Significance
Catecholamines and serotonin elicit arrhythmias in atrial trabeculae and arrhythmogenic diastolic Ca2+ releases in myocytes from patients with normal sinoatrial rhythm (SR). Arrhythmic events are greatly blunted in the myocardium of patients with chronic atrial fibrillation (AF). The mediation by cyclic AMP-dependent protein kinase of L-type Ca2+-current (ICa,L) responses to agonists, lusitropy, and protein phosphorylation are preserved in AF. The vanishing of agonist-evoked arrhythmias in AF is associated with the disappearance of an ICa,L response, facilitated by Ca2+/calmodulin-dependent kinase II (CaMKII), in myocytes from patients with SR, and decreased phosphorylation of phospholamban, but not ryanodine, by this enzyme. The disappearance of arrhythmias and associated reduction of CaMKII functions questions the use of CaMKII inhibitors in AF, as has been proposed by other groups.
Abstract
Atrial fibrillation (AF) is the most common heart rhythm disorder. Transient postoperative AF can be elicited by high sympathetic nervous system activity. Catecholamines and serotonin cause arrhythmias in atrial trabeculae from patients with sinus rhythm (SR), but whether these arrhythmias occur in patients with chronic AF is unknown. We compared the incidence of arrhythmic contractions caused by norepinephrine, epinephrine, serotonin, and forskolin in atrial trabeculae from patients with SR and patients with AF. In the patients with AF, arrhythmias were markedly reduced for the agonists and abolished for forskolin, whereas maximum inotropic responses were markedly blunted only for serotonin. Serotonin and forskolin produced spontaneous diastolic Ca2+ releases in atrial myocytes from the patients with SR that were abolished or reduced in myocytes from the patients with AF. For matching L-type Ca2+-current (ICa,L) responses, serotonin required and produced ∼100-fold less cAMP/PKA at the Ca2+ channel domain compared with the catecholamines and forskolin. Norepinephrine-evoked ICa,L responses were decreased by inhibition of Ca2+/calmodulin-dependent kinase II (CaMKII) in myocytes from patients with SR, but not in those from patients with AF. Agonist-evoked phosphorylation by CaMKII at phospholamban (Thr-17), but not of ryanodine2 (Ser-2814), was reduced in trabeculae from patients with AF. The decreased CaMKII activity may contribute to the blunting of agonist-evoked arrhythmias in the atrial myocardium of patients with AF.
Atrial fibrillation (AF) is the most common cardiac arrhythmia, associated with increased risk of death, congestive heart failure, and stroke (1). AF is characterized by an irregular, often rapid heart rate. Atria contract with reduced force, thereby favoring thrombus formation (2). AF occurs in several cardiac diseases, and its incidence is higher in woman than in men, particularly in those with valvular heart disease (SI Appendix, Table S1). Chronic AF causes structural and electrical remodeling, as well as enlarged atria (SI Appendix, Table S1), which in turn contributes to maintain AF.
Atrial contractility is reduced in isolated atrial tissues (3) obtained from patients with chronic AF, attributed to a marked decrease in L-type Ca2+ current (ICa,L) (3, 4). The inotropic responses to the agonist for β1-adrenergic receptors (β1ARs) and β2-adrenergic receptors (β2ARs), isoproterenol (ISO), but not the density of βARs and G proteins, are decreased in AF (3); however, whether the function of coexisting β1ARs and/or β2ARs is perturbed is unknown. Activation of human atrial β1ARs, β2ARs, and serotonin [5-hydroxytryptamine (5-HT)] 5-HT4 receptors hastens relaxation through phosphorylation of phospholamban (PLB) (5–7) by cAMP-dependent protein kinase (PKA) and Ca2+/calmodulin-dependent kinase II (CaMKII) and produces arrhythmias (8, 9) in atrial trabeculae in patients with sinus rhythm (SR). Catecholamines and 5-HT have been proposed to initiate AF (8, 9). The relevance of these in vitro arrhythmias is corroborated by the clinical finding that high sympathetic nervous system activity during and after cardiac surgery causes premature beats and transient postoperative AF in approximately one-third of patients (10).
Increased propensity to generate spontaneous impulses is assumed to initiate and/or maintain AF in humans. Arrhythmias may develop through spontaneous impulse generation within individual myocytes and/or reentry around nonexcitable tissue. The traveling electrical impulse of a premature atrial beat can encounter areas of refractoriness, return to its origin in a retrograde way, and through reentry initiate and maintain AF. Spontaneous impulse generation could be related to increased activity of PKA and/or CaMKII, with subsequent uncoordinated release of Ca2+ from the sarcoplasmic reticulum. Such a concept is attractive, because Ca2+ released from the “leaky” sarcoplasmic reticulum would activate the Na+-Ca2+ exchanger to extrude Ca2+ and to produce a arrhythmogenic depolarizing current, thereby explaining both the contractile dysfunction and the high recurrence rate (11–13).
If arrhythmias were initiated and maintained by increased activity of PKA and/or CaMKII, then interventions known to stimulate both kinases would be expected to evoke more arrhythmias in patients with AF. However, in vitro induction of arrhythmias and activation of PKA and CaMKII by catecholamines and 5-HT were not assessed in tissues from patients with AF. Thus, we compared the effects of endogenous agonists on force and arrhythmias in intact trabeculae, as well as the ICa,L, Ca2+ transients (CaTs), and diastolic Ca2+ release, in atrial myocytes obtained from patients with SR and patients with AF. Functional measurements, including relaxation, were supplemented by Western blot analysis of relevant targets of PKA and CaMKII. Chronic AF caused a marked decrease in agonist-evoked arrhythmias, associated with a decrease in some CaMKII-catalyzed functions but unchanged PKA functions.
Results
In AF Arrhythmias Elicited by Catecholamines, 5-HT, and Forskolin Are Markedly Reduced, and Inotropic Responses to 5-HT Are Selectively Depressed.
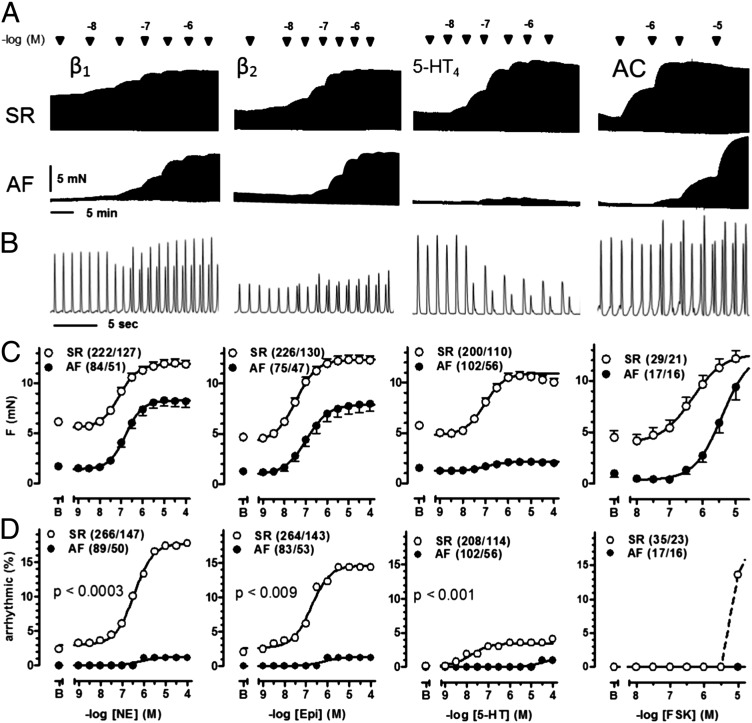
The maximum inotropic response to 5-HT, but not to norepinephrine (NE), epinephrine (EPI), or forskolin (FSK), was markedly reduced in trabeculae of patients with AF. The inotropic potencies (−log EC50 values) of the four drugs were decreased (Fig. 1 and SI Appendix, Table S2). NE, EPI, 5-HT, and FSK caused concentration-dependent arrhythmic contractions with maximum incidences of 17%, 14%, 4%, and 14%, respectively, in trabeculae of patients with SR. In trabeculae of patients with AF, the incidence of arrhythmic contractions (Fig. 1) was markedly reduced (≤2% for the agonists, abolished for FSK). Our results with 5-HT are in line with a decrease in the arrhythmogenic electrophysiological effects of 5-HT reported in atrial myocytes from patients with AF compared with patients with SR (15).
Fig. 1.
Arrhythmias, elicited by agonists and FSK, are markedly reduced and inotropic responses to 5-HT selectively depressed in AF. (A) Representative tracings of cumulative force-response curves to NE, EPI, 5-HT, and FSK, mediated through β1ARs, β2ARs, 5-HT4Rs, and AC. The AC activator FSK was used to produce receptor-independent accumulation of cAMP (14). A trabeculum of a patient with SR (Upper) and a trabeculum of a patient with AF (Lower) were set up as pairs into the same organ bath. (B) Representative arrhythmias elicited in trabeculae from patients with SR by 300 nM of each NE, EPI, 5-HT, and 10 µM FSK. (C) Inotropic data. Each data point represents mean values ± SEM. As reported previously (3), basal contractile force of atrial trabeculae was fourfold to fivefold smaller in patients with AF compared with those with SR. (D) Incidence of arrhythmias. Number of experiments is given in trabeculae/patient. P values compare arrhythmias between patients with SR and patients with AF.
Biphasic CaTs and Spontaneous Diastolic Ca2+ Releases Induced by 5-HT and FSK in Myocytes from Patients with SR Are Blunted in Patients with AF.
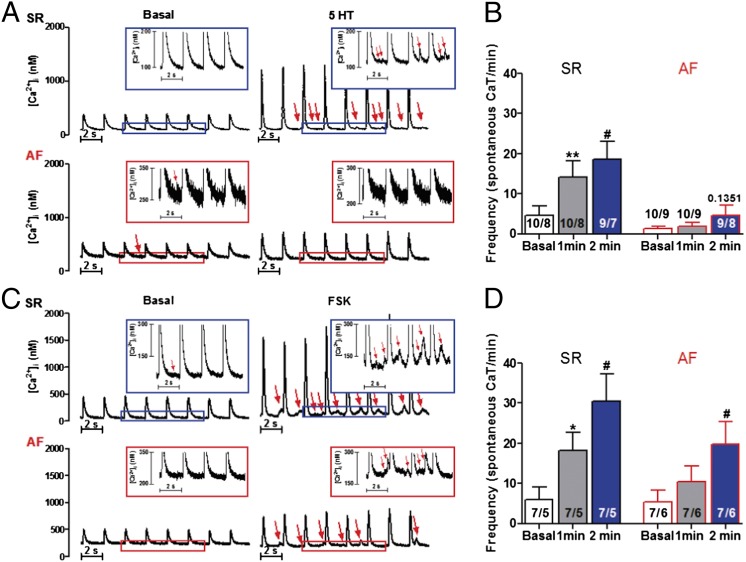
In the patients with AF, the inotropic and arrhythmic responses to 5-HT were markedly decreased, and the inotropic potency to FSK was reduced by sevenfold. FSK failed to elicit arrhythmias in AF (Fig. 1). Because ICa,L responses to 5-HT were similar to the responses of catecholamines and FSK in myocytes from patients with SR and patients with AF (see below), the blunted arrhythmias in AF suggest a decrease in the cytoplasmic arrhythmogenic Ca2+ surges in AF produced by 5-HT and FSK. CaTs in the absence of 5-HT and FSK were monophasic. In myocytes from patients with SR, both 5-HT and FSK increased CaTs, which became biphasic and often associated with spontaneous diastolic Ca2+ releases (SDCRs) (Fig. 2, and SI Appendix, Figs. S1–S3). Interestingly, ISO (1 μM) caused similar results with biphasic CaTs and SDCRs (SI Appendix, Fig. S4). In AF, the CaTs generated by 5-HT and FSK were reduced and lost their biphasic shape (SI Appendix, Figs. S1–S3). SDCRs were absent (5-HT) or reduced (FSK) in AF (Fig. 2), in line with the failure of 5-HT and FSK to elicit arrhythmias.
Fig. 2.
Effects of 5-HT and FSK on spontaneous Ca2+ release in cardiomyocytes from patients with SR and AF. (A) Examples of original CaTs traces before and after exposure to 5-HT (100 μM) in a cardiomyocyte, from a patient with SR (Upper) and a patient with AF (Lower). (Insets) Enlarged part of the traces. Arrows indicate spontaneous Ca2+ release. (B) Calculated frequency of spontaneous CaTs before and after 1 and 2 min of 5-HT application in cardiomyocytes from patients with SR and patients with AF, respectively. (C and D) Examples of original CaT traces and calculated frequency of spontaneous CaTs before and after 1 and 2 min of FSK (10 µM) application in cardiomyocytes from patients with SR and patients with AF. *P < 0.05; **P < 0.01, 1 min vs. mean basal values; #P < 0.05, 2 min vs. basal values; all paired t tests. Numbers in the columns indicate myocytes/patients.
Maximum ICa,L Responses to NE, EPI, 5-HT, and FSK Are Similarly Reduced in AF, but Control by PKA Is Preserved.
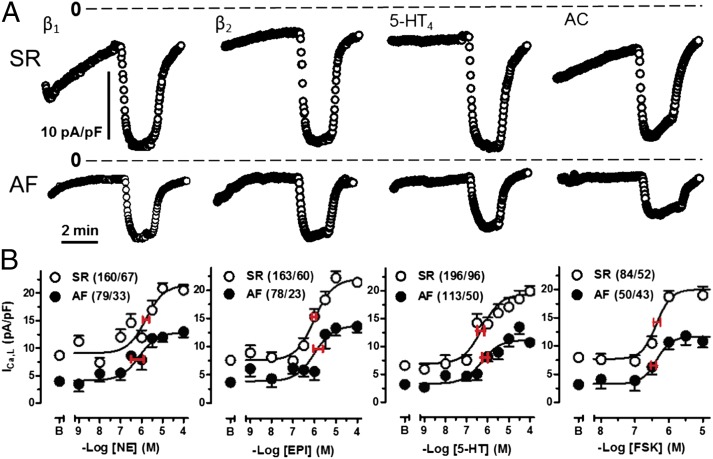
ICa,L is the initial step in electromechanical coupling, believed to contribute to increased contractile force and generation of arrhythmias (16). The maximum ICa,L responses to NE, EPI, 5-HT, and FSK were similar in patients with SR and similarly reduced by approximately one-third in patients with AF, but the potencies appeared unchanged in AF (Fig. 3 and SI Appendix, Table S3), consistent with preserved function of the receptors and adenylyl cyclase (AC). The reduced ICa,L responses in myocytes from patients with AF could be related to reduced channel density or reduced PKA-catalyzed Ca2+ channel phosphorylation and/or reduced access of cAMP to the channel domain.
Fig. 3.
Similar reductions in the ICa,L response to NE, EPI, 5-HT, and FSK in AF. (A) Representative plots for ICa,L in response to the agonists (100 µM) and FSK (10 µM) in myocytes from patients with SR or AF. Dotted line indicates 0 current level. (B) Concentration-effect curves for the agonists and FSK. Red horizontal lines through the midpoint of the curves are SEM of –logEC50s. Each data point represents mean values ± SEM in cells/patients. As reported previously (4), basal ICa,L was decreased in myocytes from patients with AF compared with patients with SR.
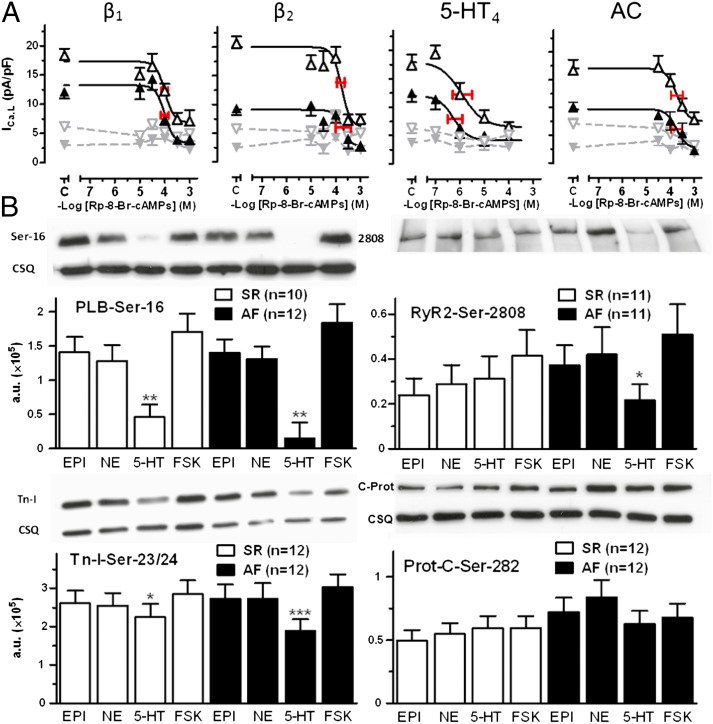
To quantitatively assess the cAMP/PKA-dependent regulation of ICa,L by the agonists and FSK, we dialyzed the cardiomyocytes with the nonhydrolyzable analog Rp-8-Br-cAMPS, which competes with cAMP for binding to the regulatory unit of PKA (17). The more cAMP produced, the greater the amount of Rp-8-Br-cAMPS needed to block PKA activation. ICa,L was not modified by Rp-8-Br-cAMPS in patients with SR or those with AF (Fig. 4A) in the absence of agonists, inconsistent with regulation of basal ICa,L by cAMP-activated PKA. Rp-8-Br-cAMPS caused concentration-dependent reductions in the ICa,L responses to the agonists. The corresponding −log IC50 values of Rp-8-Br-cAMPS were not different for the agonists and FSK in myocytes from patients with SR and from patients with AF (Fig. 4A), demonstrating that the uniformly reduced response of ICa,L to the agonists and FSK in AF is not related to reduced cAMP/PKA availability. Remarkably, however, the −log IC50 value of Rp-8-Br-cAMPS to reduce the 5-HT–evoked ICa,L response was 2 log units lower than the corresponding –log IC50 values for NE, EPI, and FSK (Fig. 4A), suggesting a 100-fold lower 5-HT–evoked cAMP production relative to ICa,L increases.
Fig. 4.
Evidence for unchanged PKA function in AF. (A) Concentration-effect curves of Rp-8-Br-cAMPS for the titration of PKA activity of ICa,L at the sarcolemmal Ca2+ channel domain under the influence of 100 µM NE, 100 µM EPI, 100 µM 5-HT, and 10 µM FSK. The midpoint and red horizontal bars in the inhibitory curves for Rp-8-Br-cAMPS represent the concentration for half-maximal inhibition (–logIC50 ± SEM) of the ICa,L responses. Open and closed symbols represent data from patients with SR and patients with AF, respectively. Gray symbols represents basal ICa,L data in the absence of agonists and FSK. Each data point represents mean ± SEM. Total number of cells/patients investigated from patients with SR vs. patients with AF were as follows: NE: 86/18 vs. 57/13; Epi: 103/24 vs. 53/12; 5-HT: 53/15 vs. 71/21; FSK: 39/8 vs. 35/8. C indicates values obtained from control experiments without Rp-8-Br-cAMPS. Note that Rp-8-Br-cAMPS was 2 log units (∼100-fold) more potent in inhibiting the ICa,L response to 5-HT compared with the responses to the catecholamines and FSK. (B) Western blots of PLB-Ser-16, RyR2-Ser-2808, Tn-I-Ser-23/24, and Prot-C-Ser-282 phosphorylation caused by agonists and FSK. Data in columns are from atria with four trabeculae of the same patient with SR (open columns) or patient with AF (black columns). *P < 0.05, **P < 0.01; ***P < 0.001 for the responses to 5-HT compared with the responses to FSK. Phosphorylation responses are shown as mean values ± SEM in arbitrary units (a.u.). CSQ, calsequestrin. NE, EPI, 5-HT, and FSK caused similar PKA-catalyzed phosphorylations in patients with SR or AF. Responses to 5-HT tended to be smaller than responses to NE, EPI, and FSK.
Lusitropy and PKA-Catalyzed Phosphorylation of Proteins by the Agonists and FSK Are Not Different Between Patients with SR and Patients with AF.
The expression of proteins involved in lusitropy, PLB, troponin-I (Tn-I), and myosin binding protein C (Prot-C), as well as SERCA2, did not differ between patients with SR and patients with AF (SI Appendix, Fig. S5). Basal phosphorylation of PLB-Ser-16 was hardly detectable in trabeculae contracting for 1 h in the organ bath used for the relaxation measurements (SI Appendix, Figs. S6 and S7), suggesting that in the absence of agonists, this protein contributes little to relaxation. In contrast, basal phosphorylation of Tn-I-Ser23/24 and Prot-C-Ser-282 was detectable under these conditions, but did not differ between patients with SR and patients with AF (SI Appendix, Figs. S6 and S7). Our data are at variance with a report of increased phosphorylation of PLB-Ser-16 and decreased phosphorylation of Prot-C-Ser-282 in atria from patients with AF, frozen in the operating theater (11). Thus, we compared the phosphorylation of PLB at Ser-16 in trabeculae frozen in the operating theater with trabeculae from the same patient that was contracted for 1 h in an organ bath. We also measured the CaMKII-catalyzed phosphorylation of PLB-Thr-17. In contrast to the trabeculae frozen in the operating theater, phosphorylation in trabeculae after contracting for 1 h in an organ bath were reduced to only marginal signals at Ser-16 and Thr-17 (SI Appendix, Fig. S8).
In contrast to a previous report (11), here the phosphorylation of PLB at Ser-16 and Thr-17 was consistently lower in trabeculae frozen in the operating theater from patients with AF than in those from patients with SR. The high basal phosphorylation levels in trabeculae frozen in the operating theater likely reflect catecholamine surges during surgery. After contracting for 1 h in the organ bath, the endogenous catecholamines dissipated from the trabeculae, as revealed by the marginal phosphorylation levels. The decreased phosphorylation of basal PLB at Ser-16 and Thr-17 in AF correspond well to the rightward shift in the concentration-effect curves for agonists and FSK in trabeculae of patients with AF (Fig. 1 and SI Appendix, Table S2). The hastening of trabecular relaxation caused by 10 µM of the agonists and FSK did not differ between patients with SR and those with AF (SI Appendix, Fig. S9). Accordingly, the agonists and FSK increased phosphorylation of PLB-Ser-16, Tn-I-Ser-23/24, and Prot-C-Ser-282 similarly in trabeculae of patients with SR and patients with AF (Fig. 4B and SI Appendix, Fig. S7). In both groups, stimulation of PLB-Ser-16 by 5-HT was less pronounced than stimulation by NE, EPI, or FSK. Our results are consistent with an early report indicating that ISO-evoked relaxation and AC stimulation do not differ between patients with SR and patients with AF (18).
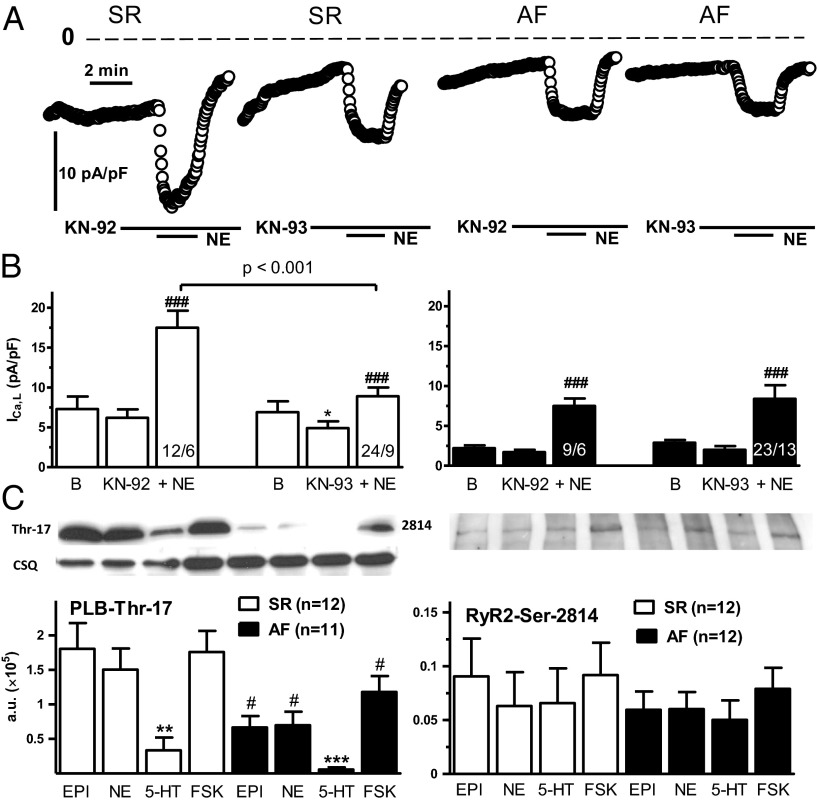
KN-93 Reduces NE-Evoked Increases of ICa,L in Patients with SR, but Not in Patients with AF.
We next compared the role of CaMKII on basal ICa,L and the activation of ICa,L by NE in myocytes from patients with SR and patients with AF. As reported previously (13, 19), the CaMKII inhibitor KN-93, but not its inactive analog KN-92, reduced basal ICa,L in myocytes from patients with SR but not in those from patients with AF. In addition, KN-93 reduced the NE-evoked ICa,L responses in myocytes from patients with SR but not in those from patients with AF, consistent with a loss in AF of the NE-facilitated phosphorylation of the L-type Ca2+ channel by CaMKII (Fig. 5 A and B).
Fig. 5.
Evidence for decreased function of CaMKII in AF. (A) Time course of ICa,L amplitude in two myocytes from patients with SR (Left) and in two myocytes from patients with AF (Right), representing the decreases in both basal ICa,L and NE-evoked response by the active CaMKII inhibitor KN-93 (20 µM) in patients with SR, but not in patients with AF. KN-92 (20 µM) was ineffective. The dotted line indicates a current level of 0. (B) Data summary from several experiments. ###P < 0.001, NE in the presence of KN-92 or KN-93, respectively. *P < 0.05, KN-93 vs. the corresponding basal. (B) Numbers of experiments are given in cells/patients. (C) Western blots of CaMKII-catalyzed phosphorylation by agonists and FSK of PLB-Thr-17 (Left) and RyR2-Ser-2814 (Right). Same design as in Fig. 4B. NE, EPI, and FSK caused similar increases of phosphorylation of PLB-Thr-17, whereas the responses to 5-HT were smaller. The phosphorylation of PLB at Thr-17 was reduced in patients with AF compared with patients with SR. **P < 0.01; ***P < 0.001, 5-HT vs. FSK. #P < 0.05, AF vs. SR; P = 0.06. AF vs. SR for 5-HT. The RyR2 phosphorylation at Ser-2814 by the agonists and FSK were not significantly different in trabeculae from patients with SR and trabeculae from patients with AF.
Reduced Agonist-Evoked Phosphorylation of PLB by CaMKII, but No Change in RyR2 Phosphorylation Catalyzed by CaMKII and PKA in AF.
NE, EPI, and FSK induced the phosphorylation of PLB at Thr-17 in contracting trabeculae of both patients with SR and patients with AF, but to a lesser degree in the latter AF (Fig. 5C and SI Appendix, Fig. S7). The effects of 5-HT were small in patients with SR, but even smaller in those with AF (Fig. 5C and SI Appendix, Fig. S7B). Increased phosphorylation of RyR2 channels at Ser-2808, catalyzed by PKA (11, 12), and Ser-2814, catalyzed by CaMKII, has been implicated in the arrhythmogenic release of Ca2+ through leaky RyR2 channels (13, 20); however, in contrast to previous reports, neither basal phosphorylation (SI Appendix, Figs. S6 and S7) nor phosphorylation stimulated by agonists of RyR2 at Ser-2808 (Fig. 4B and SI Appendix, Fig. S7A) and Ser-2814 (Fig. 5C and SI Appendix, Fig. S7B) were different between patients with SR and those with AF.
Discussion
Surprisingly, we hardly detected arrhythmic contractions in atrial trabeculae of the patients with AF. This finding is inconsistent with the idea of leaky sarcoplasmic reticulum in AF. The agonists must have produced an additional Ca2+ load, as has been reported under βAR stimulation (21). The increased Ca2+ load facilitates the release process (22), consistent with the increased CaTs observed with 5-HT, FSK, and ISO in cardiomyocytes from patients with SR. βAR stimulation increases the Ca2+ sensitivity of the RyR2 channels and reportedly increases the likelihood of RyR2 clusters opening and triggering release from neighboring clusters (22). CaTs are reportedly biphasic at room temperature (23) in the absence of agonists in human atrial myocytes. The early phase is apparently due to Ca2+-induced Ca2+ release by ICa,L from the longitudinal junctional sarcoplasmic reticulum. The late phase appears to occur through Ca2+ release from corbular reticulum, by Ca2+ released from neighboring RyR2 channels, independent of ICa,L, as suggested by recent modeling (24). In myocytes from our patients with SR, Ca2+ transients were monophasic at 37 °C; however, a late phase became conspicuous with 5-HT, FSK, and ISO at 37 °C, suggesting that these agents cause propagation of Ca2+ release from the periphery into the myocyte interior through collection of RyR2 channels, resulting in increased contractility and propensity to arrhythmias.
Arrhythmogenic, delayed afterdepolarizations (DADs) have been reported with ISO via spontaneous diastolic Ca2+ releases, with a subsequent increase in inward depolarizing current induced by the Na+-Ca2+ exchanger (25), which in turn could elicit DADs. 5-HT, FSK, and ISO caused SDCRs in myocytes of patients with SR. In patients with AF, SDCRs were absent with 5-HT and reduced with FSK, suggesting that the blunting of arrhythmias elicited by 5-HT and FSK in AF are related, at least in part, to the reduction in SDCRs.
CaMKII participates in the generation of catecholamine-evoked arrhythmias in animal models. Arrhythmogenic effects of ISO were found to be antagonized by CaMKII inhibitors in rabbit ventricle (26). Increased Ca2+ leaking and DADs have been reported in myocytes from mice overexpressing CaMKII and ISO elicited ventricular arrhythmias that were prevented by KN-93 (27). Particularly relevant to AF, KN-93 prevented ISO-evoked arrhythmic activity in rabbit pulmonary veins (28).
We have demonstrated that the agonists and FSK caused PLB phosphorylation at Thr-17 by CaMKII, conceivably activated by preceding increased cytoplasmic Ca2+ levels owing to PKA- and CaMKII-mediated increased ICa,L, as well as to Ca2+ release from the sarcoplasmic reticulum (29). SERCA, activated by the preceding PKA-catalyzed phosphorylation of PLB at Ser-16, would increase the Ca2+ load of the sarcoplasmic reticulum. SERCA’s Ca2+ pumping activity then may be further enhanced by phosphorylation of PLB at Thr-17 (29), resulting in additional Ca2+ overload of the sarcoplasmic reticulum and release and thus facilitating the appearance of SDCRs and arrhythmias observed with agonists and FSK.
Increased CaMKII-catalyzed phosphorylation of PLB and RyR2 in the absence of agonists have been reported in AF and implicated in the triggering and/or maintenance of this arrhythmia in human atrium (11, 13, 20). The reports of CaMKII-catalyzed hyperphosphorylation have suggested the therapeutic use of CaMKII inhibitors in AF (13, 30); however, our evidence indicates that CaMKII-dependent phosphorylation is either reduced or unchanged in human AF. Phosphorylation of PLB at Thr-17 in trabeculae in the operating theater (SI Appendix, Fig. S8) and in contracting atria by the agonists and FSK (Fig. 5) was reduced in AF. These findings, together with the loss of the CaMKII-facilitated component of both basal ICa,L and agonist-evoked ICa,L response, may contribute to the decrease in arrhythmias. Phosphorylation by CaMKII of RyR2 at Ser-2814 by the agonists and FSK was unchanged in patients with AF compared with patients with SR (Fig. 5), suggesting unchanged modulation of Ca2+ release through this RyR2 phosphorylation site.
The marked blunting or disappearance of atrial arrhythmias elicited by the agonists and FSK in AF also could be related in part to the more negative resting membrane potential. This hyperpolarization is caused by a twofold increase in the inward rectifying current (IK1) reported in myocytes of patients with AF compared with myocytes of patients with SR (31). Hyperpolarization in AF would reduce the likelihood of depolarizing currents reaching the threshold for arrhythmogenic depolarizations.
The maximum response of ICa,L to the catecholamines, 5-HT, and FSK was similarly reduced by one-third in the patients with AF. But although the inotropic response to the catecholamines and FSK was similar in patients with SR and those with AF, it was markedly reduced for 5-HT in patients with AF, suggesting uncoupling between the L-type Ca2+ current and contractile processes. The low 5-HT4R density compared with the density of β1AR (32) and the ∼100-fold smaller sarcolemmal cAMP/PKA signals for 5-HT compared with NE and EPI (Fig. 4A) already produce lower functional and biochemical signals in patients with SR (Figs. 4 and 5 and SI Appendix, Fig. S9). In patients with AF, the difference between reduced 5-HT signals and catecholamine signals became more pronounced (Figs. 4 and 5), possibly related to the well-known increase in atrial myocyte size in AF (3, 4). This would increase the diffusion path and dissipation of cAMP from its formation by the cell membrane-bound AC to its intracellular effectors. The especially small amounts of cAMP produced by 5-HT (Fig. 4A) would be expected to reach these effectors at even lower concentrations in myocytes from patients with AF than in myocytes from patients with SR, thereby reducing the inotropically relevant phosphorylations catalyzed by PKA.
Our evidence from human right atrium is a model for arrhythmias caused by endogenous catecholamines and 5-HT. Arrhythmias of this sort may trigger extrasystoles that could lead to paroxysmal AF or transient postoperative AF, the latter of which is often prevented by treatment with βAR blockers (10). In contrast, βAR blockers neither affect the incidence of chronic AF (33) nor convert AF into SR (34) in patients with advanced heart failure, inconsistent with a contribution of endogenous catecholamines. Our work identifies mechanisms for the vanishing of catecholamine-evoked AF in atrium that has undergone remodeling. CaMKII appears to facilitate agonist-evoked arrhythmias in atrial myocardium of patients with SR; however, once chronic AF is established, agonists fail to elicit arrhythmias, probably related to atrial remodeling, which includes decreases in CaMKII-mediated processes. Because of this pathophysiological adaptation, it appears redundant to use CaMKII inhibitors in AF, as has been proposed by others.
Materials and Methods
Right atrial appendages were collected with informed consent from patients undergoing cardiac surgery at the Department of Heart Surgery, Dresden University of Technology. These studies were approved by the Medical Faculty Ethics Committee of Dresden University of Technology (document EK790799). Patient characteristics are detailed in SI Appendix, Table S1. The methodology for experiments with atrial trabeculae, ICa,L, CaTs, and Western blots are described in SI Appendix, Materials and Methods. Data comparisons were made with ANOVA and nonpaired or paired t tests as appropriate, using GraphPad Prism and IBM SPSS 19.0.1.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Eschenhagen for reading the manuscript and Ms. Romy Kempe, Annett Opitz, Trautlinde Thurm, and Annegret Häntzschel for providing valuable technical assistance. This work was supported by the German Center of Cardiovascular Research. N.R. and U.R. received financial support from Fondation Leducq (Grant 07 CVD 03).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324132111/-/DCSupplemental.
References
- 1.Lloyd-Jones DM, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Manning WJ, et al. Impaired left atrial mechanical function after cardioversion: Relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23(7):1535–1540. doi: 10.1016/0735-1097(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 3.Schotten U, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation. 2001;103(5):691–698. doi: 10.1161/01.cir.103.5.691. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner DR, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85(5):428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 5.Molenaar P, et al. (-)-Adrenaline elicits positive inotropic, lusitropic, and biochemical effects through β2-adrenoceptors in human atrial myocardium from nonfailing and failing hearts, consistent with Gs coupling but not with Gi coupling. Naunyn Schmiedebergs Arch Pharmacol. 2007;375(1):11–28. doi: 10.1007/s00210-007-0138-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ. A 5-hydroxytryptamine receptor in human atrium. Br J Pharmacol. 1990;100(4):879–885. doi: 10.1111/j.1476-5381.1990.tb14108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gergs U, et al. Phosphorylation of phospholamban and troponin I through 5-HT4 receptors in the isolated human atrium. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):349–359. doi: 10.1007/s00210-008-0371-y. [DOI] [PubMed] [Google Scholar]
- 8.Kaumann AJ, Sanders L. Both β1- and β2-adrenoceptors mediate catecholamine-evoked arrhythmias in isolated human right atrium. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):536–540. doi: 10.1007/BF00173215. [DOI] [PubMed] [Google Scholar]
- 9.Kaumann AJ, Sanders L. 5-Hydroxytryptamine causes rate-dependent arrhythmias through 5-HT4 receptors in human atrium: Facilitation by chronic β-adrenoceptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1994;349(4):331–337. doi: 10.1007/BF00170877. [DOI] [PubMed] [Google Scholar]
- 10.Kalman JM, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60(6):1709–1715. doi: 10.1016/0003-4975(95)00718-0. [DOI] [PubMed] [Google Scholar]
- 11.El-Armouche A, et al. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114(7):670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 12.Vest JA, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111(16):2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 13.Neef S, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106(6):1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 14.Seamon KB, Padgett W, Daly JW. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pau D, Workman AJ, Kane KA, Rankin AC. Electrophysiological and arrhythmogenic effects of 5-hydroxytryptamine on human atrial cells are reduced in atrial fibrillation. J Mol Cell Cardiol. 2007;42(1):54–62. doi: 10.1016/j.yjmcc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 17.Van Haastert PJ, et al. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984;259(16):10020–10024. [PubMed] [Google Scholar]
- 18.Schotten U, et al. Atrial fibrillation-induced atrial contractile dysfunction: A tachycardiomyopathy of a different sort. Cardiovasc Res. 2002;53(1):192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 19.Christ T, et al. L-type Ca2+ current down-regulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110(17):2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 20.Voigt N, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+–Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125(17):2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain M, Orchard CH. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current, and the Ca2+ transient in rat myocytes during β-adrenergic stimulation. J Physiol. 1997;505(Pt 2):385–402. doi: 10.1111/j.1469-7793.1997.385bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogrodnik J, Niggli E. Increased Ca2+ leak and spatiotemporal coherence of Ca2+ release in cardiomyocytes during beta-adrenergic stimulation. J Physiol. 2010;588(Pt 1):225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatem SN, et al. Different compartments of sarcoplasmic reticulum participate in the excitation-contraction coupling process in human atrial myocytes. Circ Res. 1997;80(3):345–353. doi: 10.1161/01.res.80.3.345. [DOI] [PubMed] [Google Scholar]
- 24.Koivumäki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: A computational study. PLOS Comput Biol. 2011;7(1):e1001067. doi: 10.1371/journal.pcbi.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei SK, Hanlon SU, Haigney MC. Beta-adrenergic stimulation of pig myocytes with decreased cytosolic free magnesium prolongs the action potential and enhances triggered activity. J Cardiovasc Electrophysiol. 2002;13(6):587–592. doi: 10.1046/j.1540-8167.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 26.Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100(3):391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 27.Sag CM, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2(6):664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo L-W, et al. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007;571(2-3):197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 29.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: Role of CamKII. J Mol Cell Cardiol. 2010;48(2):322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokita AG, Anderson ME. New therapeutic targets in cardiology: Arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126(17):2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobrev D, et al. Molecular basis of down-regulation of G-protein–coupled inward rectifying K+ current (IK,Ach) in chronic human atrial fibrillation: Decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 32.Kaumann AJ, Lynham JA, Brown AM. Comparison of the densities of 5-HT4 receptors, β1- and β2-adrenoceptors in human atrium: Functional implications. Naunyn Schmiedebergs Arch Pharmacol. 1996;353(5):592–595. doi: 10.1007/BF00169181. [DOI] [PubMed] [Google Scholar]
- 33.Swedberg K, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: Results from COMET. Eur Heart J. 2005;26(13):1303–1308. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 34.Kao DP, et al. Effect of bucindolol on heart failure outcomes and heart rate response in patients with reduced ejection fraction heart failure and atrial fibrillation. Eur J Heart Fail. 2013;15(3):324–333. doi: 10.1093/eurjhf/hfs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.