Significance
Equine influenza viruses of the H3N8 subtype have caused outbreaks of respiratory disease in horses throughout the world since their discovery in 1963 in Florida. In 2004 an equine virus in circulation was transmitted to dogs and subsequently spread throughout the United States and to Europe. Comparative analyses of the structures of hemagglutinin glycoproteins of equine and canine viruses by X-ray crystallography locate the sites of variation on the molecules, indicate a role in determining binding specificity for an amino acid sequence difference in the receptor binding site, and describe a unique structural difference in the membrane fusion region in recent equine and canine virus HAs by comparison with all other known HAs. These differences are proposed to have facilitated cross-species transfer.
Abstract
In 2004 an hemagglutinin 3 neuraminidase 8 (H3N8) equine influenza virus was transmitted from horses to dogs in Florida and subsequently spread throughout the United States and to Europe. To understand the molecular basis of changes in the antigenicity of H3 hemagglutinins (HAs) that have occurred during virus evolution in horses, and to investigate the role of HA in the equine to canine cross-species transfer, we used X-ray crystallography to determine the structures of the HAs from two antigenically distinct equine viruses and from a canine virus. Structurally all three are very similar with the majority of amino acid sequence differences between the two equine HAs located on the virus membrane-distal molecular surface. HAs of canine viruses are distinct in containing a Trp-222→Leu substitution in the receptor binding site that influences specificity for receptor analogs. In the fusion subdomain of canine and recent equine virus HAs a unique difference is observed by comparison with all other HAs examined to date. Analyses of site-specific mutant HAs indicate that a single amino acid substitution, Thr-30→Ser, influences interactions between N-terminal and C-terminal regions of the subdomain that are important in the structural changes required for membrane fusion activity. Both structural modifications may have facilitated the transmission of H3N8 influenza from horses to dogs.
Equine influenza viruses of the hemagglutinin 3 neuraminidase 8 (H3N8) subtype were first isolated in 1963 from race horses in Miami (1). Since then they have caused numerous outbreaks of infection in horses around the world with serious disease and economic consequences (2). In 2004, again in Florida, an H3N8 virus was isolated from an outbreak of canine influenza (3) and similar viruses have since been isolated from dogs in the United States and in Europe (4, 5). Genetic comparisons indicate that the canine viruses are closely related to equine viruses that were in circulation in horses around 2000 (3, 5). In studies of differences in equine viruses isolated since 1963 (6–8) and between equine and canine viruses (3, 5), the sequences of genes for the hemagglutinin membrane glycoprotein (HA) have been compared. Sequence data for equine virus HAs indicate the evolution of four distinct lineages. The first was associated with antigenic drift, between 1963 and 1980 (6, 7, 9), and following this three separate branches formed a “Eurasian” lineage, an “American” lineage, and a divided lineage containing two clades, “Florida” clade 1 and Florida clade 2 (10, 11). The HAs of the canine viruses are most similar to those of Florida clade 1 equines. The majority of amino acid sequence changes revealed from the analyses are in the HA1 component of HA, some in regions known to be antigenically important in H3 HAs, and several near the receptor binding site (12) (Fig. 1).
Fig. 1.
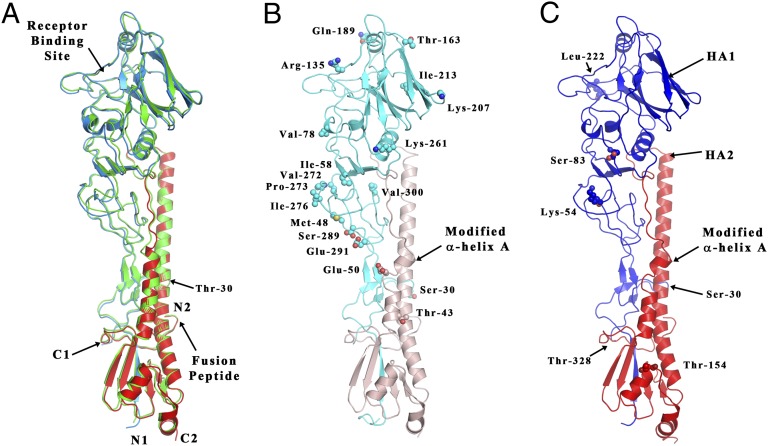
The structure of monomers of Ee, Ef, and C HAs compared with A/Duck/Ukraine/63 avian H3 HA. (A) Superposition of the A/Duck/Ukraine/63 avian H3 HA (14) (colored in green) with the Ee structure (colored in blue for the HA1 chain and in red for the HA2 chain). The position of Thr-30 of chain HA1 is also indicated. (B) Structure of the Ef HA (light shades of blue and red for the HA1 and HA2 chains, respectively). The side-chain atoms of amino acids differing from those of the Ee HA are shown as spheres. The position of Ser-30 of chain HA1 and the location of the modified HA2 α-helix are indicated. (C) Structure of the C HA (darker shades of blue and red for the HA1 and HA2 chains, respectively). The positions of the five amino acids specific to canine HAs are indicated and colored as corresponding to the chain they belong to. Also indicated is the position of HA1 Ser-30 and the location of the modified HA2 α-helix.
To understand the structural consequences of these changes, in particular those that distinguish equine from canine virus HAs, we have used X-ray crystallography to determine their structures. We have examined the HAs from two equine viruses and one canine virus: A/Equine/Newmarket/2/93, from the Eurasian lineage, “Ee”; A/Equine/Richmond/07, from Florida clade 2, “Ef”; and A/Canine/Colorado/06, “C”. Comparison of the overall structures of the three HAs with those of other H3 HAs from human and avian viruses indicates that in general they are closely similar. However, the structures of α-helix A, in the fusion subdomain (13), of the HAs of Ef and C are distinctly different from those of the HAs of all other known equine, avian, or human influenza viruses. We have defined the genetic and structural basis of the novel fusion subdomain structure by X-ray crystallography of site-specific mutant HAs and consider its possible consequences for HA stability and function in membrane fusion. Because of the importance of receptor binding by HA in virus transmission and cross-species transfer, we have used biolayer interferometry to compare the avidity and specificity of equine and canine virus binding to a range of sialoside receptor analogs. We have also used X-ray crystallography to determine the structures of equine and canine virus HAs in complex with some of these receptor analogs. From these studies we deduce the molecular basis of the observed differences in specificity and avidity and we consider their possible role in virus transmission.
Results and Discussion
Equine and Canine HA Structures.
All three structures can be seen in Fig. 1 to be very similar to each other and to other HAs of the H3 subtype described before (14). This similarity was expected from their sequence identities: Ee vs. Ef, 95%; Ef vs. C, 96%; and Ef vs. the HAs of the H3 avian and H3 human viruses, A/duck/Ukraine/63, 86%, and A/Aichi/2/68, 85%. It is also reflected in the rmsd of the α-carbon atoms shown in Table S1. Of the 20-aa sequence differences noted between Ee and Ef (Fig. 1B and Fig. S1) 19 are accessible on the surface of HA. Of these, 15 by comparison with the locations of amino acid changes in antigenic variants of human H3 HAs might result in antigenic differences (12).
In HA1, in the receptor binding site just one change, the amino acid substitution HA1 Trp-222→Leu distinguishes canine HAs from both Ee and Ef HAs. The clearest structural difference between Ee and Ef is in the fusion subdomain between HA2 residues 48 and 57. This difference is shared by Ef and C. In Ee, as in all other known HA structures, residues 48–57 are part of the five-turn α-helix A (residues 38–58), which, with the longer antiparallel α-helix B, forms a prominent α-helical hairpin in each HA subunit.
In contrast, in Ef and C HAs, α-helix A is unfolded at residues 49 and 50 and a salt bridge between group-conserved HA2 Arg-54 and conserved HA2 Glu-97 of the neighboring subunit is lost. The significance of other sequence differences in HA1 between Ef and C HAs, Asn-54→Lys, Asn-83→Ser, and Ile-328→Thr (3), is not clarified by comparison of the HA structures. Residues 83 and 54 are on the surface of HA, about 20 Å and 35 Å from the receptor binding site, respectively, toward the virus membrane. Amino acid substitutions at either position might influence HA antigenicity (Fig. 1). Residue 328 is the C terminus of HA1 (Fig. 1). The substitution Ile-328→Thr, which is conserved in canine viruses, could have been selected to ensure the required cleavage of precursor HA0 into HA1 and HA2. This could be achieved by optimizing the Gln/Glu–X–Arg− recognition site for a canine protease like tryptase Clara (15) or by increasing the ability of HA0 to compete with mucus protease inhibitors known to be present in the respiratory tract (16).
Receptor Binding Avidity and Specificity.
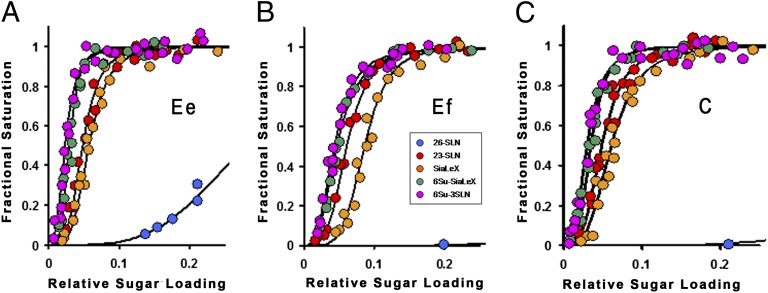
To probe the impact on receptor binding of the sequence differences between the three HAs, we determined, by biolayer interferometry, the avidity and specificity of the viruses for five different receptor analogs (Fig. 2). The strong preference observed for α2,3-linked over α2,6-linked sialosides is in agreement with previously reported results for equine viruses (17). Similarly, the greater avidities of equine viruses for analogs containing sulfated GlcNAc3 residues have been reported before (18–20). Ef virus binds 3′ sialylactosamine (3′SLN) with about 1/3rd the avidity of Ee and the other three analogs, Sialyl Lewis X (SLex), sulfated Sialyl Lewis X (Su-SLex), and Su-3′SLN, with about 1/10th the avidity of Ee (Fig. 2).
Fig. 2.
Biolayer interferometry (BLI) data for the binding of Ee, Ef, and C viruses to different receptor analogs. (A–C) Ee (A), Ef (B), and C (C) binding curves where 30-kDa polymers containing 20% mol sugar and 5% mol biotin linked to a polyacrylamide backbone were immobilized to different extents on streptavidin-coated biosensors. Data are plotted as fractional saturation of the sensor surface as a function of relative sugar loading for a fixed virus concentration of 100 pM.
In contrast, the properties of the canine virus are very similar to those of Ee, binding 3′SLN and SLex with closely similar avidity and Su-SLex and Su-3′SLN with about one-third to one-half the avidity of Ee. The difference in sequence between Ef and C HA in the receptor binding site, HA1 Trp-222→Leu, is likely to be involved in these receptor binding differences. In other respects the receptor binding sites are identical. The HAs of canine H3N2 viruses isolated in South Korea and southern China in 2005 also differed from their proposed avian HA precursor in containing the Trp-222→Leu substitution (21, 22).
H3N2 viruses generated by reverse genetics that contained either Trp-222 or Leu-222 were observed to have similarly small differences in receptor binding properties to those reported here, which led to the suggestion that this amino acid substitution could facilitate infection of dogs (23).
Structures of Equine and Canine HAs in Complex with Receptor Analogs.
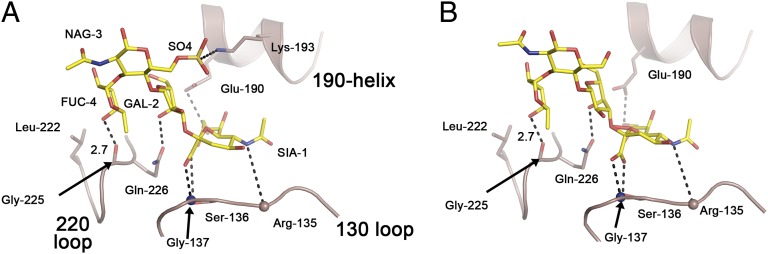
The differences detected in the structures of the complexes formed between the HAs and the receptor analogs are consistent with the results of the binding assays (Fig. 3 and Figs. S2–S4). All three HAs bind the sialosides with the Sia1–Gal2 α2,3-glycosidic linkage in trans conformation (24). Characteristically, HA1 Gln-226 interacts with α2,3-linked receptors by forming hydrogen bonds with the 4-OH group of Gal2 and the glycosidic oxygen between Sia1 and Gal2 (25). Gln-226 also assumes a slightly higher position in the complexes [up to 1.5 Å toward the 190-helix (Fig. S2)] than in unliganded HA. In the structures of C HA complexed with Su-3′SLN and Su-SLeX, ionic bonds are formed between the SO4 and the terminal amino group of Lys-193. These interactions appear to be the dominant determinants of the avidity increases observed for these analogs, as noted before for H5 HAs (26). In the complex structure formed by C HA with SLeX, the carbonyl oxygen of residue 225 forms an additional hydrogen bond with the 4-OH of GlcNAc3-linked fucose (Fig. 3). This interaction is also observed in the complex formed by Ef HA but the hydrogen bond appears longer in this case (3.1 Å instead of 2.7 Å in C HA; Fig. S4). This is probably a result of the Trp-222→Leu substitution in C vs. Ef/Ee, a probability that would be consistent with the observed importance of the size of the 222 side chain in influencing receptor binding affinity of avian HAs (19, 20). Conversely, in complexes formed by C and Ee HAs with Su-SLeX, the length of this hydrogen bond appeared very similar (2.7 Å and 2.6 Å, respectively), possibly because of the interaction of Lys-193 with the SO4 group (Fig. 3 and Fig. S4).
Fig. 3.
Receptor binding of Sialyl Lewis X (SLeX) and sulfated Sialyl Lewis X (Su-SLeX) by C HA. Su-SLeX bound to C HA (A) and SLeX bound to C HA (B). C HA HA1 main chain carbon residues are colored pink, and SLeX and Su-SLeX carbon atoms are colored yellow. Nitrogen and oxygen atoms are colored blue and red, respectively, in both balls and sticks and sphere representations. Hydrogen bonds are represented by black dashes; spheres are main chain atoms, with the exception of main chain 225 carbonyl.
The Different Structures of α-Helix A.
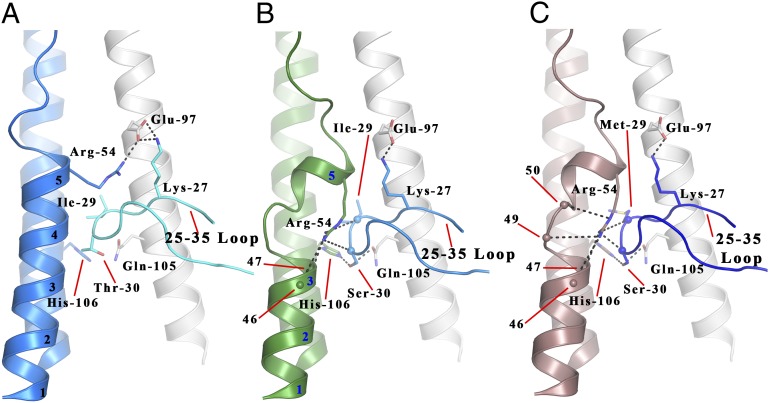
As mentioned before, the clearest structural difference between the HAs of the Ee and Ef equine viruses is in α-helix A of HA2 (Figs. 1, 4, and 5). In Ee HA, as in all human and avian H3 HAs, and probably in all equine H3 HAs of viruses isolated before 2000 (3, 5), α-helix A is five turns long (Fig. 4A). In Ef HA, in contrast, the helix is interrupted at HA2 residues 49 and 50. It is continued for one turn between residues 51 and 54 and then it terminates (Fig. 4B). HA2 residue 55 becomes the N terminus of the loop that links the C terminus of α-helix A to the N terminus of α-helix B, (residues 55–75). This structural feature of Ef HA is shared by C HA (Fig. 4C). In both cases, HA2 residues 49 and 50 are adjacent to a prominent loop of the neighboring subunit of the trimer, the 30 loop formed by HA1 residues 25–35 (Fig. 4). In this region the sequence of Ee HA differs from that of Ef and C at HA1 residue 30: threonine in Ee rather than serine in Ef and C. The HAs of all equine H3N8 viruses isolated since 2000 contained the HA1 Thr-30→Ser substitution and the HAs of all canine H3N8 viruses contained HA1 Ser-30.
Fig. 4.
The local structure formed by HA2 residues 38–55 and HA1 residues 25–35 in the Ee, Ef, and C structures. (A) Ee HA is shown with one HA2 chain colored blue and the HA1 and HA2 chains of the closest monomer colored teal and white, respectively. (B) Ef HA is shown with one HA2 chain colored green and the HA1 and HA2 chains of the closest monomer colored blue and white, respectively. (C) C HA is shown with one HA2 chain colored pink and the HA1 and HA2 chains of the closest monomer colored dark blue and white, respectively. Hydrogen bonds are represented by black dashes, and spheres represent main chain atoms. The turns of HA2 helix A are numbered and referenced.
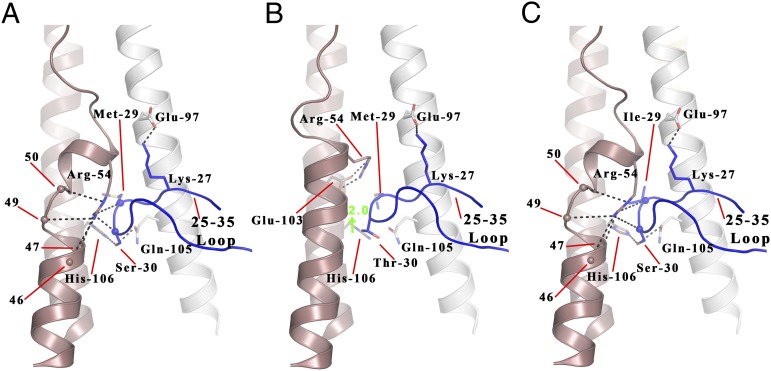
Fig. 5.
The local structure formed by HA2 residues 38–55 and HA1 residues 25–35 in the C wt, S30T, and M29I structures. (A–C) C (A), S30T mutant (B), and M29I mutant (C) HA structures for HA2 residues 38–55 and HA1 residues 25–35. C wt, M29I, and S30T mutants are shown with one HA2 chain colored pink and the HA1 and HA2 chains of the closest monomer colored dark blue and white, respectively. Hydrogen bonds are represented by black dashes, spheres represent main chain atoms. The turns of HA2 helix A are numbered and referenced.
To determine whether this sequence change is responsible for the structural difference between Ee vs. Ef and C, we analyzed the structure of a site-specific mutant C HA containing the substitution Ser-30→Thr. The results show (Fig. 5B) that in the C Ser-30→Thr mutant the α-helical structure between HA2 residues 47 and 57 is restored. In addition, HA2 Arg-54, which is conserved in all H3 HAs, is repositioned to form a salt bridge with conserved HA2 Glu-103 of the same subunit. The structural effect of the Thr-30→Ser substitution appears to be a consequence of the ability of serine to occupy the polar region formed by HA2 His-106 of α-helix B and HA2 Gln-105 of the neighboring α-helix B (Fig. 5A). Ser-30 forms hydrogen bonds with both residues. As a result, the 30 loop is lower by about 2 Å and a cavity is created that is lined by residues of α-helix A and of the 30 loop (Fig. 5A). HA2 Arg-54 occupies this cavity and interacts, through hydrogen bonds, with the main chain carbonyls of HA1 residues 29 and 30 of the neighboring 30 loop and the carbonyls of HA2 residues 46, 47, 49, and 50 of α-helix A. In contrast, in the vast majority of HAs, like Ee, that have threonine rather than serine at HA1 residue 30, the 30 loop adopts a position about 2 Å higher and the size of the cavity is reduced. In this conformation Arg-54 adopts a different rotamer that enables it to interact with Glu-97 of the neighboring subunit (Fig. 4A). We introduced the Ser-30→Thr mutation into Ef HA and observed the same recovery of α-helix A structure (Fig. S5). However, because Ef HA, like the HAs of about 10% of equine viruses isolated since 2000, contains the substitution HA2 Gly-50→Glu, the 30 loop in the Ef Ser-30→Thr mutant is restricted to a slightly lower position and HA2 Arg-54 forms a hydrogen bond with the carbonyl of HA1 29 and the side chain of Glu-50, rather than a salt bridge with HA2 Glu-97 (Fig. S5). In other respects the effects of the Ser-30→Thr substitution were similar in Ef and C site-specific mutant HAs. To complete this comparison, C HA also differs from Ef HA at HA1 residue 29, containing methionine rather than isoleucine. Our results with a Met-29→Ile site-specific mutant HA indicate that the substitution has no effect on the structure of C HA (Fig. 5C). This result was expected because Ef contains HA1 Ile-29 together with the modified α-helix A.
Potential Consequences of the Ef/C-Specific α-Helix A Structure.
Sequences of HAs from equine H3N8 viruses isolated since 1963 indicate that the Thr-30→Ser mutation was first detected in 1999 (3, 5). The change in structure of α-helix A presumably occurs coincidentally. A biological consequence of this change may be to enable transfer of the novel equine virus to dogs and between dogs. The location of HA2 residues 47–54 in the Ef and C HA structures is in a region of HA that is prominent in the structural changes in HA required for HA-mediated membrane fusion and has been highlighted in studies of mutant HAs that change structure at higher pH than wild-type HA (27, 28). Conserved HA1 Lys-27 in the 30 loop becomes susceptible to trypsin after incubation of HA at fusion pH, indicating structural rearrangement of the 30 loop (29). Group 2-conserved HA2 His-106 becomes the N terminus of a 180° turn in α-helix B, formed at fusion pH by the refolding of HA2 residues 106–111 (30). HA2 His-106 makes a hydrogen bond with conserved HA2 Lys-51 at neutral pH (31), and HA1 residues 29 and 30 around which the 30 loop turns (Fig. 4) are adjacent to HA2 residues 50 and 51 of the neighboring subunit. In addition, group 2-conserved HA2 Gln-105 makes a hydrogen bond with Ser-30 and also, via water, with conserved HA2 Asp-109, which also makes a hydrogen bond via a water molecule with the main chain amide of HA2 Leu-2, the second residue of the “fusion peptide.” Because the Thr-30→Ser substitution appears to enable these interactions, it is possible that some feature of HAs containing Ser-30 related to HA-mediated membrane fusion or its activation may be favorable for infection and transmission in dogs as well as horses.
Our analysis of the pH at which the conformational change in C and other HAs containing Ser-30 occurs (Table S2) indicates that the characteristic change in protease susceptibility of HA at fusion pH occurs 0.2 pH higher for these HAs than for Ee HA. Because fusion by Ee HA occurs at pH 5.0, which is lower than the pH at which other H3 HAs have generally been observed to fuse membranes (28), the slightly higher pH of fusion of viruses with HA1 Ser-30 is consistent with an effect of the Thr-30→Ser substitution to decrease HA stability and facilitate HA-mediated membrane fusion.
Conclusion
For the five mutations that distinguish the HAs of canine from HAs of equine H3N8 viruses (Fig. 1C) our structural and receptor binding analyses primarily provide support for a role of the Trp-222→Leu mutation in virus transmission in canines. This conclusion gains support from the established importance of residues in the 220 loop in determining receptor specificity, in particular HA1 residues 225 and 226 (32, 33). The difference in receptor binding specificity with which the substitution is associated may be a reflection of the relative abundance of sialosides on the surfaces of canine cells that are targets for influenza infection but as yet such studies have not been reported. The other mutation, for which a role in transmission can be suggested, is at the C terminus of HA1, which may be involved in recognition by a protease used to cleave the HA0 precursor in infected dogs. There is precedence for the probable involvement of such a protease in human influenza, from studies of the specificity of tryptase Clara (15). For two of the three remaining mutations in HA1, we can deduce no structural consequences. This is also the case for the single substitution in HA2, Asn-154→Thr, which results in the loss of an otherwise strictly conserved carbohydrate side chain. A role for this side chain in maintenance of HA structure or chaperone recognition is therefore not necessary for canine influenza replication. However, a role for HA1 Ser-30 in modulating HA stability or in membrane fusion activity or activation is a distinct possibility. This also leads to the suggestion that a combination of mutations that involve changes in receptor binding, HA1 Leu-222, and membrane fusion properties, HA1 Ser-30, may be necessary for equine to canine cross-species infection. In this regard, it is noteworthy that the HA of an H5N1 aerosol-transmissible mutant also had mutations that modified its receptor binding specificity and affinity, its stability, and probably its membrane fusion activity (34, 35). Our studies of the structure of the aerosol-transmissible H5 mutant HA (36) indicate that the HA1 Thr-318→Ile substitution gains increased stability for the aerosol-transmissible mutant HA through hydrophobic interactions between HA1 Ile-318 and α-helix A residues HA2 Val-48 and Val-52. The results of both studies indicate the region in which HA1 Ser-30 of canine and recent equine HAs interacts with HA2 residues of α-helix A and α-helix B is an important contributor to the structure and stability of H3 hemagglutinins.
Materials and Methods
Influenza Viruses.
Wild-type influenza viruses A/Equine/Newmarket/2/93, Ee; A/Equine/Richmond/1/07, Ef; and A/Canine/Colorado/17864/06, C were grown in hens’ eggs and purified by sucrose density gradient centrifugation, according to standard protocols (37).
Biolayer Interferometry.
Virus binding to receptor analogs was measured on an Octet RED instrument (ForteBio) as previously described (38). Biotinylated 3′SLN (Neu5Acα2–3Galβ1–4GlcNAcβ) and 6′SLN (Neu5Acα2–6Galβ1–4GlcNAc) were purchased from Lectinity Holdings. Su-3′SLN [Neu5Acα2–3Galβ1–4(6-HSO3)GlcNAcβ], Su-SLeX [Neu5Acα2–3Galβ1–4(Fucα1–3)-(6-HSO3)GlcNAcβ] and SLeX [Neu5Acα2–3Galβ1–4(Fucα1–3)GlcNAcβ] were obtained from N. Boivin at the Shemyakin Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow. Binding of viruses (100 pM) was measured at 25 °C in a 30- to 50-min association step. All solutions also included 10 µM oseltamivir carboxylate (Roche) and 10 µM zanamivir (GSK) to prevent cleavage of the sugar analogs by viral neuraminidase. The (relative) amount of virus bound to the biosensor at different relative sugar loadings was calculated from the amplitude of the response at the end of the association step.
Expression and Purification of HAs.
HAs for wild-type A/Equine/Newmarket/2/93, A/Equine/Richmond/1/07, and A/Canine/Colorado/17864/06 along with A/Equine/Richmond/1/07 mutant (S30T) and A/Canine/Colorado/17864/06 mutant (S30T) were subcloned, as previously described, into a modified pAcGP67A vector that carries a TEV protease site, a trimerization foldon domain, and a 6× His tag (38). Large-scale protein expression was performed with 2–3 L of SF9 cells. Seventy-two hours postinfection cells were removed by centrifugation, supernatant concentrated, and loaded onto a talon cobalt column (Clontech). HA fractions were pooled and buffer was exchanged by concentrating into 20 mM Tris, pH 8.0, 150 mM NaCl. Concentrated protein was digested with trypsin [10:1 HA:trypsin (wt:wt ratio); 4 °C, 16 h] to remove the foldon and the His tag. The digestion was stopped using soybean trypsin inhibitor. HAs were further purified by gel filtration chromatography, using a superdex-200 16/60 column (GE) in 20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl. HA fractions were pooled and buffer was exchanged into 10 mM Tris⋅HCl, pH 8.0, 50 mM NaCl and concentrated to 12 mg/mL for crystallization.
Crystallization of HAs.
Canine HA crystals were obtained from 29% (vol/vol) pentaerythritol propoxylate (PEP) 17/8, 0.1 M Tris, pH 8.07, 0.2 M ammonium sulfate. Canine HA–receptor complexes were prepared by soaking HA crystals in crystallization solution [35% (vol/vol) PEP 5/4, 0.1 M Hepes, 0.2 M ammonium sulfate] supplemented with 40 mM receptor analog 3′SLN and 12 mM of analogs Su-3′SLN, Su-SLeX, and SLeX. Canine S30T mutant crystals were obtained from 45% (vol/vol) PEP 17/8, 0.1 M Hepes, pH 7.5, 0.3 M potassium chloride. Canine M29I mutant crystals were obtained from 35% (vol/vol) PEP 629, 0.1 M Hepes, pH 7.5, 0.2 M ammonium sulfate.
A/Equine/Richmond/1/07 HA crystals were obtained from 12% PEG 2KMME, 0.1 M Mes, pH 5.7. Crystals were cryoprotected with crystallization solution plus 25% ethylene glycol and HA–receptor analog complexes were prepared by soaking HA crystals in cryoprotected crystallization solution supplemented with 40 mM 3′SLN and 12 mM SLeX. A/Equine/Richmond/1/07 S30T mutant was crystallized from 18% PEG 2KMME, 0.1 M Cacodylate, pH 6.5.
A/Equine/Newmarket/2/93 crystals were obtained from 30% PEG400, 0.1 M Tris⋅HCl, pH 8.0. HA–receptor complexes were obtained from soaking HA crystals in crystallization solution supplemented with 24 mM Su-3′SLN and Su-SLeX.
Structure Determination.
All crystals were frozen by direct immersion in liquid nitrogen and diffraction datasets were collected at 100 K at the IO2 and IO4 beamlines at the Diamond light source (Harwell, UK). Datasets were indexed and integrated with XDS (39) and imported in the CCP4 suite of programs (40), and structures were solved and refined using CCP4 or Phenix (41). Crystallographic statistics are summarized in Tables S3 and S4.
Tryptic Digestion After Low-pH Incubation.
HA solutions (0.15 mg/mL) were incubated at various pH values (obtained by adding 0.15 M citrate buffer, pH 3.5), adjusted to neutral pH by using 1 M Tris⋅HCl, pH 8.0, and digested with trypsin at a HA/trypsin ratio of 20:1 (wt:wt) for 30 min at room temperature. The digestion was stopped using an equal amount, to trypsin, of soybean trypsin inhibitor. Tryptic digestion products were analyzed by SDS/PAGE.
Supplementary Material
Acknowledgments
We thank Debra Elton and Jenny Mumford of the Animal Health Trust for supplying viruses A/Equine/Newmarket/2/93, A/Equine/Richmond/1/07, and A/Equine/South Africa/4/03. We thank Dr. Takashi Yamanaka of the Equine Research Institute, Japan Racing Association, for supplying A/Equine/Ibaraki/1/07. We thank Dr. Edward J. Dubovi and Dr. Colin Parrish (Cornell University) for providing A/Canine/Colorado/17864/06. We thank Nicolai Boivin at the Shemyakin Institute of Bioorganic Chemistry for supplying sulphated sialosides. We are grateful to Dr. Stephen Wharton and Dr. John McCauley, Division of Virology, Medical Research Council (MRC) National institute for Medical Research, for assistance during the project. We thank members of the National Institute for Medical Research World Health Organization Collaborating Centre for Reference and Research on Influenza for their assistance throughout the studies. We are grateful to staff at the Diamond Light Source Synchrotron for assistance and beamline access under Proposal 7707. This work was funded by the MRC through Programmes U117584222 and U117570592.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4UNW–4UNZ, 4UO0–4UO9, and 4UOA).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406606111/-/DCSupplemental.
References
- 1.Waddell GH, Teigland MB, Sigel MM. A new influenza virus associated with equine respiratory disease. J Am Vet Med Assoc. 1963;143:587–590. [PubMed] [Google Scholar]
- 2.Elton D, Bryant N. Facing the threat of equine influenza. Equine Vet J. 2011;43(3):250–258. doi: 10.1111/j.2042-3306.2010.00357.x. [DOI] [PubMed] [Google Scholar]
- 3.Crawford PC, et al. Transmission of equine influenza virus to dogs. Science. 2005;310(5747):482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- 4.Daly JM, et al. Transmission of equine influenza virus to English foxhounds. Emerg Infect Dis. 2008;14(3):461–464. doi: 10.3201/eid1403.070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payungporn S, et al. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg Infect Dis. 2008;14(6):902–908. doi: 10.3201/eid1406.071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels RS, Skehel JJ, Wiley DC. Amino acid sequences of haemagglutinins of influenza viruses of the H3 subtype isolated from horses. J Gen Virol. 1985;66(Pt 3):457–464. doi: 10.1099/0022-1317-66-3-457. [DOI] [PubMed] [Google Scholar]
- 7.Kawaoka Y, Bean WJ, Webster RG. Evolution of the hemagglutinin of equine H3 influenza viruses. Virology. 1989;169(2):283–292. doi: 10.1016/0042-6822(89)90153-0. [DOI] [PubMed] [Google Scholar]
- 8.Bryant NA, et al. Antigenic and genetic variations in European and North American equine influenza virus strains (H3N8) isolated from 2006 to 2007. Vet Microbiol. 2009;138(1–2):41–52. doi: 10.1016/j.vetmic.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw VS, et al. Analysis of antigenic variation in equine 2 influenza A viruses. Bull World Health Organ. 1983;61(1):153–158. [PMC free article] [PubMed] [Google Scholar]
- 10.Daly JM, et al. Antigenic and genetic evolution of equine H3N8 influenza A viruses. J Gen Virol. 1996;77(Pt 4):661–671. doi: 10.1099/0022-1317-77-4-661. [DOI] [PubMed] [Google Scholar]
- 11.Lai AC, et al. Diverged evolution of recent equine-2 influenza (H3N8) viruses in the Western Hemisphere. Arch Virol. 2001;146(6):1063–1074. doi: 10.1007/s007050170106. [DOI] [PubMed] [Google Scholar]
- 12.Knossow M, Skehel JJ. Variation and infectivity neutralization in influenza. Immunology. 2006;119(1):1–7. doi: 10.1111/j.1365-2567.2006.02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 14.Ha Y, Stevens DJ, Skehel JJ, Wiley DC. X-ray structure of the hemagglutinin of a potential H3 avian progenitor of the 1968 Hong Kong pandemic influenza virus. Virology. 2003;309(2):209–218. doi: 10.1016/s0042-6822(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 15.Kido H, et al. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. A possible activator of the viral fusion glycoprotein. J Biol Chem. 1992;267(19):13573–13579. [PubMed] [Google Scholar]
- 16.Kido H, et al. The human mucus protease inhibitor and its mutants are novel defensive compounds against infection with influenza A and Sendai viruses. Biopolymers. 1999;51(1):79–86. doi: 10.1002/(SICI)1097-0282(1999)51:1<79::AID-BIP9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 18.Gambaryan AS, et al. H5N1 chicken influenza viruses display a high binding affinity for Neu5Acalpha2-3Galbeta1-4(6-HSO3)GlcNAc-containing receptors. Virology. 2004;326(2):310–316. doi: 10.1016/j.virol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Gambaryan AS, et al. 6-sulfo sialyl Lewis X is the common receptor determinant recognized by H5, H6, H7 and H9 influenza viruses of terrestrial poultry. Virol J. 2008;5:85. doi: 10.1186/1743-422X-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambaryan AS, et al. Receptor-binding profiles of H7 subtype influenza viruses in different host species. J Virol. 2012;86(8):4370–4379. doi: 10.1128/JVI.06959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song D, et al. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008;14(5):741–746. doi: 10.3201/eid1405.071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect Genet Evol. 2010;10(8):1286–1288. doi: 10.1016/j.meegid.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, et al. Mutation tryptophan to leucine at position 222 of haemagglutinin could facilitate H3N2 influenza A virus infection in dogs. J Gen Virol. 2013;94(Pt 12):2599–2608. doi: 10.1099/vir.0.054692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen MB, Sabesan S, Skehel JJ, Wiley DC. Binding of the influenza A virus to cell-surface receptors: Structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography. Virology. 1997;232(1):19–31. doi: 10.1006/viro.1997.8526. [DOI] [PubMed] [Google Scholar]
- 25.Ha Y, Stevens DJ, Skehel JJ, Wiley DC. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc Natl Acad Sci USA. 2001;98(20):11181–11186. doi: 10.1073/pnas.201401198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong X, et al. Recognition of sulphated and fucosylated receptor sialosides by A/Vietnam/1194/2004 (H5N1) influenza virus. Virus Res. 2013;178(1):12–14. doi: 10.1016/j.virusres.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Thoennes S, et al. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology. 2008;370(2):403–414. doi: 10.1016/j.virol.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels RS, et al. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- 29.Skehel JJ, et al. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci USA. 1982;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 31.Russell RJ, et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology. 2004;325(2):287–296. doi: 10.1016/j.virol.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 32.Gamblin SJ, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303(5665):1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 33.Rogers GN, et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 34.Imai M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herfst S, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong X, et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature. 2013;497(7449):392–396. doi: 10.1038/nature12144. [DOI] [PubMed] [Google Scholar]
- 37.Skehel JJ, Schild GC. The polypeptide composition of influenza A viruses. Virology. 1971;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- 38.Lin YP, et al. Evolution of the receptor binding properties of the influenza A(H3N2) hemagglutinin. Proc Natl Acad Sci USA. 2012;109(52):21474–21479. doi: 10.1073/pnas.1218841110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.