Significance
EBV is a human herpesvirus that is associated with several forms of cancer. It can transform B lymphocytes into proliferating lymphoblastoid cell lines yet establishes a benign lifetime latent infection in resting memory B cells in virtually all human beings. EBV encodes for ∼40 micro-RNAs, small RNAs that modulate the activity of cellular genes. A subset of these is highly expressed in latently infected memory B cells in vivo. Here, we show that one of them, 18-5p, suppresses the expression of MAP kinase kinase kinase 2 (MAP3K2). We further show that MAP3K2, a central molecule in many cellular signaling pathways, mediates the signals that initiate viral replication. Thus, 18-5p favors latency in vivo by suppressing viral replication through reduction of MAP3K2.
Keywords: viral reactivation, EBV miRNA, MAPK pathway
Abstract
EBV is an oncogenic human herpesvirus that has the ability to infect and transform B cells latently in vitro. However, the virus also establishes a lifetime, benign, persistent latent infection in resting memory B cells in vivo, where the virus is quiescent (i.e., expresses none of the known latent proteins). The virus encodes ∼40 micro-RNAs (miRNAs), most of which are transcribed from the BamH1 fragment A rightward transcript (BART) region of the virus. We have shown previously that a subset of these miRNAs is present at high copy numbers in latently infected memory B cells in vivo, suggesting a role in maintaining latency. Here, we describe the role of one of these miRNAs, BART 18-5p. We show that it targets the 3′UTR of the mRNA, encoding the important cellular signaling molecule MAP kinase kinase kinase 2 (MAP3K2), at exactly the same site as the oncogenic cellular miRNA mir–26a-5p. To our knowledge, this is the first report of a virus encoding a miRNA that suppresses a target in the MAP kinase signaling cascade, a central signal transduction pathway that governs a broad spectrum of biological processes. We further show that MAP3K2 is an intermediary in the signaling pathways that initiate lytic viral replication. Thus, 18-5p expression in latently infected B cells has the effect of blocking viral replication. We propose that the role of 18-5p is to maintain latency by reducing the risk of fortuitous reactivation of the virus in latently infected memory B cells.
EBV is a human herpesvirus that establishes a lifetime persistent latent infection in long-lived memory B cells (1). It is a transforming virus for B cells in culture and is associated with several forms of cancer, including nasopharyngeal carcinoma, gastric carcinoma, Burkitt lymphoma, and Hodgkin disease. Like many viruses that persist in the infected host, EBV replicates and resides in different cellular compartments with either lytic or latent gene expression programs. Whereas the lytic program gives rise to the production of infectious viral particles, the latent program enables the virus to express a limited number of viral genes that maintain the viral reservoir in the face of specific immune responses. Unique for EBV is the expression of four different latency programs representing different levels of viral silencing in different stages of B-cell differentiation (2, 3). The generally accepted model is that EBV initially infects naive B cells and expresses the full set of latent proteins and RNAs known collectively as the growth program. The role of this program is to activate and drive proliferation of the B cells, which then migrate into and through the germinal center to become resting memory B cells analogous to the pathway for an antigen-activated B cell. In the emerging resting memory B cells, the virus is quiescent and expresses no latent proteins. By comparison, in vitro infection of B cells also results in long-term latent infection; however, in this case, the infection process stops at the growth program and the activated blasts continue to proliferate in culture as lymphoblastoid cell lines expressing the growth program (4).
It is believed that, in vivo, the trigger for the virus to start replicating is the differentiation of latently infected memory cells to become plasma cells (5). In vitro, the virus can be reactivated from EBV-positive cell lines by several stimuli, including phorbol esters, butyrate, and cross-linking of surface Ig. However, this process is always inefficient. Viral replication is initiated through expression of two virally encoded immediate early (IE) transcription factors: BamH1 fragment Z left reading frame 1 (BZLF1) and BamH1 fragment R left reading frame 1 (BRLF1) (4). In turn, these transcription factors activate a series of genes that are involved primarily in replicating the viral DNA, referred to as early (E) antigens. Subsequent to replication of the viral DNA, the viral structural proteins are synthesized, referred to as late (L) antigens, including the major envelope glycoprotein gp350/220 (6, 7), leading to virion assembly and release.
Recently, it has become apparent that EBV encodes for a large number of micro-RNAs (miRNAs). miRNAs are small, 19- to 25-nt long, evolutionarily conserved noncoding RNAs that act posttranscriptionally to regulate gene expression. To date, more than 40 mature EBV miRNAs have been identified (8). These miRNAs are encoded in two major regions of the EBV genome: the BamH1 fragment A rightward transcript (BART) gene (mir–BART1-22) and BamH1 fragment H right reading frame 1 (BHRF1) gene (mir-BHRF1 1-3). BHRF1 miRNAs are expressed in the growth program but not in the default or latency program. However, the BART miRNAs are expressed in all forms of EBV latency. The function of most EBV miRNAs is largely unknown. EBV lacking the BART region is able to immortalize B cells efficiently, suggesting that the BART miRNAs are not essential for transformation (9). Nevertheless, it is thought that EBV miRNAs may promote viral latency or cancer development by targeting both viral and cellular genes.
In a previous study, we observed that some miRNAs were more highly expressed in latently infected resting memory B cells (latency/EBV nuclear antigen 1 only program) than in lymphoblastoid cell lines (growth program), and vice versa (10). BART 18-5p is one of the miRNAs that are abundantly expressed in latently infected resting memory B cells in vivo, with ∼300 copies per cell. Because EBV does not express any proteins in latently infected memory B cells, high levels of 18-5p expression may suggest a role for this miRNA in maintenance of viral latency. In this paper, we report on the identification of a target gene for 18-5p that implicates it in maintaining latency by suppressing viral replication.
Results
BART 18-5p Targets Cellular Genes Functionally Related to Viral Replication.
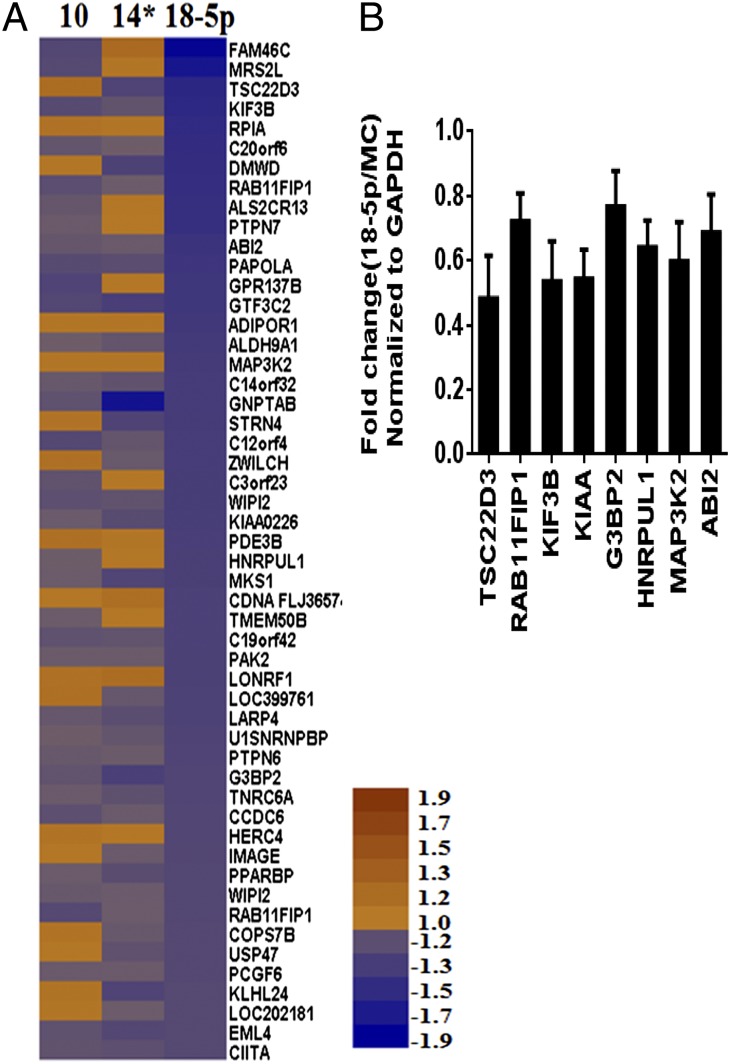
We have observed previously that 18-5p is more highly expressed in latently infected resting memory and germinal center B cells than in lymphoblastoid cell lines (Table 1). To understand the role of 18-5p better, we attempted to identify cellular genes that it regulates by expressing miRNA mimics in two different EBV-negative B-cell lines, BL2 and BJAB, followed by microarray analysis. Gene arrays have been used extensively to identify putative targets for miRNAs. A list of consensus genes down-regulated in both BL2 and BJAB cells by 18-5p was generated and aligned with the same set of genes from cells expressing other miRNAs (miRNA 10 and 14*). As shown by a heat map (Fig. 1A), 18-5p exhibits a distinct pattern of down-regulated genes compared with miRNA 10 and 14*. Interestingly, many of these genes are functionally related to autophagy, viral replication, viral budding, and endocytic protein recycling, including MAP kinase kinase kinase 2 (MAP3K2). To validate down-regulation of these genes, 18-5p was expressed in a third EBV-negative B-cell line, Akata 2A8, and the mRNA levels were measured by quantitative RT-PCR (qRT-PCR) (Fig. 1B), with typical reductions in the range of 20–60%. Akata 2A8 and its EBV-positive counterpart, Akata 2A8.1, were used in subsequent studies to analyze the function of 18-5p.
Table 1.
Representative examples of differential EBV BART miRNA expression in different tissues
| BART miRNA | Lymphoblastoid (growth program latency III) | Memory B (latency program latency 1/0) | Germinal center B (default program latency II) |
| Copy number per cell | |||
| 10 | 38 | <1 | <1 |
| 14* | 96 | <1 | <1 |
| 18-5p | 42 | 294 | 279 |
Fig. 1.
BART 18-5p targets cellular genes related to viral replication. (A) Heat map of consensus genes down-regulated in BL2 and BJAB cells by 18-5p in comparison to the same gene set for miRNA 10 and 14*. Brown and purple indicate up-regulation (up to 1.9-fold) and down-regulation (down to −1.9-fold), respectively. The microarray data can be accessed in the National Center for Biotechnology Information Gene Expression Omnibus database (accession no. GSE58224). (B) qRT-PCR validation of selected genes down-regulated in Akata 2A8 cells expressing 18-5p.
BART 18-5p Specifically Down-Regulates MAP3K2.
We decided to focus on MAP3K2 because MAPK family members are known to be important in herpesvirus replication (11–18). To test if 18-5p specifically targets MAP3K2, we either transiently expressed an 18-5p miRNA mimic or stably expressed an 18-5p primary miRNA (pri-miRNA; pGIZ–18-5p) in EBV-negative Akata 2A8 cells and measured the inhibition of MAP3K2 at both the mRNA and protein levels by real-time qRT-PCR and Western blot (Fig. 2 A and B). In contrast to control miRNAs [mimic control (MC) and pGIZ-MC], 18-5p expressed transiently or stably suppressed MAP3K2 gene expression by ∼40% or ∼30%, respectively. A parallel decrease in MAP3K2 at the protein level (48% and 24%, respectively) was also observed. The effect was specific, because other EBV miRNAs (BART10 and BART14*) had no effect on MAP3K2 expression (Fig. S1).
Fig. 2.
BART 18-5p specifically targets MAP3K2 in B cells. (A) mRNA level of MAP3K2 in EBV-negative Akata 2A8 cells transfected with miRNA mimics (MC and 18-5p) or cells infected with lentiviruses that express primary miRNAs (pGIZ-MC and pGIZ–18-5p). (B) Protein level of MAP3K2 from the same experiments as in A shown by Western blot. (C) Knockdown of Akata 2A8 cells transfected with an 18-5p miRNA mimic using 100 or 200 pmol of anti–18-5p LNA reversed the reduction of MAP3K2 by 18-5p at the RNA level. (D) Protein levels of MAP3K2 in the same experiment as C. (E) 18-5p miRNA expression in EBV-positive Akata 2A8.1 cells treated with the anti–18-5p inhibitor or NC LNA. (F and G) LNA knockdown of endogenous 18-5p expression leads to elevated levels of MAP3K2 in the EBV-positive BL cell lines Akata 2A8.1 and BL36.
To validate the specificity of the MAP3K2 knockdown by 18-5p further, locked nucleic acids (LNAs) were used to inactivate 18-5p, followed by measurement of MAP3K2 expression again in the EBV-negative line, Akata 2A8. LNAs are modified, complementary oligonucleotides that hybridize with their target to form stable duplexes with a high melting point, essentially inactivating the miRNA. As expected, cotransfection of 18-5p with either 100 or 200 pmol of scrambled LNA [negative control (NC) LNA] again led to a significant decrease in MAP3K2 at the mRNA level, but introduction of the specific anti–18-5p LNA was able to rescue this decrease (Fig. 2C). This reversal was even more striking at the protein level, with a two- to threefold increase in MAP3K2 protein in the presence of the 18-5p LNA inhibitor compared with the control (Fig. 2D).
To confirm that the activity of 18-5p was representative of the endogenous miRNA, the anti–18-5p LNA was introduced into Akata 2A8.1 cells, an EBV-positive counterpart of the Akata 2A8 cell line, which expresses 18-5p endogenously. Anti–18-5p LNA dramatically suppressed the levels of free endogenous 18-5p miRNA (Fig. 2E), leading to a significantly higher level (40% increase) of MAP3K2 mRNA (Fig. 2F) To confirm this observation in a second, independent cell line, we tested the EBV-positive Burkitt lymphoma line BL36. In this case, we saw an even more striking effect, with a 70% increase in MAP3K2 mRNA upon suppression of the endogenous 18-5p (Fig. 2G). We conclude that MAP3K2 is a specific target for 18-5p.
18-5p Specifically Interacts with the MAP3K2 Transcript in the RNA-Induced Silencing Complex.
Mature miRNAs stably associate with the Argonaute (Ago) proteins in the RNA-induced silencing complex (RISC) before targeting the mRNA. Overexpression of exogenous miRNAs, followed by immunoprecipitation of Ago proteins, permits the rapid isolation and identification of miRNA-bound mRNAs. To test if MAP3K2 is a physiological target for 18-5p, endogenous Ago2 was immunoprecipitated from Akata 2A8 cells stably expressing either pGIZ–18-5p or pGIZ-MC and examined for enrichment of MAK3K2 mRNA by qRT-PCR. Specific precipitation of Ago2 resulted in a 400-fold enrichment of 18-5p (Fig. S2 A and B). The enrichment was specific, because a positive control cellular miRNA, mir-200c, was also enriched by nearly 130-fold in the Ago2 complexes, whereas the small, noncoding NC RNA U1 was not associated with Ago2 (Fig. S2 C and D). When the level of mRNAs was measured, it was possible to demonstrate a 3.5-fold enrichment of the MAP3K2 mRNA in the pGIZ–18-5p/Ago2 precipitates (Fig. 3A). This association was specific, because no enrichment was seen in the pGIZ–18-5p/Ago2 precipitates for the mRNA of a control gene, beta-actin. The mRNA for AB12, another putative 18-5p target from the microarray analysis, was not significantly enriched in the Ago2 complex, suggesting that it might be an indirect target for 18-5p. As a further control, we analyzed ZEB1, a known target of mir-200c. No enrichment of ZEB1 mRNA was observed in the pGIZ–18-5p/Ago2 precipitates (Fig. 3A). This confirms the specificity of the MAP3K2 mRNA association with the 18-5p/Ago2 complex. We conclude that endogenous MAP3K2 interacts with 18-5p in the RISC.
Fig. 3.
BART 18-5p physically interacts with the 3′UTR of MAP3K2. (A) BART 18-5p interacts with MAP3K2 in the RISC complex. Fold enrichment of MAP3K2, beta-actin (ACTB), ZEB1, and abl-interactor 2 (ABI2) mRNAs in the immunoprecipitated 18-5p/Ago2 complex from Akata 2A8 cells stably expressing pGIZ–18-5p. (B) Potential 18-5p binding site in the 3′UTR of the MAP3K2 mRNA. The 18-5p miRNA has identical nucleotides (blue) in the seed region as the human miRNA 26a-5p. The seed region of 18-5p has a complementary binding site in the 3′UTR (red) but not the 5′UTR or coding region. For the mutated 3′UTR (mut), the potential binding nucleotides were replaced to disrupt the complementarity (red). (C) Luciferase assays in HEK 293 cells cotransfected by luciferase reporter vectors encoding 3′UTR WT (wt), 3′UTR mut, 5′UTR, and the coding region with or without LNA inhibitors. Open bars represent vector alone, dotted bars represent vector + MC miRNA, black bars represent vector + 18–5p, gray bars represent vector + 18-5p + anti–18-5p LNA, and striped bars represent vector + 18–5p + anti-MC LNA. *not significant (N.S); **P < 0.01.
18-5p Physically Interacts with the MAP3K2 Transcript at the 3′UTR.
We performed bioinformatics analysis on the MAP3K2 gene using the miRNA target prediction software MiRanda in an attempt to identify potential binding site(s) for 18-5p. This analysis revealed a single complementary site that was located in the 3′UTRs of the MAP3K2 mRNA (Fig. 3B). This region had eight nucleotides of perfect complementarity with the seed region of mature 18-5p (positions 2–8 at the 5′ end), the region believed to be the key for posttranscriptional repression of mRNA targets by miRNAs. Interestingly, this is the identical seed sequence targeted by the oncogenic human mir–26a-5p, which also down-regulates MAP3K2 (19). To validate the functionality of the putative 18-5p binding site, the 3′UTR (3,018 bp), 5′UTR (457 bp), and coding regions (1,860 bp) of the MAP3K2 mRNA were cloned downstream of the Renilla luciferase coding region in the psiCheck2 vector. HEK 293 cells, highly transfectable cells routinely used for luciferase assays, were cotransfected with this vector and either the control (MC) or 18-5p miRNA, together with either the NC LNA or anti–18-5p LNA. Luciferase activity was measured at 24 h posttransfection. As shown in Fig. 3C, 18-5p significantly reduced the activity of the luciferase vector containing the full-length 3′UTR (40% down-regulation; P < 0.01), whereas the MC had no effect. In contrast, 18-5p mimics had no effect on the luciferase expression of cells expressing the empty reporter vector, 5′UTR, or the coding region of MAP3K2. The reduction of luciferase activity in the 3′UTR group was directly proportional to 18-5p expression, because treatment with a specific anti–18-5p inhibitor, but not NC LNA, fully restored luciferase expression (1.89-fold increase; P < 0.01). Finally, to confirm that 18-5p exerted its effect specifically through the putative binding site identified in the 3′UTR, all seven bases that matched the seed region of 18-5p were mutated (Fig. 3B). This completely abolished the inhibitory effects of 18-5p, because no difference in luciferase expression was observed between MC and 18-5p–expressing cells. We conclude therefore that 18-5p directly binds to this sequence in the 3′UTR to target the MAP3K2 mRNA.
18-5p Impairs EBV Lytic replication upon Reactivation by B-Cell Receptor Cross-Linking.
MAP3K2 has been implicated previously as a crucial mediator of replication by the mouse gammaherpesvirus Murid herpesvirus 68 (MHV 68) (11). Thus, the ability of 18-5p to repress MAP3K2 raises the possibility that the function of 18-5p could be to block EBV lytic replication. No fully permissive culture system for EBV replication is available. Current experimental models are usually based on the reactivation of latently infected cell lines with inducing chemicals or B-cell receptor (BCR) stimulation. To determine if 18-5p disrupts the BCR signaling pathway, we transiently overexpressed 18-5p mimics in Akata 2A8.1, followed by anti-Ig treatment. Overexpression of 18-5p down-regulated MAP3K2 expression in Akata 2A8.1 (Fig. S3A). In parallel, the transcription of lytic genes, including the IE BZLF1 and the L glycoprotein gp350, acting as indicators for viral replication, was examined by qRT-PCR. We observed a substantial repression of viral replication by 18-5p. BZLF1 was reduced fourfold, and gp350 was reduced twofold (P < 0.05 and P < 0.01, respectively) (Fig. 4A). In parallel, cell-free virions (DNase-resistant) in the culture supernatant, representative of freshly encapsidated virus, were measured by gp350 DNA PCR. The level of extracellular virus was reduced sixfold by 18-5p (1.8 × 105 copies per milliliter vs. 3.3 × 104 copies per milliliter; P < 0.05) (Fig. 4B).
Fig. 4.
BART 18-5p impairs viral lytic replication upon reactivation. (A) Lytic genes (IE BZLF1 and L gp350) were quantified by qRT-PCR in Akata 2A8.1 cells transfected with MC or 18-5p mimics upon reactivation by BCR cross-linking for 72 h. (B) Copy numbers of EBV virions in the supernatants from A. (C) BZLF1 and gp350 expression in Akata 2A8.1 cells transfected with NC LNA or anti–18-5p LNA upon reactivation by BCR cross-linking for 72 h. (D) Copy numbers of EBV virions in the supernatant collected from C. (E) BZLF1 and gp350 expression in a B95.8-infected LCL transfected with miRNA mimics and LNAs upon TPA/BA treatment for 48 h. (F) Copy numbers of EBV virions in the supernatant collected from E.
Because 18-5p is expressed endogenously in Akata 2A8.1 cells, to demonstrate the direct action of endogenous 18-5p on the induction of viral replication by endogenous MAP3K2, we knocked down endogenous 18-5p with the anti–18-5p LNA. In this case, lytic gene transcription was enhanced fivefold for BZLF1 and threefold for gp350 (Fig. 4C), and virus production was increased twofold (Fig. 4D). Taken together, we observed a 20-fold change in BZLF1, a sixfold change in gp350, and a 12-fold change in virion production associated with the difference between suppression of endogenous 18-5p and ectopic expression of 18-5p.
18-5p Impairs EBV Lytic Replication upon Reactivation by 12-o-Tetradecanoylphorbol-13-Acetate/Sodium Butyrate.
An alternate pathway for reactivating EBV is through induction with 12-o-tetradecanoylphorbol-13-acetate (TPA) and sodium butyrate (BA). To test the effect of 18-5p on chemical induction of the virus, we induced a lymphoblastoid cell line (LCL; previously selected for maximal inducibility) transformed with the B95-8 strain of virus (known to lack 18-5p). Overexpression of 18-5p specifically down-regulated MAP3K2 expression in the LCL (Fig. S3B). After 48 h of TPA/BA stimulation, transcription of the lytic genes BZLF1 and gp350 was induced 12-fold and sevenfold, respectively, compared with the uninduced cells (Fig. 4E). However, the induction was significantly disrupted by the presence of 18-5p, as evidenced by the reduced levels of gene transcription of both lytic genes (two- to 2.5-fold) in the presence of 18-5p and its reversal with the 18-5p LNA inhibitor (Fig. 4E). In parallel, we observed a 60% reduction in released virus by 18-5p (15 × 103 copies per milliliter with MC vs. 5.1 × 103 copies per milliliter with 18-5p; P < 0.05) (Fig. 4F), which, again, was reversed with the specific inhibitory LNA. Similar results were obtained with the parental B95.8 cell line (Fig. S4).
We conclude therefore that 18-5p significantly inhibits EBV lytic replication in response to both TPA/BA and surface Ig activation.
MAP3K2 Mediates Reactivation Signaling and Rescues 18-5p–Mediated Repression.
To test if MAP3K2 indeed mediates viral reactivation, we have overexpressed MAP3K2 in the B95-8 LCL (Fig. S5A) and observed the effect upon reactivation by TPA/BA. Overexpression of MAP3K2 markedly enhanced viral replication as judged by both lytic gene expression (Fig. 5A) and virion production (Fig. 5B). To confirm the relationship between 18-5p, MAP3K2, and viral replication, we performed add-back experiments with MAP3K2 in B95.8-LCL cells ectopically expressing 18-5p and observed the effect on viral replication. We observed that overexpression of MAP3K2 (Fig. S5B) did indeed significantly restore the expression of BZLF1 (increased from 2.1-fold to 12.8-fold after TPA/BA induction; P < 0.01) and gp350 (from 3.6-fold to 10-fold; P < 0.01) (Fig. 5C). However, we failed to detect an increase in virions (Fig. S6). The failure to recover virus production suggests that the reduction of EBV lytic replication by 18-5p is only partially mediated through MAP3K2 and that 18-5p interferes with other genes associated with viral assembly and budding (Fig. 1).
Fig. 5.
Overexpression of MAP3K2 enhances viral replication. (A) BZLF1 and gp350 expression in B95.8-LCL cells transfected with empty vector or MAP3K2 alone, followed by TPA/BA induction. (B) Copy numbers of EBV virions in the supernatant collected from A. (C) BZLF1 and gp350 expression in B95.8-LCL cells expressing 18-5p with or without MAP3K2 upon TPA/BA induction.
Discussion
In this study, we have demonstrated that the EBV-encoded BART 18-5p miRNA targets the mRNA for the MAP kinase MAP3K2. We further demonstrate the relevance of this finding by showing that this kinase plays a central role in the signaling cascade that drives reactivation and replication of the virus. Consequently, the effect of 18-5p is to reduce the level of viral replication. The 18-5p miRNA had an impact on viral replication induced by two different in vitro signals, TPA and BA, which primarily act through the PKC signaling pathway, and by BCR cross-linking, which involves four signaling pathways: PI3K, Rac1, Ras, and phospholipase C. As can be seen in Fig. 6, both pathways converge on MAP3K2, the now-identified target of 18-5p, explaining why the miRNA has an impact on both signaling mechanisms.
Fig. 6.
Proposed model for 18-5p–mediated inhibition of viral reactivation through the MAPK signal transduction pathway. Briefly, TPA triggers the PKC signaling pathway, and BCR stimulation activates the PI3K, PLC, Ras, and Rac1 pathways. Both pathways are mediated through MAP3K2 and activate downstream effector transcription factors that can activate the BZLF1 promoter (Zp), followed by BZLF1 protein expression and induction of the lytic cycle gene cascade. BART 18-5p represses MAP3K2 via binding to its 3′UTR, and thereby inhibits viral reactivation.
Interestingly, we have also shown that 18-5p shares a high degree of sequence homology at the 5′ end with the human oncogenic miRNA mir–26a-5p, especially in the seed sequence. The mir–26a-5p also down-regulates MAP3K2 by targeting exactly the same region in its 3′UTR. The coincidence is satisfying, because it further strengthens our conclusion that MAP3K2 is a bona fide target for 18-5p and supports the idea that EBV miRNAs, like some other virus-encoded miRNAs, exploit the host by using homologous seed sequences (20, 21). This seems to be a common strategy, because it was estimated that 26% of annotated virus-derived miRNAs have seed regions that are identical to host miRNAs (22). It has been shown previously that mir–26a-5p inhibits JNK-dependent apoptosis in human glioblastoma cells, thereby promoting tumor growth (19). Although there is no known association of EBV with glioblastoma, it is interesting to note that 18-5p is highly expressed in all of the EBV-associated tumors and that through analogy with mir–26a-5p, it may play a role in oncogenesis.
Activation of the MAPK signaling pathway is required for efficient viral replication, especially the initiation of the lytic cascade, for several gammaherpesvirinae, including EBV, Kaposi sarcoma-associated herpesvirus, and MHV68 (11, 13, 15). The full process of EBV lytic replication has three stages: initial induction of the lytic gene cascade, viral assembly and transport, and egress of infectious progeny. The lytic gene cascade, in turn, has three stages: induction of the IE transcription factors that, in turn, activate the E genes responsible for initiating viral DNA replication and, finally, expression of the L genes, including the virion structural proteins. Transcription factors, such as CREB, ATF1-2, and c-JUN, downstream of the MAPK pathway bind to the promoter regions of the IE gene BZLF1 (8), which encodes a transcriptional activator that is sufficient to drive the entire lytic replication program in both B cells and epithelial cells (23). Thus, 18-5p–mediated down-regulation of MAP3K2 should have an impact on the IE stage of lytic replication, as verified by the observed down-regulation of BZLF1 with a consequent decrease in L antigen and virion production. However, the inhibitory effect of 18-5p on viral replication is only partially mediated by MAP3K2, because reconstitution of MAP3K2 reversed the decreased expression of the IE and L genes tested but did not increase the production of virions. This implies that 18-5p also targets other cellular genes involved in the production of virions. Indeed, in our initial microarray screen for 18-5p targets, we noticed that many of the genes are functionally related to autophagy, viral replication, viral budding, and endocytic protein recycling (Fig. 1). For instance, RAB11FIP1, KIF3B, and KIAA are key players in regulating endocytic protein transport and autophagy, with potential involvement in virion packaging (24–27). ABI2 is associated with virus assembly and viral release for Arenavirus, hepatitis B virus, Sendai virus, and HIV (28–31). G3BP2 plays a pivotal role in Sindbis virus replication via interacting with virus-encoded nonstructural proteins (32). HNRPUL1 is recruited to the viral replication center, and thus modulates adenovirus replication (33). Therefore, 18–5p may have global effects that suppress multiple functions related to viral replication. Down-regulation of these genes by 18-5p only ranged from 20 to 40%, consistent with the current belief that miRNAs typically exert subtle inhibitory effects on many mRNAs embedded within intricate regulatory networks, thereby augmenting the effect and producing a stronger response.
Limiting lytic replication would confer a selective survival advantage to the EBV-infected cells, especially in the long-lived memory B-cell population. The production of virus triggers a potent cytotoxic T-cell and neutralizing antibody response, which controls virus spread (34). This provides a strong selective pressure for EBV to develop molecular strategies to evade host attack and only reactivate at a specific place and time. These strategies include limited expression of potentially immunogenic viral proteins (e.g., shutoff of all latent proteins in EBV-infected memory B cells) (35), expression of genes with the ability to block presentation of viral antigens (36), and avoidance of unnecessary viral replication. In this study, the identification of 18-5p as having a role in restricting viral replication in the memory compartment falls into the last category and is in accord with the observation that viral reactivation is restricted to the oropharyngeal lymphoid tissues and is not seen in the memory compartment (5, 37).
In conclusion, we have shown that a previously reported but uncharacterized EBV miRNA, BART 18-5p, can inhibit viral replication in EBV latently infected B cells upon in vitro reactivation through repression of an important cellular signaling molecule, MAP3K2. To our knowledge, this is the first report of a virus encoding a miRNA that suppresses a target in the MAPK signaling cascade, a central signal transduction pathway that governs a broad spectrum of biological processes. The recognition of cross-talk between viral miRNAs and cellular genes is of particular importance because it provides insight into the function of viral components in establishing and maintaining latency.
Materials and Methods
Cell Lines.
BL2 and Akata 2A8 are EBV-negative BL lines. Akata 2A8.1 is an EBV-positive variant of Akata 2A8. BJAB is an EBV-negative B-cell line, B95-8 is an EBV-positive marmoset B-cell line, and B95.8-LCL cells are human peripheral B cells transformed with B95.8 virus.
MiRNA Mimics, Inhibitors, and Cell Transfection.
Synthetic miRNA mimics (Thermo Scientific) and LNAs (Exqion) were transfected into 1.5–2.5 × 106 cells using an Amaxa nucleofector, the Kit V solution, and the program C-009 (all from Lonza). Luciferase plasmids and pGIZ plasmids were transiently transfected into HEK 392 T cells using Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer’s manual. In total, 2 × 106 B95.8-LCL cells were electroporated with 3 μg of pCMV6-MAP3K2 (human origin) and pCMV6-XL5 control plasmids (a gift from Ren Sun, University of California, Los Angeles, Los Angeles) using the nucleofector.
Cloning.
The lentivirus plasmid pGIZ vector (Thermo Scientific) was used to express miRNAs stably (details are provided in SI Materials and Methods). Region 145,921–146,050 on the EBV genome (European Molecular Biology Laboratory AJ507799.2) encompassing the pri–mir–18-5p sequence was used. For the NC, the Caenorhabditis elegans miR-67 stem-loop sequence was used (mirBase accession ID MI0000038). For luciferase studies, the MAP3K2 cDNA was isolated from the BL36 cell line and 3′UTR (19), coding region, 5′UTR, and mutated 3′UTR were cloned into the psiCHECK2 dual-luciferase vector (Promega) (details are provided in SI Materials and Methods).
Microarray Analysis.
EBV miRNAs and MC were transiently expressed in the EBV-negative B cells BL2 and BJAB, followed by microarray analysis 24 h later using the Affymetrix Human HGU133 Plus 2.0 Array (Broad Institute) (details are provided in SI Materials and Methods).
qRT-PCR and Virion DNA PCR.
Analysis of mature miRNA expression was performed using miRNA-specific qRT-PCR as described previously (10). Gene expression was assessed by qRT-PCR. The housekeeping gene GAPDH was used as an internal control for normalization. For the EBV virion PCR assay, real-time DNA PCR for the gp350 gene was used (details are provided in SI Materials and Methods). Absolute virion copy numbers were calculated based on a standard curve of serial 10-fold dilutions of EBV (B95-8 strain)-quantitated viral DNA (Advanced Biotechnologies).
Western Blot.
Western blot analysis was carried out by standard procedures (details are provided in SI Materials and Methods).
Ago2 Immunoprecipitation.
Ago2 immunoprecipitation was performed using the Magna RIP Kit (Millipore) according to the manufacturer’s instructions.
Luciferase Reporter Assay.
Assays were carried out in triplicate (details are provided in SI Materials and Methods). Luciferase activity was measured using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions.
Viral Reactivation and EBV Virion Isolation.
B95.8 or B95.8-LCL cells were treated in replicate with TPA and BA, and Akata 2A8.1 cells were treated with anti-human IgG/IgM antibodies (Jackson ImmunoResearch) (details are provided in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Francisco Navarro and Judy Lieberman for assistance with the initial microarray analysis and Dr. Ren Sun for the pCMV6-MAP3K2 and pCMV6-XL5 control plasmids. This work was supported by Public Health Service Grants R01 CA65883 and R01 AI18757 (to D.A.T.-L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE58224).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406136111/-/DCSupplemental.
References
- 1.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 2.Thorley-Lawson DA. Epstein-Barr virus: Exploiting the immune system. Nat Rev Immunol. 2001;1(1):75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 3.Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190(4):567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2603–2654. [Google Scholar]
- 5.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79(2):1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA, Edson CM. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979;32(2):458–467. doi: 10.1128/jvi.32.2.458-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorley-Lawson DA, Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc Natl Acad Sci USA. 1980;77(9):5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinke O, Feederle R, Delecluse HJ. Genetics of Epstein-Barr virus microRNAs. Semin Cancer Biol. 2014;26C:52–59. doi: 10.1016/j.semcancer.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Kempkes B, Pich D, Zeidler R, Sugden B, Hammerschmidt W. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J Virol. 1995;69(1):231–238. doi: 10.1128/jvi.69.1.231-238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu J, et al. A novel persistence associated EBV miRNA expression profile is disrupted in neoplasia. PLoS Pathog. 2011;7(8):e1002193. doi: 10.1371/journal.ppat.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, et al. Tpl2/AP-1 enhances murine gammaherpesvirus 68 lytic replication. J Virol. 2010;84(4):1881–1890. doi: 10.1128/JVI.01856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H, Xie J, Ye F, Gao SJ. Modulation of Kaposi’s sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J Virol. 2006;80(11):5371–5382. doi: 10.1128/JVI.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamson AL, et al. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74(3):1224–1233. doi: 10.1128/jvi.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh T, Hoshikawa Y, Satoh Y, Kurata T, Sairenji T. The interaction of mitogen-activated protein kinases to Epstein-Barr virus activation in Akata cells. Virus Genes. 1999;18(1):57–64. doi: 10.1023/a:1008021402908. [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. Reactivation of Kaposi’s sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371(1):139–154. doi: 10.1016/j.virol.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl JA, et al. Phosphoproteomic analyses reveal signaling pathways that facilitate lytic gammaherpesvirus replication. PLoS Pathog. 2013;9(9):e1003583. doi: 10.1371/journal.ppat.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J, Cahir-McFarland E, Zhao B, Kieff E. Virus and cell RNAs expressed during Epstein-Barr virus replication. J Virol. 2006;80(5):2548–2565. doi: 10.1128/JVI.80.5.2548-2565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Ikuta K, Tajima M, Sairenji T. 12-O-tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-kappaB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology. 2001;286(1):91–99. doi: 10.1006/viro.2001.0965. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci USA. 2010;107(5):2183–2188. doi: 10.1073/pnas.0909896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh Z, Mallick B, Chakrabarti J. Cellular versus viral microRNAs in host-virus interaction. Nucleic Acids Res. 2009;37(4):1035–1048. doi: 10.1093/nar/gkn1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottwein E, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450(7172):1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kincaid RP, Sullivan CS. Virus-encoded microRNAs: An overview and a look to the future. PLoS Pathog. 2012;8(12):e1003018. doi: 10.1371/journal.ppat.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonteich E, et al. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121(Pt 22):3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunaga K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11(4):385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 26.Sir D, Ou JH. Autophagy in viral replication and pathogenesis. Mol Cells. 2010;29(1):1–7. doi: 10.1007/s10059-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavignac Y, Esclatine A. Herpesviruses and autophagy: Catch me if you can! Viruses. 2010;2(1):314–333. doi: 10.3390/v2010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie T, Nagata N, Yoshida T, Sakaguchi T. Recruitment of Alix/AIP1 to the plasma membrane by Sendai virus C protein facilitates budding of virus-like particles. Virology. 2008;371(1):108–120. doi: 10.1016/j.virol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Strack B, Calistri A, Craig S, Popova E, Göttlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114(6):689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 30.Shtanko O, Watanabe S, Jasenosky LD, Watanabe T, Kawaoka Y. ALIX/AIP1 is required for NP incorporation into Mopeia virus Z-induced virus-like particles. J Virol. 2011;85(7):3631–3641. doi: 10.1128/JVI.01984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, et al. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA. 2007;104(24):10205–10210. doi: 10.1073/pnas.0704000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cristea IM, et al. Host factors associated with the Sindbis virus RNA-dependent RNA polymerase: Role for G3BP1 and G3BP2 in virus replication. J Virol. 2010;84(13):6720–6732. doi: 10.1128/JVI.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackford AN, et al. A role for E1B-AP5 in ATR signaling pathways during adenovirus infection. J Virol. 2008;82(15):7640–7652. doi: 10.1128/JVI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Virology. 5th Ed. Vol 2. New York: Lippincott Williams & Wilkins; 2007. pp. 2655–2700. [Google Scholar]
- 35.Hochberg D, et al. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004;101(1):239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo J, et al. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. J Virol. 2011;85(4):1604–1614. doi: 10.1128/JVI.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decker LL, Klaman LD, Thorley-Lawson DA. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J Virol. 1996;70(5):3286–3289. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.