Significance
Marijuana abusers show lower positive and higher negative emotionality scores than controls, which is consistent, on one hand, with lower reward sensitivity and motivation and, on the other hand, with increased stress reactivity and irritability. To investigate this aspect of marijuana’s impact on the human brain, we compared the brain’s reactivity in marijuana abusers vs. controls when challenged with methylphenidate (MP). We found that marijuana abusers display attenuated dopamine (DA) responses to MP, including reduced decreases in striatal distribution volumes. These deficits cannot be unambiguously ascribed to reduced DA release (because decreases in nondisplaceable binding potential were not blunted) but could reflect a downstream postsynaptic effect that in the ventral striatum (brain reward region) might contribute to marijuana’s negative emotionality and addictive behaviors.
Keywords: nucleus accumbens, amotivation, cannabinoid 1 receptors, brain imaging, midbrain
Abstract
Moves to legalize marijuana highlight the urgency to investigate effects of chronic marijuana in the human brain. Here, we challenged 48 participants (24 controls and 24 marijuana abusers) with methylphenidate (MP), a drug that elevates extracellular dopamine (DA) as a surrogate for probing the reactivity of the brain to DA stimulation. We compared the subjective, cardiovascular, and brain DA responses (measured with PET and [11C]raclopride) to MP between controls and marijuana abusers. Although baseline (placebo) measures of striatal DA D2 receptor availability did not differ between groups, the marijuana abusers showed markedly blunted responses when challenged with MP. Specifically, compared with controls, marijuana abusers had significantly attenuated behavioral (“self-reports” for high, drug effects, anxiety, and restlessness), cardiovascular (pulse rate and diastolic blood pressure), and brain DA [reduced decreases in distribution volumes (DVs) of [11C]raclopride, although normal reductions in striatal nondisplaceable binding potential (BPND)] responses to MP. In ventral striatum (key brain reward region), MP-induced reductions in DVs and BPND (reflecting DA increases) were inversely correlated with scores of negative emotionality, which were significantly higher for marijuana abusers than controls. In marijuana abusers, DA responses in ventral striatum were also inversely correlated with addiction severity and craving. The attenuated responses to MP, including reduced decreases in striatal DVs, are consistent with decreased brain reactivity to the DA stimulation in marijuana abusers that might contribute to their negative emotionality (increased stress reactivity and irritability) and addictive behaviors.
Despite the high prevalence of marijuana consumption, the effects of marijuana abuse in the human brain are not well understood. Marijuana, like other drugs of abuse, stimulates brain dopamine (DA) signaling in the nucleus accumbens (1, 2), which is a mechanism believed to underlie the rewarding effects of drugs (3–5) and to trigger the neuroadaptations that result in addiction (reviewed in ref. 6). Indeed, in humans, imaging studies have shown that drugs of abuse increase DA release in striatum (including the nucleus accumbens), and these increases have been associated with the subjective experience of reward (7–9). However, for marijuana, the results have been inconsistent: One study reported striatal DA increases during intoxication (10); two studies showed no effects (11, 12); and one study reported DA increases in individuals with a psychotic disorder and in their relatives, but not in controls (13). Imaging studies of the brain DA system in marijuana abusers have also shown different findings from those reported for other types of substance abusers. Specifically, substance abusers (cocaine, methamphetamine, alcohol, heroin, and nicotine), but not marijuana abusers (14–16), show reduced baseline availability of DA D2 receptors in striatum (reviewed ref. 6). Similarly, cocaine abusers (17, 18) and alcoholics (19, 20), but not marijuana abusers (16), show attenuated DA increases in striatum when challenged with a stimulant drug, although marijuana abusers with comorbid schizophrenia or risk for schizophrenia showed blunted DA increases to stimulants (21) and to stress (22). However, prior studies are limited by their small sample sizes (ranging from six to 16 subjects). Also, prior studies did not control for the potential confounds that the changes in cerebral vascular resistance associated with marijuana abuse (23–25) could have on the delivery of the radiotracer to the brain when using a stimulant drug as pharmacological challenge, because stimulants decrease cerebral blood flow (26). Thus, the extent to which there are changes in brain DA signaling in marijuana abusers is still unclear.
Here, we compared brain DA reactivity in healthy controls and marijuana abusers on a larger sample than that in prior studies and measured arterial concentration of nonmetabolized radiotracer to control for differences in radiotracer delivery to brain. We used PET and [11C]raclopride (radioligand that binds to D2/D3 receptors not occupied by DA) to assess the effects of methylphenidate (MP) on the nondisplaceable binding potential [BPND; ratio of the distribution volume (DV) in striatum to that in cerebellum], which is the most frequent model parameter used to estimate DA changes (27), in 24 healthy controls and 24 marijuana abusers. We also quantified the DV, which corresponds to the equilibrium measurement of the ratio of the concentration of the radiotracer in tissue to that in arterial plasma, to control for potential changes in radiotracer delivery that could confound group comparisons of stimulant-induced changes in BPND. We used MP, which is a stimulant drug that blocks DA transporters, because it induces robust and reproducible DA increases in the human brain (28, 29). We predicted that MP’s behavioral effects in marijuana abusers would be attenuated, consistent with preclinical findings (30), and that decreased DA reactivity in ventral striatum would be associated with higher scores in negative emotionality (neuroticism), which mediates genetic risk for marijuana dependence (31), and with addiction severity.
Materials and Methods
Participants.
Twenty-four marijuana abusers and 24 healthy controls completed the studies. Participants were recruited from advertisements in local newspapers (demographics are provided in Table 1). At least two clinicians interviewed the patients, using a semistructured standardized interview, to ensure that they met the Diagnostic Statistical Manual IV diagnostic criteria for marijuana abuse or dependence. Marijuana abusers were excluded if they had a history of substance abuse or dependence (other than marijuana and nicotine), history of other psychiatric or neurological diseases, medical conditions that might alter cerebral function (i.e., cardiovascular, endocrinological, oncological, autoimmune diseases), current use of prescribed or over-the-counter medications, and/or head trauma with loss of consciousness for more than 30 min. All subjects had Hamilton’s Anxiety and Depression scores <19 (32). Exclusion criteria for controls were as for the marijuana abusers, other than allowance for regular marijuana use (exclusion for controls was smoking more than one joint of marijuana per month). All subjects had a physical, psychiatric, and neurological examination. Drug screens were done on the days of the scans to exclude the use of psychoactive drugs (other than marijuana in marijuana abusers). Subjects were instructed to discontinue any over-the-counter medication 2 wk before the days scheduled for the scans. Food and beverages (except for water) were discontinued at least 4 h prior to the study, and tobacco cigarettes were discontinued for at least 2 h before the study. This study was approved by the Committee on Research Involving Human Subjects at Stony Brook University, and written informed consent was obtained from all subjects.
Table 1.
Demographics, clinical characteristics, and personality scores (positive emotionality, negative emotionality, and constraint) of participants, and the significance for the comparisons between healthy controls and marijuana abusers
| Parameter | Healthy controls(n = 24) | Marijuana abusers(n = 24) | P |
| Age | 28.2 ± 6 | 26.9 ± 7 | NS |
| Sex | 50% males | 50% males | |
| Education | 13.9 ± 2 | 13.2 ± 1 | NS |
| Body mass index | 24.3 ± 3 | 24.1 ± 4 | NS |
| Tobacco | 3 active | 10 active | 0.02 |
| 1 former | 2 former | ||
| Marijuana initiation | 15 ± 3 y of age | ||
| Days per week | 4.9 ± 3 | ||
| Joints per day | 4.8 ± 3 | ||
| Years of abuse | 10.5 ± 2 | ||
| Scores on MDQ | 5.4 ± 3 | ||
| Positive emotionality | 52.3 ± 6 | 47.2 ± 10 | 0.05 |
| Negative emotionality | 13.7 ± 9 | 22.4 ± 9 | 0.001 |
| Constraint | 51.3 ± 10 | 47.7 ± 8 | NS |
MDQ, marijuana dependency questionnaire; NS, not significant.
Behavioral and Cardiovascular Measures.
Subjective ratings (high, restlessness, drug effects, and anxiety) were measured using self-reports (range: 1–10) that were recorded before administration of placebo or MP and periodically over the duration of the [11C]raclopride scans (33). At the end of the study, participants were asked to rate the drug’s potency (scale of 1–10).
Heart rate and blood pressure were recorded before administration of placebo or MP and periodically over the duration of the study. MP concentration in plasma was measured using capillary GC/MS (34).
A factorial repeated ANOVA was used to assess the effects of MP on the behavioral measures (drug main effect) and to assess if responses differed between controls and marijuana abusers (drug × group interaction effect). Post hoc t tests were done to determine the direction of the findings. For comparison purposes, we averaged the behavioral and cardiovascular measures obtained during placebo and compared them against the averaged measures during MP. For the behavioral measures, we also compared the “peak scores” between controls and marijuana abusers.
Personality Measures and Dependency Questionnaires.
Participants completed the Multidimensional Personality Questionnaire, which provides ratings for three main factors: positive emotionality, negative emotionality, and constraint (35). Positive emotionality is a combination of scores for well-being (reward sensitivity), social potency, achievement (motivation), and social closeness; negative emotionality is a combination of scores for stress reaction, alienation, and aggression; and constraint is a combination of scores for self-control, harm avoidance, and traditionalism. The marijuana abusers also completed the Marijuana Dependency Questionnaire, which scores seven symptoms of dependence, each on a range from 0 to 3. These questionnaires were obtained at the time of screening.
PET Scans.
Studies were done with a Siemens HR+ tomograph (resolution: 4.5 × 4.5 × 4.5 mm FWHM) in 3D mode. Each subject underwent two [11C]raclopride scans done on two separate days. Five minutes before injection of [11C]raclopride, subjects were i.v. injected with placebo (3 mL of saline) on one day and with MP (0.5 mg/kg) on another day. The order of administration was randomized. The study was a single-blind design (subjects were blinded to the drugs received). Dynamic scans were started immediately after injection of 4–10 mCi of [11C]raclopride (specific activity of 0.5–1.5 Ci/μM at the end of bombardment) for a total of 60 min using previously published procedures (36). Blood sampling was obtained from a catheter placed in the radial artery, which was used to measure the concentration of the nonmetabolized radiotracer in plasma. During the scanning period, subjects remained in a supine position with their eyes open in a darkly lit room and noise was kept to a minimum, except for the periodic assessment of drug effects.
Analysis.
The [11C]raclopride images were transformed to DV images and to BPND images. These images were analyzed using statistical parametric mapping (SPM8; Wellcome Trust Centre for Neuroimaging), which enabled us to make comparisons on a voxel-by-voxel basis (37). We estimated the DV for each voxel, which corresponds to the equilibrium measurement of the ratio of the radiotracer’s tissue concentration to that of its plasma concentration using a graphical analysis technique for reversible systems (38). A custom Montreal Neurological Institute (MNI) template, which we previously developed using the DV images from 34 healthy subjects acquired with [11C]raclopride and the same scanning sequence (39), was used for the spatial normalization of the DV images. For the BPND images, we normalized the DV in each voxel to that of the DV in cerebellum [left and right regions of interest (ROIs)], which corresponds to BPND. The DV and BPND images were then spatially smoothed using an 8-mm Gaussian kernel to minimize the variability of the brain anatomy across subjects.
Statistical group analyses were based on a factorial repeated ANOVA SPM8 model with two groups (marijuana abusers and controls) and two conditions (placebo and MP) and a covariate [parts per million levels of carbon monoxide (CO), a marker for tobacco smoking (40)]. We used CO as a covariate because the groups differed in smoking prevalence (Table 1). A mask of a priori selected regions (dorsal and ventral striatum) and those from thalamus and midbrain (including the subthalamic nucleus) was created using the digital anatomical brain atlases provided with the MRIcro software (www.cabiatl.com/mricro/). Specifically, the voxels corresponding to striatum (caudate, putamen, and ventral striatum) and thalamus were defined in the MNI stereotactic space using the Automated Anatomical Labeling atlas (41); thresholding, the simplest method of image segmentation, was used to identify midbrain voxels on a T1-weighted image (ch2.img; an average of 27 T1-weighted scans of the same individual that is included in the MRIcro template folder). The statistical significance of group differences on MP-induced changes in DV and in BPND within the mask of a priori selected regions (striatum and thalamus) was set by a voxel-level threshold PFWE < 0.05, corrected for multiple comparisons with the family-wise error (FWE). For the midbrain region, significance was set by a voxel-level threshold PFWE < 0.05, corrected for multiple comparisons with the FWE and small volume correction (SVC) (FWE-SVC; 10-mm diameter spherical searching volume).
Independent ROIs were computed to corroborate the SPM findings using procedures previously described (27). These ROIs were also used to assess the correlation between MP-induced changes on DV and on BPND, with the clinical characteristics (personality measures, dependency scores in marijuana abusers, and drug history) and the craving responses triggered by MP.
Results
Participant Characteristics.
Tobacco smoking was more prevalent in marijuana abusers than controls; otherwise, there were no differences in demographics between groups (Table 1). However, the groups differed significantly in personality measures; marijuana abusers had significantly lower scores in positive emotionality (P = 0.05) and higher scores in negative emotionality (P = 0.002) than controls (Table 1).
Correlation analysis between scores in negative emotionality and history of marijuana abuse showed a negative correlation between age of initiation of marijuana abuse and negative emotionality scores (r = 0.58, P = 0.003) such that the younger the initiation, the higher the scores. The correlations with reported daily doses of marijuana and negative emotionality were not significant. The correlations with positive emotionality and history of marijuana abuse were not significant.
Plasma Concentrations of MP and Behavioral and Cardiovascular Effects.
MP concentrations in plasma (nanograms per milliliter) did not differ between groups at 10 min (controls, 195 ± 51; abusers, 194 ± 45), 25 min (controls, 125 ± 24; abusers, 121 ± 19), or 40 min (controls, 102 ± 25; abusers, 94 ± 15).
MP had significant behavioral effects, and these effects were attenuated in marijuana abusers compared with controls (Fig. 1A). Specifically, MP significantly increased scores on self-reports (averaged measures), and the effects differed between groups, with controls reporting a more robust “high” (drug effect: F = 92, P = 0.0001; interaction: F = 6.2, P = 0.02), “restlessness” (F = 35, P = 0.0001; interaction: F = 5.8, P = 0.02), “anxiety” (F = 7, P = 0.01; interaction: F = 5.8, P = 0.02), and “drug effects” (F = 100, P = 0.0001; interaction F = 4, P = 0.05) than marijuana abusers. Also, comparisons of “peak” behavioral effects to MP were significantly stronger for controls for high (P = 0.01), restlessness (P = 0.003), anxiety (P = 0.03), and drug effects (P = 0.02), than for the marijuana abusers. The potency of MP was also reported to be stronger by the controls than by the marijuana abusers (8.3 ± 2 vs. 5.8 ± 3; t = 3.4, P = 0.002). In marijuana abusers, MP increased self-reports of marijuana craving (Placebo: 4.0 ± 3–MP: 6.3 ± 3; P = 0.006) and tobacco craving (Placebo: 2.4 ± 2–MP: 3.8 ± 4; P = 0.05).
Fig. 1.
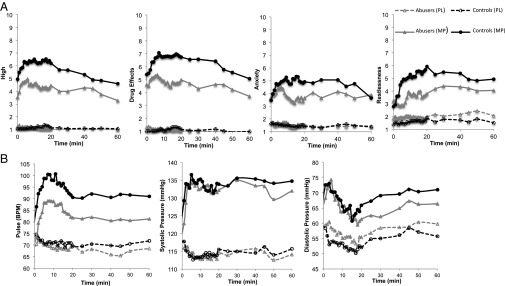
Behavioral (A) and cardiovascular (B) effects in healthy controls (black symbols) and marijuana abusers (gray symbols) after placebo (PL; dashed lines) and after MP (continuous lines). (A) MP-induced increases in self-reports of high, drug effects, anxiety, and restlessness were significantly lower for marijuana abusers than controls (P < 0.05). (B) MP-induced increases in heart rate and diastolic blood pressure were lower for marijuana abusers than controls (P < 0.05). BPM, beats per minute.
MP increased heart rate (F = 98, P = 0.0001) and systolic (F = 153, P = 0.0001) and diastolic (F = 65, P = 0.0001) blood pressure in both groups, and MP’s effects differed between groups for heart rate (interaction effect; F = 4.6, P = 0.04) and diastolic blood pressure (interaction effect: F = 4.0, P = 0.05), but not for systolic blood pressure (Fig. 1B). Post hoc t tests revealed that MP-induced increases in heart rate and diastolic pressure were significantly stronger (P < 0.05) in controls than in marijuana abusers.
Effects of MP on the DVs of [11C]Raclopride.
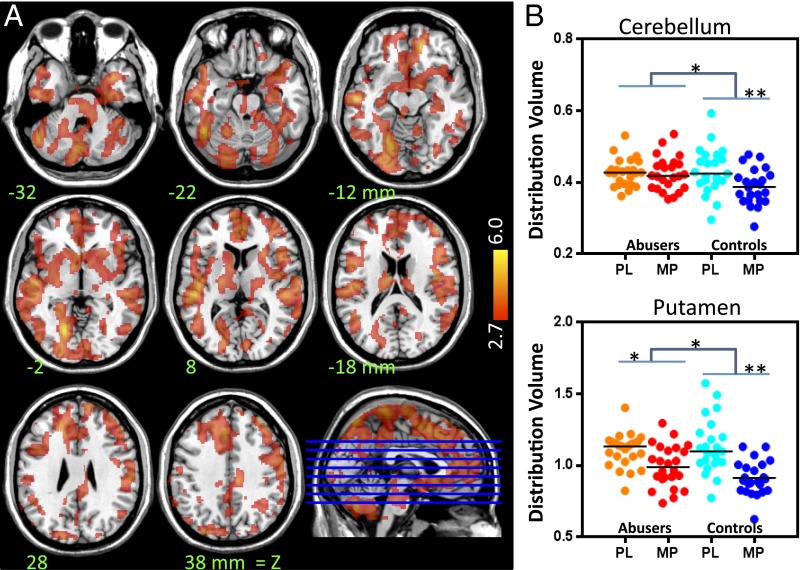
The SPM analysis showed no group differences in baseline measures of DV. It also showed that MP significantly decreased DV in brain and that the effects were significantly larger in controls than in marijuana abusers (Fig. 2). Individual plotting of MP-induced changes in DV showed that MP-induced changes in cerebellum were decreased in controls but not in marijuana abusers and that there were larger decreases of MP-induced changes in striatum in controls than in marijuana abusers (Fig. 2).
Fig. 2.
(A) SPM results for the comparison of MP’s effects on DVs (delta measures) between controls and marijuana abusers. The figure shows the contrast controls > abusers, indicating stronger MP-induced decreases in DV in controls (P < 0.005), and color bars indicate t scores. There were no regions where marijuana abusers showed greater decreases than controls. (B) Individual DV values in cerebellum and putamen after placebo (PL) and after MP for the marijuana abusers and the controls. *P < 0.05; **P < 0.005.
The ROI analysis corroborated that MP decreased the DV in cerebellum and striatum and that the effects were larger for controls than abusers. For cerebellum, the drug (F = 15, P = 0.0004) and drug × group interaction (F = 8.2, P = 0.007) were significant; post hoc t tests showed larger decreases in controls (13 ± 11%) than abusers (1.4 ± 16%) (P = 0.01). For caudate, the drug (F = 41, P = 0.0001) and interaction (F = 4.8, P = 0.04) were significant; post hoc t tests revealed larger decreases in controls (22 ± 18%) than abusers (9 ± 22%) (P = 0.05). For putamen, drug (F = 93, P = 0.0001) and interaction (F = 6.9, P = 0.02) were significant; post hoc t tests showed larger decreases in controls (30 ± 16) than abusers (16 ± 21%) (P = 0.02). For ventral striatum, drug (F = 56, P = 0.0001) and interaction (F = 7.3, P = 0.01) were significant; post hoc t tests showed greater decreases in controls (25 ± 18%) than abusers (11 ± 25%) (P = 0.02). A group (controls vs. abusers) by region (delta DV in caudate, putamen, ventral striatum, and cerebellum) comparison revealed that group differences differed between regions (F = 3.5, P = 0.02); post hoc analysis showed that group differences in cerebellum were larger than in putamen (P = 0.02) and ventral striatum (P = 0.02), and showed a trend in caudate (P = 0.07). This finding is significant; it confounds group comparisons of BPND because the latter measure is normalized to the DV in cerebellum. Note that attenuated decreases in cerebellar DV with MP in the marijuana abusers could result in an overestimation of their DA increases, reflecting an apparent lower striatal-DV/cerebellar-DV ratio (BPND) with MP (see below).
Correlations Between MP-Induced Changes in DV and Clinical Measures.
Correlation analysis revealed that MP-induced decreases in DV in ventral striatum were negatively associated with scores in negative emotionality (r = 0.51, P = 0004), and weaker correlations were observed in putamen (r = 0.37, P = 0.02) and caudate (r = 0.35, P = 0.02) such that the larger the DV decreases, the lower were the scores of negative emotionality. Correlation with positive emotionality and constraint were not significant.
MP-induced craving for marijuana in the marijuana abusers was negatively associated with DV decreases in putamen (r = 0.46, P = 0.03) and ventral striatum (r = 0.51, P = 0.01) such that participants with the smallest decreases had the most intense craving.
Baseline Measures of D2/D3 Receptor Availability (BPND).
For the baseline (placebo) measures, the SPM analysis revealed no group differences in BPND (D2/D3 receptor availability). When we decreased the threshold of significance to uncorrected P < 0.05, SPM showed lower values in marijuana abusers than in controls in ventral striatum (0, −2, −8; statistical t values = 2.59, P uncorrected = 0.007).
The ROI analysis also showed a nonsignificant trend toward lower baseline BPND in marijuana abusers than in controls in ventral striatum (controls, 3.20 ± 0.3; abusers, 2.97 ± 0.59; P = 0.11) and no differences in caudate (controls, 2.80 ± 0.36; abusers 2.76 ± 0.57) or putamen (controls, 3.42 ± 0.41; abusers, 3.35 ± 0.57).
Effects of MP on BPND.
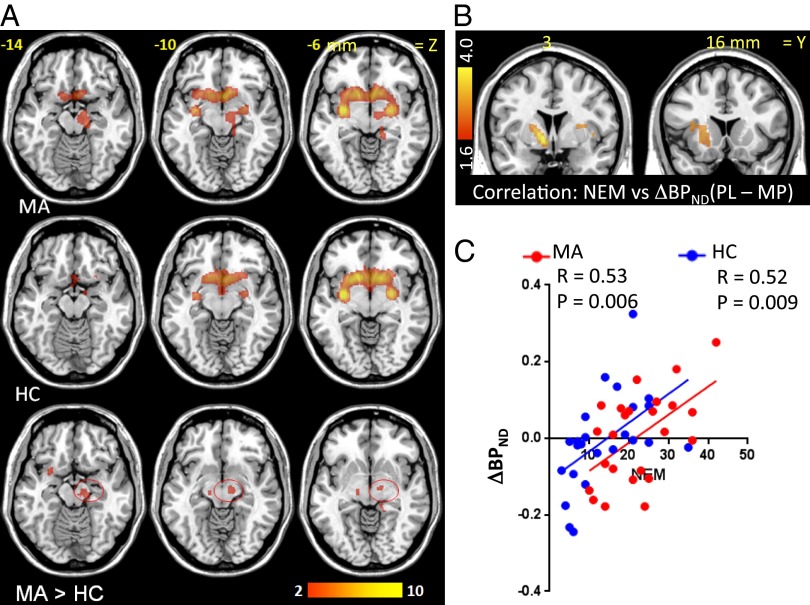
The SPM analysis revealed significant decreases in BPND with MP compared with placebo (interpreted as reflecting DA increases) in striatum in both controls and marijuana abusers (Fig. 3 and Table 2). The SPM analysis revealed no group differences in MP-induced decreases in BPND in striatum but unexpectedly revealed larger BPND decreases in marijuana abusers than in controls in midbrain (region centered in susbtantia nigra that also encompassed subthalamic nucleus; center of cluster left: 12, −14, −10, and 132 voxels, t = 3.1; center of cluster right: 14, −18, −8, and 27 voxels; t = 2.9; PFWE < 0.05; SVC = 10 voxels) (Fig. 3 and Table 2).
Fig. 3.
(A) SPM results for the comparison of MP vs. placebo on the BPND images from [11C]raclopride in marijuana abusers (MA) and in healthy controls (HC) (Puncorr < 0.005) and group comparisons for the effects of MP (ΔBPND) (P < 0.01, cluster size of 10 voxels). The contrast MA > HC indicates that MP induced greater decreases in BPND in midbrain in marijuana abusers (red circles), and color bars indicate t scores. There were no regions where MP decreased BPND more in controls than marijuana abusers. (B) SPM results for the voxel-wise correlation between MP-induced decreases in BPND (ΔBPND) and scores in negative emotionality (NEM). (C) Regression slopes for the correlation between MP-induced changes in BPND (ΔBPND) in the ventral striatum and NEM in healthy controls (blue) and in marijuana abusers (red). The larger the decreases in BPND, the lower were the scores in NEM.
Table 2.
Statistical information for clusters showing significant changes for BPND in marijuana abusers and in healthy controls for the contrast placebo BPND > MP BPND, and for clusters showing significant differences for ΔBPND for the contrast marijuana abusers > controls (A > C)
| Brain region | MNI coordinates, mm | Cluster size (k) # voxels | Abusers, t score |
Controls, t score |
A > C, t score |
||
| x | y | z | |||||
| Placebo BPND > MP BPND | |||||||
| Putamen | 30 | −6 | 0 | 930 | 11.7 | 14.4 | |
| Ventral striatum | 14 | 14 | −6 | 7.3 | 9.3 | ||
| Globus pallidum | −24 | −4 | 0 | 818 | 10.2 | 12.3 | |
| Ventral striatum | −4 | 12 | −8 | 7.8 | 7.2 | ||
| Caudate | −14 | 20 | 4 | 6.6 | 5.9 | ||
| Abusers ΔBPND > Controls ΔBPND | |||||||
| Left midbrain | −12 | −14 | −10 | 132 | 3.5 | NS | 3.1 |
| Right midbrain | 14 | −18 | −8 | 27 | NS | −3.4 | 2.9 |
Statistical threshold for comparisons of placebo > MP: t score = 5 (PFWE < 0.05); statistical threshold for comparisons abusers > controls: t score = 2.4 (P < 0.01, uncorrected; 10 voxels).
The ROI analysis corroborated a significant group × drug interaction in midbrain (F = 14, P = 0.0006), and post hoc t test analyses showed that whereas in marijuana abusers, MP decreased BPND in midbrain (−3.5 ± 8%; F = 5.4, P = 0.03), MP increased BPND in controls (4 ± 6%; F = 9.2, P = 0.006).
Correlations Between MP-Induced Changes on BPND and Clinical Measures.
Voxel-wise correlation analysis revealed that MP-induced decreases in BPND in ventral striatum were inversely associated with scores in negative emotionality (Fig. 3 B and C) such that the larger the BPND decreases, the lower the scores. The striatal correlations with positive emotionality and constraint were not significant.
Because the SPM revealed a significant group difference in MP-induced changes in midbrain BPND, we also performed correlations with this brain region and showed a significant correlation with positive emotionality (r = 0.42, P = 0.003) such that the greater the BPND decreases, the lower the scores. In the marijuana abusers, MP-induced decreases in BPND in midbrain were correlated with increases in marijuana (r = 0.40, P = 0.05) and tobacco (r = 0.45, P = 0.03) craving, as well as with the dependency scores (r = 0.43, P = 0.04), such that the greater the decreases in BPND, the higher was the craving triggered by MP and the higher were the dependency scores.
Discussion
Here, we show that marijuana abusers had attenuated behavioral and cardiovascular responses and blunted reductions in striatal DV (although normal reductions in BPND) when challenged with MP compared with controls, which is consistent with decreased brain reactivity to DA stimulation. We also corroborate prior findings (14–16) of no significant differences in baseline striatal D2/D3 receptor availability between controls and marijuana abusers and provide preliminary evidence of abnormal midbrain DA reactivity in marijuana abusers.
DA D2/D3 Receptor Availability in Striatum.
Only four brain imaging studies (totaling 42 marijuana abusers) have measured DA D2/D3 receptors (14–16, 42). These studies showed no differences in striatal D2/D3 receptors between marijuana abusers and controls, but their generalizability is limited by the small sample sizes (samples ranged from n = 6 to n = 16). Thus, our results showing no differences in D2/D3 receptor availability (except for a trend in ventral striatum), using a larger sample (24 marijuana abusers) than that used for studies that identified reductions in striatal D2/D3 receptors in alcoholics and cocaine abusers, indicate that marijuana abusers, different from other drug abusers, do not show significant striatal D2/D3 receptor reductions. This difference could reflect marijuana’s agonist properties at cannabinoid 1 (CB1) receptors, which heteromerize with D2 receptors, antagonizing their effects (43). Both CB1 and D2 receptors couple to Gi-o proteins and inhibit adenylyl-cyclase, whereas their costimulation results in Gs protein-dependent activation of adenylyl-cyclase (44, 45). Moreover, CB1 receptor agonists and antagonists counteract and potentiate, respectively, D2 receptor agonist effects (46–49), although D2 and CB1 receptor interactions might differ between rodents and primates (50, 51). It is therefore possible that in marijuana abusers, chronic CB1 receptor stimulation prevented the striatal D2/D3 receptor down-regulation observed with repeated drug use (reviewed in ref. 6). However, it should be noted that the marijuana abusers studied in the present and prior studies have been at least 10 y younger than the cocaine abusers and alcoholics studied by prior PET studies, which is relevant because striatal D2/D3 receptors decrease with age (52), and it is hypothesized that drugs accelerate the effects of brain aging (53). Thus, studies in older marijuana abusers are needed to clarify this.
MP-Induced Changes in DV.
In controls but not in marijuana abusers, MP reduced cerebellar DV. To ensure that the DV responses in the controls were consistent with prior findings, we performed a secondary analysis on the effects of MP on the cerebellar DV in an independent cohort of controls, which showed a 12% reduction, and in a sample of adults with attention deficit hyperactivity disorder (ADHD), which also showed an 11% reduction (for controls of the current cohort, the cerebellar DV decrease was 13 ± 11%). The mechanism underlying the lack of an effect of MP in cerebellar DV in abusers is unclear but could reflect the effects of chronic marijuana on cerebrovascular reactivity (increased cerebral vascular resistance) (23–25), which might have prevented MP-induced vasoconstriction and associated reductions in radiotracer delivery to the brain. The attenuated decreases in DV with MP in the marijuana abusers were observed throughout the brain but were most accentuated in cerebellum. The higher sensitivity of the cerebellum to what we interpret to reflect changes in vascular reactivity with marijuana abuse is consistent with clinical findings that report strokes associated with marijuana abuse are more frequently localized in the posterior circulation and ischemia is most frequently observed in cerebellum (25, 54–56). Cerebellar arteries express CB1 receptors in the smooth muscle layer (57), but because comparisons with arteries in other brain regions have not been done, it is not possible to determine if higher levels of CB1 receptors in cerebellar arteries underlie their higher sensitivity to vascular effects from marijuana.
However, CB1 receptors in cerebellum are also expressed in neurons and glia (58), and the cerebellum is a region that is affected in marijuana abusers (59–61); thus, we cannot rule out the possibility that other factors contribute to the lack of an effect of MP on the cerebellar DV in the marijuana abusers.
MP also decreased the DV in striatum to a greater extent in controls than in abusers (Fig. 2). In ventral striatum, these decreases were associated with negative emotionality and with marijuana craving such that the lower the response, the higher the negative emotionality and the craving. This would suggest that these attenuated responses might reflect reduced striatal DA reactivity in marijuana abusers compared with controls even though there were no group differences in MP-induced decreases in BPND (see below). This is consistent with findings from an imaging study with [18F]-dopa that reported lower than normal DA synthesis capacity in the striatum of marijuana abusers (62).
MP-Induced Changes in BPND.
We showed no group differences in MP-induced changes in BPND in striatum, which is the standard measure for assessing DA changes. Similarly, a prior study reported no differences in amphetamine-induced decreases in BPND between marijuana abusers and controls (16). However, the significant group differences in MP’s effects on the DV in cerebellum confound the findings because BPND uses the cerebellum as a reference region to normalize for nonspecific binding. Because the DV in cerebellum was not decreased by MP in marijuana abusers but was decreased in controls, this would result in an overestimation of the decrease in BPND with MP (cerebellar denominator would have a relatively larger value) and an overestimation of DA increases in marijuana abusers compared with controls.
Interestingly, an imaging study comparing DA increases using BPND and 4-propyl-9-hydroxynaphthoxazine ([11C]PHNO) (radiotracer with >20-fold higher affinity for D3 over D2 receptors, and presumably more sensitive to competition with endogenous DA) (63, 64) in response to a stressor in individuals at high risk for schizophrenia showed that those who abused marijuana had a blunted response, consistent with decreased DA signaling (22). Because the study used cognitive stress as a challenge, it was not confounded by potential group differences in stimulant-induced changes in cerebellar radiotracer delivery.
Unexpectedly, SPM revealed that MP decreased BPND in midbrain (centered in substantia nigra) in marijuana abusers but not in controls. Although the mechanism(s) underlying this group difference is unclear, we speculate that because the midbrain has a high concentration of D3 receptors (65), which are more sensitive to endogenous DA than D2 receptors (66), it could reflect up-regulation of D3 receptors in marijuana abusers. Indeed, in rodents, chronic Δ (9)-tetrahydrocannabinol (THC; the main psychoactive ingredient of marijuana) increased D3 receptors in midbrain (30). In the marijuana abusers, an MP-induced decrease in midbrain BPND correlated with craving and with dependency scores. A similar finding was reported in methamphetamine abusers, in whom up-regulation of D3 receptors in midbrain (assessed with [11C]PHNO) correlated with amphetamine-induced craving (30, 67). This, along with preclinical studies showing that D3 receptor antagonists interfere with drug seeking and cue- and stress-induced reinstatement (68), suggest that up-regulated D3 receptor signaling in midbrain might contribute to drug craving and to decreased sensitivity to reward in marijuana abusers (see below). However, because the midbrain finding was unexpected, we report it as a preliminary finding in need of replication.
Blunted Behavioral and Cardiovascular Responses to MP in Marijuana Abusers.
Behavioral and cardiovascular effects of MP have been associated with MP-induced DA increases in striatum (9, 69), so the blunted responses in the marijuana abusers are also consistent with decreased striatal reactivity to DA signaling. Although, to our knowledge, this is the first clinical report of an attenuation of the effects of MP in marijuana abusers, a preclinical study had reported that rats treated chronically with THC exhibited attenuated locomotor responses to amphetamine (2.5 mg/kg administered i.p.) (30). Such blunted responses to MP could reflect neuroadaptations from repeated marijuana abuse, such as down-regulation of DA transporters (70). The attenuation of MP’s effects could also reflect abnormal D2 receptor function, as was previously suggested to explain findings in marijuana-abusing schizophrenic patients, who, despite displaying low DA release, showed increases in psychotic symptoms when challenged with amphetamine (21). Finally, it is also possible that the attenuated responses reflect blunting of MP’s noradrenergic effects because MP blocks both DA and norepinephrine transporters.
Our findings of blunted responses to MP in marijuana abusers have clinical implications because they suggest that individuals with ADHD who abuse marijuana might be less responsive to the therapeutic benefits derived from stimulant medications.
Reduced Positive Emotionality and Increased Negative Emotionality in Marijuana Abusers.
Marijuana abusers showed lower scores on positive emotionality and higher scores on negative emotionality than controls, consistent, on the one hand, with lower reward sensitivity and motivation and, on the other hand, with increased stress reactivity and irritability. These characteristics overlap with the amotivational syndrome (71) and with the enhanced sensitivity to stress associated with marijuana abuse and other addictions (72, 73). Positive emotionality was inversely associated with MP-induced increases in midbrain DA, which could reflect the fact that in midbrain, D2 and D3 are autoreceptors; therefore, their stimulation would result in decreased DA release in striatum (including accumbens) (74), leading to decreased sensitivity to reward and amotivation (75). In contrast, MP-induced DA increases in ventral striatum were negatively associated with scores on negative emotionality, which is consistent with the protective role of DA signaling in negative emotions (76). The association between negative emotionality and age of initiation of marijuana abuse is consistent with prior findings of worse outcomes with earlier initiation of marijuana abuse (77).
Study Limitations.
The main limitation of this study was the inadequacy of BPND for comparing the DA increases between controls and marijuana abusers due to the group differences on the effects of MP on cerebellar DV. Also, [11C]raclopride cannot distinguish between D2 and D3 receptors, so studies with D3 receptor ligands are needed to determine if the increased midbrain DA response in marijuana abusers reflects D3 receptor up-regulation. The relatively poor spatial resolution of PET limits accuracy in the quantification of small brain regions, such as midbrain. Our study cannot ascertain if group differences reflect chronic use of marijuana rather than premorbid differences, and whether marijuana abusers will recover with detoxification. Although attenuation of the effects of MP could reflect interference from CB1 receptor stimulation by marijuana, this is unlikely because marijuana abusers reported that their last use of marijuana was 1–7 d before the study when cannabinoids in plasma are still detectable but at concentrations unlikely to have pharmacological effects (78). However, future studies done after longer periods of withdrawal are needed to control for potential confounds from THC and its metabolites in plasma and to determine if the blunted responses recover.
We did not obtain MRI scans on the participants. However, this is unlikely to have affected the results because measures of [11C]raclopride binding are equivalent when using a region extracted from an MRI scan or from the [11C]raclopride scan (79), and there is no evidence that marijuana abusers have striatal or cerebellar atrophy (reviewed in ref. 80). Finally, the groups differed in smoking status, but this is unlikely to account for the group differences because CO levels were used as a covariate in the analysis and there were no differences in the effects of MP between marijuana abusers who smoked cigarettes and those who did not.
Conclusions
The significantly attenuated behavioral and striatal DV response to MP in marijuana abusers compared with controls, indicates reduced brain reactivity to DA stimulation that in the ventral striatum might contribute to negative emotionality and drug craving.
Supplementary Material
Acknowledgments
We thank Lisa Muench, Colleen Shea, and Youwen Xu for radiopharmaceutical preparation and quality control, Pauline Carter and Barbara Hubbard for subject care and protocol oversight, Karen Apelskog for protocol coordination, Michael Schueller for cyclotron operations, and Ruben Baler for assistance in manuscript preparation. We also thank the subjects who volunteered to participate in this study. Research was supported by the National Institute of Health’s Intramural Research Program (National Institute on Alcohol Abuse and Alcoholism) and was carried out using the infrastructure of Brookhaven National Laboratory under Contract DE-AC02-98CH10886.
Footnotes
No author conflict of interest response is available.
References
- 1.French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226(3):159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- 2.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets WC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 8.Laruelle M, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36(7):1182–1190. [PubMed] [Google Scholar]
- 9.Volkow ND, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291(1):409–415. [PubMed] [Google Scholar]
- 10.Bossong MG, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 11.Barkus E, et al. Does intravenous Δ9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol. 2011;25(11):1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- 12.Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM. Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage. 2009;48(1):186–190. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Kuepper R, et al. Delta-9-tetrahydrocannabinol-induced dopamine release as a function of psychosis risk: 18F-fallypride positron emission tomography study. PLoS ONE. 2013;8(7):e70378. doi: 10.1371/journal.pone.0070378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrecht DS, et al. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend. 2013;128(1-2):52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes PR, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2012;26(1):144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- 16.Urban NB, et al. Dopamine release in chronic cannabis users: A [11c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71(8):677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez D, et al. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 19.Martinez D, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JL, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry. 2013;18(8):909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizrahi R, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39(6):1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducros A, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130(Pt 12):3091–3101. doi: 10.1093/brain/awm256. [DOI] [PubMed] [Google Scholar]
- 24.Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;64(3):488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- 25.Singh NN, Pan Y, Muengtaweeponsa S, Geller TJ, Cruz-Flores S. Cannabis-related stroke: Case series and review of literature. J Stroke Cerebrovasc Dis. 2012;21(7):555–560. doi: 10.1016/j.jstrokecerebrovasdis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang GJ, et al. Methylphenidate decreases regional cerebral blood flow in normal human subjects. Life Sci. 1994;54(9):PL143–PL146. doi: 10.1016/0024-3205(94)00873-6. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16(4):255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: Therapeutic implications. Synapse. 2002;43(3):181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 30.Ginovart N, et al. Chronic Δ⁹-tetrahydrocannabinol exposure induces a sensitization of dopamine D₂/₃ receptors in the mesoaccumbens and nigrostriatal systems. Neuropsychopharmacology. 2012;37(11):2355–2367. doi: 10.1038/npp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jutras-Aswad D, et al. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: Case-control study. PLoS ONE. 2012;7(6):e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GJ, et al. Behavioral and cardiovascular effects of intravenous methylphenidate in normal subjects and cocaine abusers. Eur Addict Res. 1997;3:49–54. [Google Scholar]
- 34.Srinivas NR, Hubbard JW, Quinn D, Korchinski ED, Midha KK. Extensive and enantioselective presystemic metabolism of dl-threo-methylphenidate in humans. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15(2):213–220. doi: 10.1016/0278-5846(91)90083-d. [DOI] [PubMed] [Google Scholar]
- 35.Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14(2):150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993;34(4):609–613. [PubMed] [Google Scholar]
- 37.Friston KJ, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 38.Logan J, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 39.Wang GJ, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 41.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 42.Sevy S, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197(4):549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferré S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol Pharmacol. 2005;67(5):1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 46.Andersson M, et al. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25(37):8432–8438. doi: 10.1523/JNEUROSCI.1289-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2(4):358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 48.Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp Neurol. 1997;148(1):265–270. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- 49.Marcellino D, et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54(5):815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Meschler JP, Clarkson FA, Mathews PJ, Howlett AC, Madras BK. D(2), but not D(1) dopamine receptor agonists potentiate cannabinoid-induced sedation in nonhuman primates. J Pharmacol Exp Ther. 2000;292(3):952–959. [PubMed] [Google Scholar]
- 51.Meschler JP, Conley TJ, Howlett AC. Cannabinoid and dopamine interaction in rodent brain: effects on locomotor activity. Pharmacol Biochem Behav. 2000;67(3):567–573. doi: 10.1016/s0091-3057(00)00390-7. [DOI] [PubMed] [Google Scholar]
- 52.Volkow ND, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 53.Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: A fast-track for brain ageing? Mol Psychiatry. 2013;18(2):134–135. doi: 10.1038/mp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics. 2004;113(4):e365–e370. doi: 10.1542/peds.113.4.e365. [DOI] [PubMed] [Google Scholar]
- 55.Mateo I, Infante J, Gómez Beldarrain M, García-Moncó JC. [Cannabis and cerebrovascular disease] Neurologia. 2006;21(4):204–208. Spanish. [PubMed] [Google Scholar]
- 56.Wolff V, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: A prospective study in 48 consecutive young patients. Stroke. 2011;42(6):1778–1780. doi: 10.1161/STROKEAHA.110.610915. [DOI] [PubMed] [Google Scholar]
- 57.Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cerebrovascular cannabinoid CB1 receptor protein. J Cardiovasc Pharmacol. 2004;44(5):517–519. doi: 10.1097/00005344-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez-Cueto C, et al. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br J Pharmacol. 2014;171(6):1472–1489. doi: 10.1111/bph.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sneider JT, et al. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur Neuropsychopharmacol. 2008;18(8):612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkow ND, et al. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67(1):29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- 62.Bloomfield MA, et al. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75(6):470–478. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 63.Parker C, Clarke K, Gee AD, Gee A, Rabiner E. In vitro characterisation of the high affinity D2/D3 dopamine agonist (+)PhNO. Neuroimage. 2006;31(Suppl 2):T29. [Google Scholar]
- 64.Wilson AA, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48(12):4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 65.Tziortzi AC, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. Neuroimage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 66.Sokoloff P, et al. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: Comparison with D2 receptor. Eur J Pharmacol. 1992;225(4):331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 67.Boileau I, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: A positron emission tomography study. J Neurosci. 2012;32(4):1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heidbreder CA, et al. The role of central dopamine D3 receptors in drug addiction: A review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49(1):77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volkow ND, et al. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology (Berl) 2003;166(3):264–270. doi: 10.1007/s00213-002-1340-7. [DOI] [PubMed] [Google Scholar]
- 70.Leroy C, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: A high-resolution PET study. Addict Biol. 2012;17(6):981–990. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- 71.Campbell I. The amotivational syndrome and cannabis use with emphasis on the Canadian scene. Ann N Y Acad Sci. 1976;282:33–36. doi: 10.1111/j.1749-6632.1976.tb49882.x. [DOI] [PubMed] [Google Scholar]
- 72.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyman SM, Sinha R. Stress-related factors in cannabis use and misuse: Implications for prevention and treatment. J Subst Abuse Treat. 2009;36(4):400–413. doi: 10.1016/j.jsat.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 75.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 2011;1(2):109–118. doi: 10.1002/brb3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynskey MT, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289(4):427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 78.Bergamaschi MM, et al. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin Chem. 2013;59(3):519–526. doi: 10.1373/clinchem.2012.195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang GJ, et al. MR-PET image coregistration for quantitation of striatal dopamine D2 receptors. J Comput Assist Tomogr. 1996;20(3):423–428. doi: 10.1097/00004728-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 80.Batalla A, et al. Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PLoS ONE. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]