, the study does provide a snapshot on more than 300 million Indians who are middle class and provides a glimpse into the future of the country.19 Fourthly, the INTERHEART study reported that high ratio of apo B to apo A is a more important lipid risk factor in South Asian subjects.38 We did not study these apolipoproteins. The predictive value of standard dyslipidemias, evaluated in the present study, vis a vis abnormal apolipoproteins should be prospectively studied in India before the latter are widely adopted.
In conclusion this multisite survey among urban population in India shows that although total cholesterol and LDL cholesterol levels are low among Asian Indian men and women, triglyceride levels are high and similar to Caucasian and Chinese populations. Components of atherogenic dyslipidemias (borderline high LDL cholesterol, low HDL cholesterol and high triglycerides) are the most prevalent lipid abnormalities. Very low awareness, treatment and control rates for hypercholesterolemia in this literate middle-class urban population indicates significant gaps in prevention of cardiovascular diseases in the country. Large scale public health interventions are required to increase awareness and management of lipid abnormalities to control these dyslipidemias.
Authors' contributions
RG, PCD and AG designed the study and developed the protocol, they were also involved in obtaining funding, investigator training, and supervised the whole study. SG and RG jointly wrote the first and subsequent drafts of the article. AB, AM, AG, BKG, BS, JS, and VA were the site investigators and supervised the conduct of the study locally. They were involved in data collection and provided inputs for the article and critically reviewed the whole article and provided suggestions. RG, SG, IM and KKS were involved in study supervision, data management and statistical analyses. All the authors have read the manuscript and agree to its contents.
Conflicts of interest
All authors have none to declare.
Acknowledgment
The study was funded by South Asian Society of Atherosclerosis and Thrombosis, Bangalore, India and Minneapolis, USA. The funding agency had no contribution to study design, data collection, data interpretation and writing of the manuscript.
References
- 1.Burke G.L., Bell R.A. National and international trends in cardiovascular disease: incidence and risk factors. In: Blumenthal R.S., Foody J.M., Wong N.D., editors. Preventive Cardiology: A Companion to Braunwald's Heart Disease. Saunders-Elsevier; Philadelphia: 2011. pp. 14–32. [Google Scholar]
- 2.Prospective Studies Collaboration. Lewington S., Whitlock G., Clarke R. Blood cholesterol and vascular mortality by age, sex and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factor Collaboration. Di Angelantonio E., Sarwar N., Perry P. Major lipids, apolipoproteins and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Patel A., Horibe H., Asia Pacific Cohort Studies Collaboration Cholesterol, coronary heart disease and stroke in the Asia Pacific region. Int J Epidemiol. 2003;32:563–572. doi: 10.1093/ije/dyg106. [DOI] [PubMed] [Google Scholar]
- 5.Fuster V., Kelly B.B., Board for Global Health . Institute of Medicine; Washington: 2010. Promoting Cardiovascular Health in Developing World: A Critical Challenge to Achieve Global Health. [PubMed] [Google Scholar]
- 6.Gupta R., Guptha S., Sharma K.K., Gupta A., Deedwania P.C. Regional variations in cardiovascular risk factors in India: India Heart Watch. World J Cardiol. 2012;4:112–120. doi: 10.4330/wjc.v4.i4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–953. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.McQueen M.J., Hawken S., Wang X. INTERHEART study investigators. Lipids, lipoproteins and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 9.Durrington P. Hyperlipidemia: Diagnosis and Management. 3rd ed. Hodder Arnold; London: 2007. [Google Scholar]
- 10.Carroll M.D., Lacher D.A., Sorlei P.D. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 11.Carroll M.D., Kit B.K., Lacher D.A., Shero S.T., Mussolino M.E. Trends in lipids and lipoproteins in US adults, 1988-2010. JAMA. 2012;302:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . World Health Organization; Geneva: 2011. Global Status Report of Non-Communicable Diseases 2010. [Google Scholar]
- 13.Farzadfar F., Finucane M.M., Danaei G., Global Burden of Metabolic Risk Factors for Chronic Diseases Collaborating Group (Cholesterol) National, regional and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 14.Reddy K.S., Prabhakaran D., Chaturvedi V. Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bull WHO. 2006;84:461–469. doi: 10.2471/blt.05.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinra S., Bowen L.J., Lyngdoh T. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010;341:c4974. doi: 10.1136/bmj.c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah B., Mathur P. Surveillance of cardiovascular disease risk factors in India: the need and scope. Indian J Med Res. 2010;132:634–642. doi: 10.4103/0971-5916.73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey R.M., Gupta R., Misra A. Determinants of urban-rural differences in cardiovascular risk factors in middle-aged women in India: a cross-sectional study. Int J Cardiol. 2013;163:157–162. doi: 10.1016/j.ijcard.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R., Deedwania P.C., Sharma K.K. Association of education, occupation and socioeconomic status with cardiovascular risk factors in Asian Indians: a cross-sectional study. PLoS One. 2012;7:e44098. doi: 10.1371/journal.pone.0044098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma P.K. Penguin India; New Delhi: 2007. The Great Indian Middle Class. [Google Scholar]
- 20.Das G. Allen Lane and Penguin India; New Delhi: 2012. India Sleeps at Night; pp. 1–18. [Google Scholar]
- 21.Luepkar R.V., Evans A., McKeigue P.M., Reddy K.S. 3rd ed. World Health Organization; Geneva: 2002. Cardiovascular Survey Methods. [Google Scholar]
- 22.McKinsey Global Institute . McKinsey and Company; New York: 2010. India's Urban Awakening: Building Inclusive Cities, Sustaining Economic Growth. [Google Scholar]
- 23.Gupta R., Guptha S., Gupta V.P., Agrawal A., Gaur K., Deedwania P.C. Twenty year trends in cardiovascular risk factors in India and influence of educational status. Eur J Prev Cardiol. 2012;19:1258–1271. doi: 10.1177/1741826711424567. [DOI] [PubMed] [Google Scholar]
- 24.Anonymous. Thyrocare: World's Largest Thyroid Testing Laboratory: Laboratory Services. Available at: http://www.thyrocare.com/assets/fig/about_labtour.htm. Accessed 15.10.12.
- 25.National Cholesterol Education Program Detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 26.Deedwania P., Barter P., Carmena R. Reduction of low density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–928. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 27.WHO Expert Consultation Appropriate body-mass index for Asian population and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 28.Alberti K.G., Eckel R.H., Grundy S.M. International diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; international association for the study of obesity. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung and blood Institute: American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 29.Grundy S.M. Small LDL, atherogenic dyslipidemia and the metabolic syndrome. Circulation. 1997;95:1–4. doi: 10.1161/01.cir.95.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Misra A., Luthra K., Vikram N.K. Dyslipidemia in Asian Indians: determinants and significance. J Assoc Physicians India. 2004;52:137–142. [PubMed] [Google Scholar]
- 31.Misra A., Vikram N.K. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20:482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Thankappan K.R., Shah B., Mathur P. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J Med Res. 2012;131:53–63. [PubMed] [Google Scholar]
- 33.Tunstall-Pedoe H. World Health Organization; Geneva: 2003. MONICA: Monograph and Multimedia Sourcebook. [Google Scholar]
- 34.He J., Gu D., Rynolds K. Serum total and lipoprotein cholesterol levels and awareness, treatment and control of hypercholesterolemia in China. Circulation. 2004;110:405–411. doi: 10.1161/01.CIR.0000136583.52681.0D. [DOI] [PubMed] [Google Scholar]
- 35.Yang W., Xiao J., Yang Z. Serum lipids and lipoproteins in Chinese men and women. Circulation. 2012;125:2212–2221. doi: 10.1161/CIRCULATIONAHA.111.065904. [DOI] [PubMed] [Google Scholar]
- 36.Hyre A.D., Muntner P., Menke A., Raggi P., He J. Trends in ATP-III defined high blood cholesterol, awareness, treatment and control among US adults. Ann Epidemiol. 2007;17:548–555. doi: 10.1016/j.annepidem.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Rydin Y., Bleahu A., Davies M. Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet. 2012;379:2079–2108. doi: 10.1016/S0140-6736(12)60435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karthikeyan G., Teo K.K., Islam S. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART study. J Am Coll Cardiol. 2009;53:244–253. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]


