Abstract
Background
Enterotoxigenic Bacteroides fragilis (ETBF), a molecular subclass of the common human commensal, B. fragilis, has been associated with inflammatory bowel disease. ETBF colitis is characterized by the activation of Stat3 and a Th17 immune response in the colonic mucosa. This study was designed to investigate the time course and cellular distribution of Stat3 activation in ETBF-colonized mice.
Methods
C57BL/6 wild-type, C57BL/6Stat3ΔIEC, or Rag-1 mice were inoculated with saline, nontoxigenic B. fragilis or ETBF. Histologic diagnosis and mucosal Stat activation (immunohistochemistry, Western blot, and/or electrophorectic mobility shift assay) were evaluated over time (6–24 h, 1–7 d, and 1–18 mo after inoculation). Mucosal permeability was evaluated at 16 hours, 1 day, and 3 days. Mucosal immune responses were evaluated at 1 week, and 12 and 18 months.
Results
ETBF induced rapid-onset colitis that persisted for up to 1 year. Stat3 activation (pStat3) was noted in the mucosal immune cells within 16 hours, with colonic epithelial cell activation evident at 24 hours after inoculation. ETBF-induced increased mucosal permeability was first observed at 24 hours after inoculation, after which the initial immune cell pStat3 activation was noted. Immune cell pStat3 was present in the absence of epithelial pStat3 (C57BL/ 6Stat3ΔIEC). Epithelial pStat3 was present in the absence of T and B cells (Rag-1 mice). pStat3 persisted in the epithelial and immune cells for 1 year, characterized by isolated pStat3-positive cell clusters, with varying intensity distributed through the proximal and distal colon. Similarly, mucosal Th17 immune responses persisted for up to 1 year. Loss of fecal ETBF colonization was associated with the loss of mucosal pStat3 and Th17 immune responses.
Conclusions
ETBF rapidly induces immune cell pStat3, which is independent of epithelial pStat3. This occurs before ETBF-induced mucosal permeability, suggesting that ETBF, likely through B. fragilis toxin and its action on the colonic epithelial cell, triggers mucosal immune cell Stat3 activation. Peak mucosal Stat3 activation (immune and epithelial cells) occurs subsequently when other colonic bacteria may contribute to the ETBF-initiated immune response due to barrier dysfunction. ETBF induces long-lived, focal colonic Stat3 activation and Th17 immune responses dependent on the ongoing ETBF colonization. Further study is needed to evaluate the early mucosal signaling events, resulting in epithelial Stat3 activation and the sequelae of long-term colonic Stat3 activation.
Keywords: Bacteroides fragilis, enterotoxigenic Bacteroides fragilis, colitis, Stat3, murine colitis
Inflammatory bowel disease (IBD) is postulated to result from a dysregulated immune response to the host microbiota in genetically susceptible individuals.1,2 Although no specific pathogen(s) has been clearly implicated, enteric bacteria seem to play a significant role in IBD pathogenesis.1,3 Increased epithelial permeability in IBD patients is believed to enhance direct contact between colonic flora and the mucosal immune system, possibly serving as a critical factor in disease initiation. In fact, IBD patients have increased the secretion of IgG antibodies against their microbiota into the intestinal lumen.4 Furthermore, there seems to be diminished intestinal mucosal tolerance because there is an increase in systemic adaptive immune responses against antigens, like flagellin, of the bacteria.5,6
Signal transducers and activators of transcription (Stat) proteins are transcription factors that once activated play a critical role in the regulation of immune responses.7 This family of transcription factors (Stats 1–6) undergoes activation in the cytosol by tyrosine phosphorylation before nuclear translocation. The contribution of Stat protein activation to epithelial and immune cell function is complex. Within T cells, activated Stat proteins (pStats) induce and maintain T-cell subset identity through tran-scriptional activation of subset-specific transcription factors and cotranscriptional regulation of subset-specific cytokine genes. For example, Stat1, Stat2, and Stat4 are required for the generation of Th1 cells, Stat6 for Th2 cells, and Stat3 for Th17 cells. Recent studies have highlighted the contribution of Th17-mediated inflammation in the pathogenesis of IBD.8 In genome-wide analyses, single-nucleotide polymorphisms of the Stat3 protein have been identified in patients with Crohn’s disease and ulcerative colitis. Furthermore, mutations in the IL-23 receptor gene are some of the host mutations most highly associated with IBD.9 Although IL-23 has several roles in immune regulation, one of its actions is to promote the expansion and stabilization of Th17 cell populations.10
A dense, adherent biofilm mass predominately consisting of Bacteroides fragilis group of organisms has been reported to adhere to the inflamed mucosal surface of IBD patients.11 B. fragilis sensu stricto is a common colonic symbiote estimated to colonize up to 90% of individuals.12 Colonization with B. fragilis is proposed to play a pivotal role in the regulation of mucosal and systemic immunity.13 However, B. fragilis can act as opportunistic pathogens being the leading anaerobe in bloodstream infections and intra-abdominal abscesses.14 Enterotoxigenic B. fragilis (ETBF) is a molecular subset of B. fragilis that secrete a zinc-dependent metalloprotease toxin termed the B. fragilis toxin (BFT, also known as fragilysin).15 ETBF is associated globally with pediatric and adult diarrheal illnesses and, in limited data, with active IBD and colon cancer.16–19 Previously, we reported that ETBF induce symptomatic acute and asymptomatic chronic colitis in conventional C57Bl/6 mice.20,21 Furthermore, in multiple intestinal neoplasia (Min) mice that are heterozygous for the adenomatous polyposis coli (Apc) gene, ETBF colonization induces colonic inflammation with rapid-onset colonic tumor formation. This process is characterized by the selective activation of Stat3 by day 2 after colonization with a subsequent colonic mucosal Th17 response. Blockade of IL-17 alone or IL-17 and the IL-23 receptor in this model diminishes colon tumor formation, indicating that Stat3/Th17 adaptive immunity contributes to ETBF carcinogenesis.
In this study, we further characterized Stat3 activation in ETBF-induced murine colitis in C57Bl/6, C57BL/6Stat3ΔIEC, and Rag-1 mice. We also tested whether BFT, the only known ETBF virulence factor, activates colonic epithelial cell Stat3. Our results show that ETBF rapidly activates mucosal immune cell Stat3. Subsequently, increased mucosal permeability occurs, together with increased mucosal immune cell Stat3 activation and Stat3 activation in colonic epithelial cells. In ETBF-colonized mice, Stat3 activation persists for months along with a mucosal Th17 response. Our findings indicate that ETBF-induced Stat3 activation in mucosal immune cells precedes detectable increased mucosal permeability or colonic epithelial Stat3 activation, suggesting that ETBF, likely through BFT, triggers rapid release of early epithelial cell mediators that contribute to early immune Stat3 activation. Peak mucosal Stat3 activation (immune and epithelial cells) occurs later when other colonic bacteria may contribute to the immune response due to barrier dysfunction.
MATERIALS AND METHODS
Bacteriology
ETBF strain 86–5443-2-2 (piglet) and nontoxigenic B. fragilis (NTBF) strain NCTC9343 (human) were used in this study.22 B. fragilis strains were cultured and grown anaerobically on brain heart infusion (BHI) medium plates containing 37 g of BHI base (Difco Laboratories, Detroit, MI) per liter along with 5 g of yeast extract (Difco Laboratories), 0.1 mg of vitamin K per liter, 0.5 mg of hemin per liter, 50 mg of L-cysteine, and 6 mg of clindamycin per liter (all from Sigma, St Louis, MO). A single colony was inoculated into BHI broth and grown anaerobically overnight at 378C. Washed bacteria were resuspended in 0.1N sodium bicarbonate buffer and adjusted to an optical density corresponding to approximately 109 colony-forming units (CFU) per milliliter for mouse inoculations. Serial dilutions of murine fecal samples were cultured periodically after inoculation including the day of killing on BHI plates with clindamycin to quantify strain colonization. Inoculated B. fragilis strains were either naturally resistant to clindamycin or resistant due to transformation with plasmid pFD340.
Mice
C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) or the National Cancer Institute at approximately 3 weeks of age or were bred at the Johns Hopkins using C57Bl/6 breeder pairs from the same sources. Villin Cre C57Bl/6 mice22 (kindly provided by Dr Elaine Lin, Albert Einstein College of Medicine, New York, NY) were crossed to Stat3 Flox/Flox mice23 (kindly provided by the Dr Drew Pardoll, Johns Hopkins School of Medicine, Baltimore, MD) to generate mice with a Stat3 deletion in the intestinal epithelial cell (C57BL/6Stat3ΔIEC). Rag-1 mice were purchased from Jackson Laboratories. Mice were treated for 3 to 5 days with 5 g per liter of streptomycin (Sigma) and 100 mg per liter of clindamycin (Pharmacia, Kalamazoo, MI) in their drinking water before bacterial strain inoculation. All inoculated strains were resistant to these antibiotics. As described in the Results section, in some experiments, antibiotic-containing water was discontinued after bacterial inoculation, whereas in other experiments, the antibiotic-containing water was continued throughout the experiments.20 To date, no differences in the time course or severity of the colitis induced by ETBF have been observed in mice provided short-term or continuous antibiotic-containing water. Orogastric inoculation with bacterial strains (0.1–0.2 mL/bacterial strain or approximately 109 CFU/mouse) or the buffer sodium bicarbonate (0.1–0.2 mL) was performed. Mice were euthanized using carbon dioxide euthanasia at time points as described in the Results section. Gross findings were recorded, and the gastrointestinal tract as well as, in some experiments, the liver and spleen were harvested for analyses.
Histologic Assessment
The mouse gastrointestinal tract was dissected and preserved in 10% buffered formalin. Histologic examination was performed after hematoxylin and eosin staining of 5-µm paraffin-embedded sections. To facilitate examination, the colon and, in some mice, the small bowel were “Swiss rolled” before embedding and sectioning. Inflammation was scored using a scale of 0 (normal mucosa), +1 (mild increase in inflammatory cells; no mucosal changes), +2 (moderate increase in inflammatory cells; mild scattered proliferation 6 focal loss of crypt architecture), +3 (severe increase in inflammatory cells; diffuse or nearly diffuse proliferation, focally extensive loss of crypt architecture), and +4 (complete or nearly complete mucosal destruction).
Western Blot and Electrophorectic Mobility Shift Assay Analyses of Stat Proteins
Five-millimeter punch biopsies of various regions in the gastrointestinal tract were obtained at time points described in the Results section. Specimens were flash frozen and stored at 2808C until processed. After being mechanically homogenized, samples were washed twice in a solution of phosphate-buffered solution (PBS, 1×) and phosphatase inhibitor (P5726, Sigma) followed by washing in a hypotonic buffer composed of 20 mM HEPES buffer, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, phosphatase inhibitor, protease inhibitor (Complete Mini EDTA-Free Protease inhibitor cocktail; Roche Diagnostics, Indianapolis, IN), 1 mM dithiothreitol, and 0.5 mM phenylmethanesulphonylfluoride. After centrifugation (20,200 G, 5 min, 4°C), the precipitate was resuspended again in hypotonic buffer with Igepal CA-360 (1:50 dilution; Sigma) followed by centrifugation (5 min, 20,200 G, 4°C) and removal of the supernatant (i.e., cytoplasmic extract). The nuclei-containing pellet was reconstituted and rocked (4°C, 30 min) in a hypertonic buffer composed of 420 mM NaCl, 20 mM HEPES, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, phosphatase inhibitor, protease inhibitor, 1 mM dithiothreitol, 0.5 mM phenylmethanesulphonylfluoride, and 75% glycerol. After centrifugation (20,200 G, 4°C, 30 min), the supernatant (nuclear extract) was stored in aliquots at −80°C for Western blot and electrophorectic mobility shift assay (EMSA) analyses. Protein concentrations of the nuclear extracts were determined by the Bradford assay (Biorad) or Pierce protein assay.
Optimized antibody dilutions of anti-pStat3 (Cell Signaling, Danvers, MA) and anti-β actin (Sigma) were used for Western blot analysis. pStat3 bands were detected using goat antirabbit secondary horseradish peroxidase (HRP) antibody (Jackson Immune Research, West Grove, PA) with Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Protein concentrations and HRP-labeled β-actin antibody followed by a goat antimouse secondary HRP antibody were used for the normalization of protein content in samples. For EMSA, 5 to 10 mg of protein were incubated with the 32P-labeled high-affinity SIE probe (5’-AGCTTCATTTCCCGTAAATCCCTA-3’) derived from the c-fos gene promoter that binds Stat1, Stat3, and Stat5, as described.24 Protein–DNA complexes were resolved on 5% nondenaturating polyacrylamide gels and analyzed by autoradiography using Kodak film. Supershift binding reaction was performed using anti-Stat3, rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA).
Phosphorylated Stat3 Immunohistochemistry
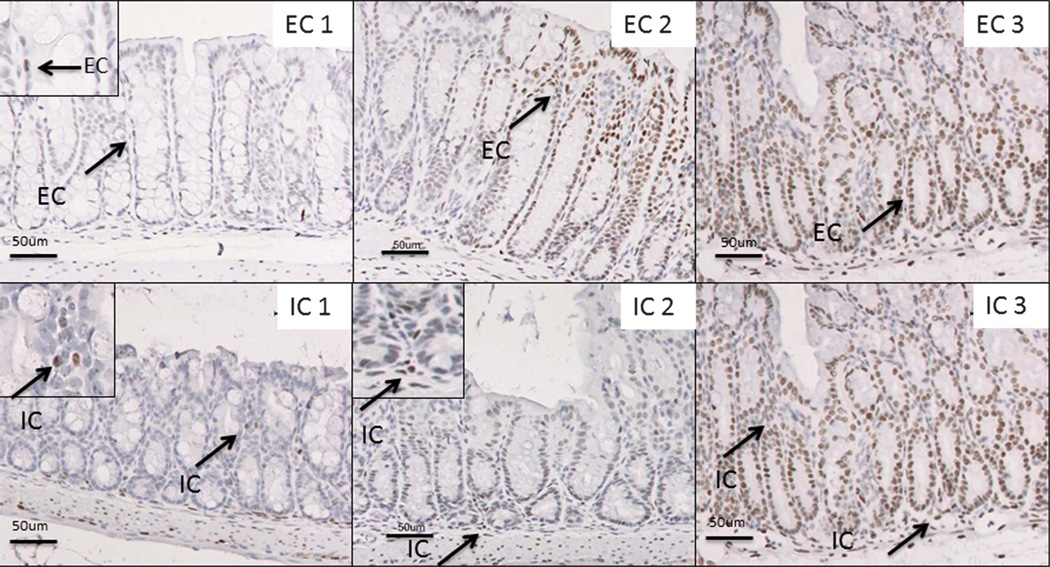
Paraffin-embedded tissues heated at 608C for 20 minutes were deparaffinized in xylene for 5 minutes. Hydrogen peroxide (0.3%) was used to quench endogenous peroxidase. Optimized antigen retrieval was performed with boiling 0.01 M citrate buffer (pH 6.0) and 0.025% trypsin in a 50 mM Tris/0.05% CaCl2 solution (pH 7.6). Goat serum (2%) was used to block nonspecific binding followed by overnight incubation of the samples at 48C in a humidified chamber with the optimized dilution of primary anti-pStat3 antibody. PowerVision HRP mouse antirabbit (Leica Microsystems, Bannockburn, IL) (30 min at room temperature) was applied as the secondary antibody, followed by 3.3’-diaminobenzidine to detect the antigen with hematoxylin tissue counterstain. Primary antibody was omitted as a control for nonspecific binding of the secondary antibody. Slides were counterstained with hematoxylin, dehydrated, and mounted with resinous media for viewing. After the initial development of the 0 to 3 grading scale (Table 1; Fig. 1), slides were scored independently by 2 individuals (E.C.W. and C.L.S.). Slides were evaluated at ×20 and ×40 on a Nikon E800 microscope.
TABLE 1.
Grading Scale Used to Evaluate pStat3 IHC
| Grade | Finding |
|---|---|
| 0 | No staining |
| 0.5 | Single, scattered immune cells and/or epithelial cells |
| 1 | Small, scattered foci immune cells and/or epithelial cells |
| 2 | Moderate sized, scattered foci immune cells and/or epithelial cells |
| 3 | Many and/or large areas immune cells and/or epithelial cells |
FIGURE 1.
Grading scale used to evaluate pStat3 IHC. Representative images of pStat3 staining in immune cell and colonic epithelial cell compartments (arrows highlight Stat3-positive immune cells [IC] and epithelial cells [EC]). Magnification, ×20 with ×40 inset in EC1, IC1, and IC2.
Mucosal Permeability
Colonic permeability was assessed by administering fluorescein isothiocyanate–dextran (MW 4000) by gavage and then measuring levels in the blood at 16 hours, 1 day, and 3 days after ETBF inoculation, as described previously. 22 mg/mL of fluorescein isothiocyanate–dextran in 20 mL/kg body weight of PBS was administered by gavage 4 hours before killing. Mice were killed at 16 hours, 1 day, and 3 days, and a blood sample was collected by cardiac puncture. The blood sample was centrifuged (3000 rpm at 4°C) for 20 minutes. 50 µL plasma was mixed with 50 µL PBS and added to 96-well microplate. Fluorescein concentration was determined by spectrofluorometer (excitation 485 nm and emission 530 nm). A standard of serially diluted marker was used to determine the serum concentration.
Flow Cytometry
Mucosal intraepithelial and lamina propria lymphocytes were isolated from the colon of ETBF-infected C57BL/6 mice at 1 week, 12 months, and 18 months, as previously described.25 C57BL/6Stat3ΔIEC mice were similarly evaluated at 1 week. Mononuclear cells were isolated by Percoll gradient (GE Healthcare Life Science, Pittsburgh, PA) separation. Splenocytes were isolated from enzymatically dissociated spleen, as previously described,25 and using LymphoPrep density gradient (Accurate Chemical & Scientific Corporation, Westbury, NY). Resulting cell suspensions were stimulated 4 hours with phorbol 12-myristate 13-acetate (30 nM), ionomycin (1 mM) in presence of golgistop (BD Biosciences, San Jose, CA), and then stained for cell surface markers followed by intracellular cytokines using a fixation/permeabilization procedure (Cytofix/Cytoperm; BD Biosciences). We used LSRII cytometer (BD Biosciences) for flow cytometry and analyzed data with DIVA software (BD Biosciences). We used antibodies to the following proteins: IFN-γ (clone XMG1.2), IL-17A (clone eBio17B7), CD4 (clone RM4.5), CD8a (clone 53-6.7), and CD3e (clone 145-2C11).
Cell Lines and BFT Treatment
HT29/C1 cells (a clone from parental HT29 cells derived from a human colon carcinoma, obtained from Dr Daniel Louvard, Institute Pasteur, Paris, France) were grown under subconfluent or polarized conditions, as previously described.26,27
All culture media and reagents were purchased from GIBCO BRL Life Technologies (Rockville, MD). BFT was purified from the culture supernatants of B. fragilis strain 86–5443-2-2, as previously described.28 All experiments with BFT were performed in serum-free media. Cells were incubated with BFT (100 ng/mL) or IL-6 (20 ng/mL) for 30 to 60 minutes.
Statistics
Comparison of means was performed by unpaired Mann– Whitney U test. A P value of ≤0.05 was considered to designate a significant difference.
Ethical Considerations
In accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care International, mice were maintained under specific pathogen-free conditions and studied according to protocols approved by the Johns Hopkins University Animal Care and Use Committee.
RESULTS
ETBF Rapidly Activates Stat3 Throughout the Gastrointestinal Tract
Stat3 signaling is required for Th17 cell generation while, concomitantly, being able to suppress Th1-mediated inflammation.27 We have previously shown that ETBF-induced murine colitis is rapid in onset (<24 h) and that ETBF colonization and colitis persists in C57Bl/6 mice up to 16 months of age.20 Furthermore, ETBF colitis is accompanied by selective Stat3 activation by 2 days after the initial colonization of multiple intestinal neoplasia mice with an increase in IL-17–producing CD3+CD4+ T cells at 1 week after inoculation.25
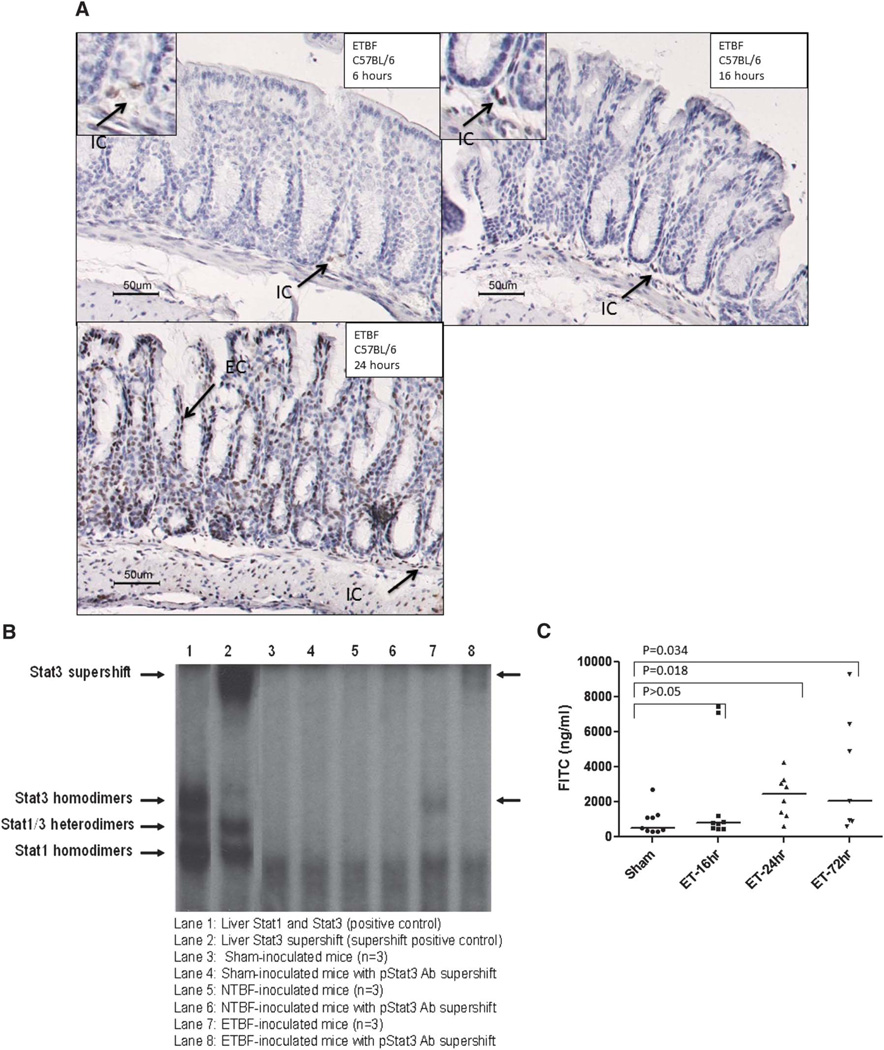
Activation of other Stat proteins except for limited pStat4 was not detected at 2 days and 1 week after NTBF or ETBF colonization of C57Bl/6 mice (data not shown). To further understand the relationship between ETBF-induced colitis and Stat3 activation, we assessed the early time course and anatomical extent of Stat3 signaling in the gastrointestinal tract of C57BL/6 mice after ETBF colonization compared with sham or NTBF-colonized mice. By 24 hours, there was colitis in the ETBF-colonized mice on histopathologic findings compared with the NTBF- and/or sham-inoculated mice, as previously reported (Rhee et al20, data not shown). In contrast, inflammation in the upper gastrointestinal tract (esophagus, stomach, mid and distal small bowel) and liver was not observed. Stool cultures revealed similar colonization in the ETBF- and NTBF-inoculated mice (approximately 108–109 CFU/gm stool). Activated Stat3 (pStat3) was noted in the ETBF-colonized mice at all time points compared with the sham-inoculated mice from the mid small bowel to the distal colon with the most intense signals noted in the cecum to distal colon. Liver and spleen samples did not demonstrate a difference in Stat3 activation in ETBF compared with shamor NTBF-colonized mice by Western blot analysis, despite the presence of splenomegaly in the ETBF-colonized mice at 1 week (data not shown, Rhee et al20). Stat3 activation was evident in the intestinal colonic immune cells (8 of 9 ETBF mice with positive immune cell staining) as early as 6 hours, but this was not consistently greater than that observed in sham (1 of 3 mice with positive immune cell staining) or NTBF (2 of 2 mice with positive immune cell staining) mice. By 16 hours after ETBF inoculation, Stat3 activation in the immune cells was noted in all mice (Table 2; Fig. 2A). Rare immune cell activation was noted in sham or NTBF controls. In ETBF mice, epithelial cell Stat3 activation, both colonic and small intestinal, was noted by 16 hours and both intestinal immune cell and epithelial cell Stat3 intensified between 16 and 24 hours. Table 2 illustrates the early time course for Stat3 activation in the colon of wild-type ETBF-colonized mice compared with sham controls as assessed by immunohistochemistry (IHC). Limited analyses of the small intestine suggested that Stat3 was activated transiently, during the first week after inoculation. We further confirmed nuclear pStat3, but not pStat1 or pStat5 in the distal colon, by EMSA at day 4 after inoculation of C57Bl/6 mice with ETBF but not in NTBF- or sham-inoculated mice (n = 3 mice/group; Fig. 2B and data not shown). Measurable increases in mucosal permeability were noted at 24 hours and were still elevated at 3 days, suggesting that initial immune cell Stat3 activation (16 h) is likely mediated by early epithelial cell events triggered by the initial interactions of colonic epithelial cells with ETBF (likely through BFT), whereas peak mucosal Stat3 activation (immune and epithelial cells) occurs at a later time point when other colonic bacteria may contribute to the immune response due to barrier dysfunction (Fig. 2C).
TABLE 2.
Evaluation of Early Stat3 Activation in the Colons of ETBF-colonized C57Bl/6 Mice Compared with Controls
| Sham |
NTBFa |
ETBF |
||||
|---|---|---|---|---|---|---|
| Time Point | ICs | CECs | ICs | CECs | ICs | CECs |
| 6 h | ||||||
| Mean ± SEMa | 0.67 ± 0.4 | 0.0 | 1.1 | 0.0 | 0.61 ± 0.1 | 0.0 |
| Range | 0–1.5 | N/A | N/A | N/A | 0–1.5 | N/A |
| N | 3 | 3 | 2 | 2 | 9 | 9 |
| 16 h | ||||||
| Mean ± SEM | 0.5 ± 0.3 | 0.0 | 0.1 | 0.0 | 1.0 ± 0.2b | 0.0 |
| Range | 0–1 | N/A | N/A | N/A | 0.5–2 | N/A |
| N | 3 | 3 | 2 | 2 | 8 | 8 |
| 24 h | ||||||
| Mean ± SEM | 0.17 ± 0.17 | 0.0 | 0.5 ± 0.5 | 0 ± 1 | 2.3 ± 0.4c | 2.3 ± 0.7 |
| Range | 0–0.5 | N/A | N/A | N/A | 0–3 | 1– 3 |
| N | 3 | 3 | 2 | 2 | 5 | 5 |
All results from 2 independent experiments.
For sample sizes of 2, individual values are shown.
P = 0.06 (6 h versus 16 h IC).
P = 0.17 (16 h versus 24 h IC).
ICs, mucosal immune cells; CECs, colonic epithelial cells; N/A, not applicable.
FIGURE 2.
Stat3 activation and mucosal permeability in ETBF-colonized C57BL/6 mice. A, Early ETBF-induced Stat3 activation. Representative pStat3 staining on distal colonic tissue from early time points (6, 16, and 24 h after ETBF inoculation) demonstrating early pStat3 in immune cells (6 h, 16 h, arrows) followed by later pStat3 in colonic epithelial cells (24 h, arrows). Sham-treated mice had none to rare pStat3 in the immune or epithelial cell compartment (not shown). Magnification, ×20 with ×40 insets in the 6- and 16-hour images. B, EMSA demonstrates increased Stat3 activation in ETBF-inoculated mice at 4 days (lanes 7 and 8). Stat3 activation is not detected in sham-inoculated (lanes 3 and 4) or NTBF-inoculated (lanes 5 and 6) mice. Results are representative of 3 mice in each group evaluated (sham, NTBF, ETBF). C, Mucosal permeability assay demonstrates increased permeability in ETBF-inoculated mice at 24 hours and 3 days as compared with sham-inoculated mice. Experiment was conducted twice with 3 to 4 mice per group.
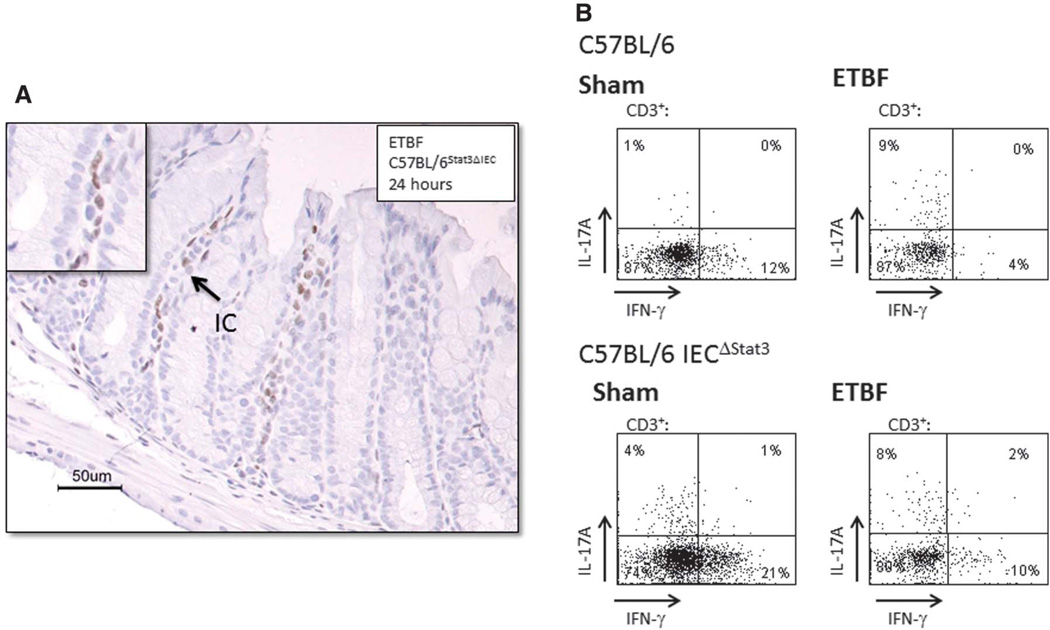
To determine if the rapid onset of the immune cell Stat3 activation in ETBF mice was dependent on epithelial cell Stat3 activation, we evaluated ETBF-infected C57BL/6Stat3ΔIEC mice. Figure 3A demonstrates that immune compartment Stat3 activation is unimpeded by the loss of epithelial cell Stat3 signaling (n = 6 mice). Loss of epithelial pStat3 did not inhibit the development of ETBF colitis; histologic inflammation scores from the proximal and distal colon were similar at 1, 3, and 7 days in ETBF-infected C57BL/6Stat3ΔIEC and C57BL/6 mice (Table 3). In ETBF-C57BL/6Stat3ΔIEC mice, the mucosal IL-17 expression was maintained (Fig. 3B).
FIGURE 3.
Epithelial pStat3 activation contributes to ETBF colitis. A, Stat3 activation in mucosal immune cells 24 hours after ETBF colonization in C57BL/6 mice Stat3ΔIEC. Magnification, ×20 with ×40 insets. (Representative photograph from experiment with 7 mice.) B, Flow cytometry analysis of intracellular staining for IL-17 and IFN-γ in isolated CD3+ mucosal immune cells from the distal colon of C57BL/6 mice and C57BL/6Stat3ΔIEC colonized with ETBF for 1 week (n = 1, experiment repeated twice).
TABLE 3.
Evaluation of Proximal and Distal Colon Inflammation in ETBF-colonized Rag-1 and C57Bl/6Stat3ΔIEC Mice Compared with ETBF-colonized C57BL/6 Mice
| ETBF C57Bl/6Stat3ΔIEC |
ETBF-Rag-1 |
ETBF-C57Bl/6 |
Sham-C57Bl/6Stat3ΔIEC |
Sham Rag-1 |
Sham-C57Bl/6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | PC | DC | PC | DC | PC | DC | PC | DC | PC | DC | PC | PC |
| 1 d | ||||||||||||
| Mean ± SEMa | 1.25 ± 0.3 | 0.5 ± 0.25 | 0.0 | 0.0 | 1.22 ± 0.2 | 0.89 ± 0.3 | 0.1 | 0.0 | 0.0 | 0.33 ± 0.33 | 0.1 | 0.0 |
| Range | 0–2 | 0–1 | N/A | N/A | 0–2 | 0–2 | 0–1 | N/A | N/A | 0–1 | 0–1 | N/A |
| N | 8 | 8 | 5 | 5 | 9 | 9 | 2 | 2 | 3 | 3 | 2 | 2 |
| 2 d | ||||||||||||
| Mean ± SEM | ND | ND | 0.25 ± 0.18 | 0.25 ± 0.18 | ND | ND | ND | ND | 0.0 | 0.0 | ND | ND |
| Range | — | — | 0–1 | 0–1 | — | — | — | — | N/A | N/A | — | — |
| N | — | — | 4 | 4 | — | — | — | — | 2 | 2 | — | — |
| 3 d | ||||||||||||
| Mean ± SEM | 2.14 ± 0.14 | 0.57 ± 0.2 | ND | ND | 2.71 ± 0.18 | 0.57 ± 0.2 | 0.0 | 0.1 | ND | ND | 0 | 0 |
| Range | 2–3 | 0–1 | — | — | 2–3 | 0–1 | N/A | 0–1 | — | — | N/A | N/A |
| N | 7 | 7 | — | — | 7 | 7 | 2 | 2 | — | — | 1 | 1 |
| 7 d | ||||||||||||
| Mean ± SEM | 1.75 ± 0.25 | 0.75 ± 0.16 | 1.83 ± 0.30 | 1 ± 0.26 | 1.78 ± 0.4 | 0.89 ± 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Range | 1–3 | 0–1 | 0–3 | 0–2 | 0–3 | 0–2 | N/A | N/A | N/A | N/A | N/A | N/A |
| N | 8 | 8 | 6 | 6 | 9 | 9 | 2 | 2 | 5 | 5 | 1 | 1 |
SEM, standard error of the mean, for sample sizes of 2, individual values are shown.
ICs, mucosal immune cells, CECs, colonic epithelial cells; N/A, not applicable; ND, not done.
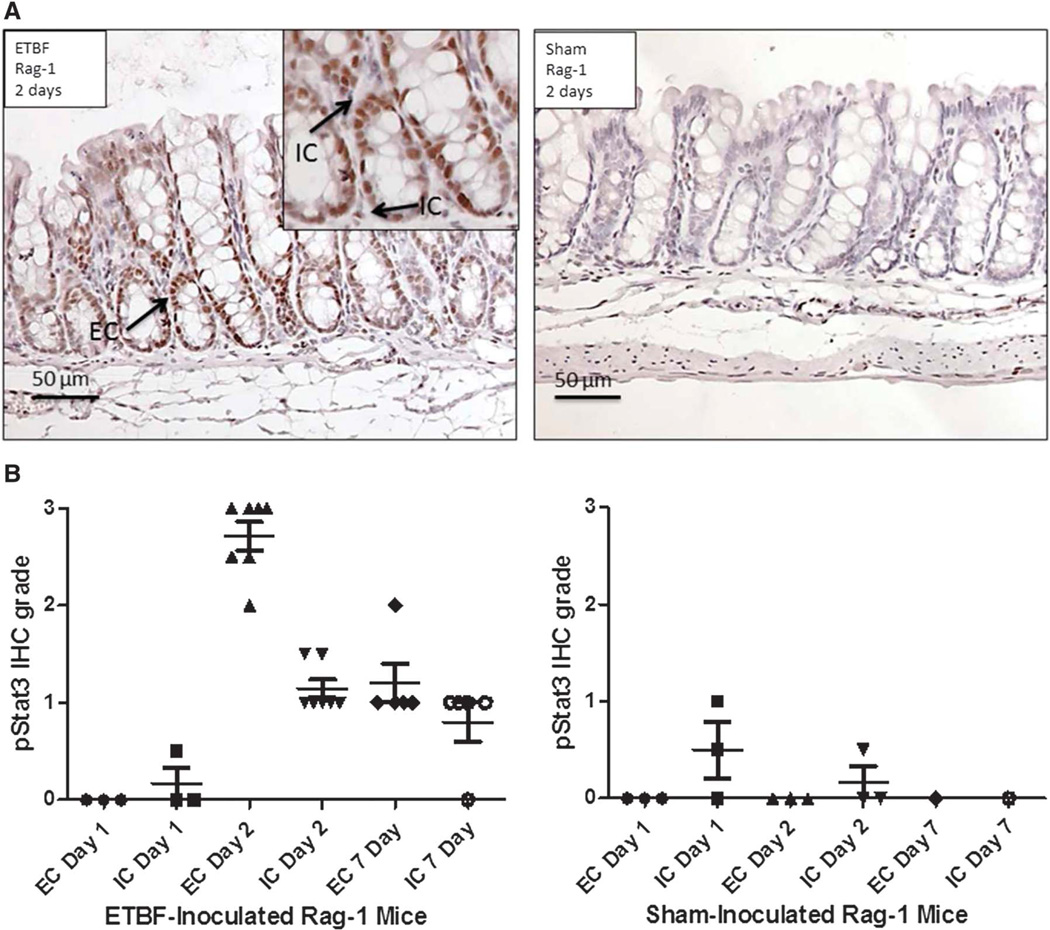
To determine if T and B cells are required for ETBF-induced colonic inflammation and epithelial pStat3 activation, we evaluated ETBF-colonized Rag-1 mice at 1 and 2 days and 1 week. Both intestinal inflammation and epithelial cell pStat3 activation occurred in the absence of T and B cells but was delayed. No intestinal inflammation was noted by histologic assessment in the ETBF Rag-1 mice at 1 day and minimal at 2 days (Table 3). By 7 days, similar levels of inflammation were noted in Rag-1 and C57BL/6 mice colonized with ETBF. Low levels of immune cell pStat3 activation noted at 1 day in ETBF Rag-1 mice was similar to that seen at 16 hours in ETBF C57BL/6 mice (n = 3 mice at 1 d). Consistently elevated levels of mucosal immune and epithelial cell Stat3 activation was noted by 2 days and persisted at 7 days in ETBF Rag-1 mice (Fig. 4A, B). These results suggest that adaptive immune cells are not required for the induction of ETBF colonic inflammation.
FIGURE 4.
ETBF-induced epithelial pStat3 activation occurs in the absence of T and B cells. A, Stat3 activation in the epithelial and immune cells of ETBF-colonized Rag-1 mice colonized with ETBF versus sham for 2 days. Magnification, ×20 with ×40 insets. B, pStat3 by IHC in the colons of ETBF-colonized and sham Rag-1 mice at 1,2, and 7 days (n = 1–7 mice per condition). IC, immune cell; EC, epithelial cell.
ETBF-induced Stat3 Activation Persists Chronically in the Colon
To assess if ETBF induces persistent activation of Stat3, we examined Stat3 activation over the longer time course of ETBF colitis using IHC and/or Western blot (Table 4). In C57Bl/6 mice colonized for 1 month with ETBF, Western blot did not demonstrate pStat3 (n = 4 of 4 mice, data not shown); however, patchy dense areas of positive pStat3 signal were detected in the proximal and distal colon by IHC of some ETBF-colonized mice (n = 10 of 14 mice with detectable pStat3 in distal colon; Fig. 5). Furthermore, while clusters of pStat3-positive immune cells alone could be identified, clusters of pStat3-positive colonic epithelial cells were invariably associated with subepithelial pStat3-positive immune cells, consistent with the early pattern noted where Stat3 activation in the colonic immune compartment preceded Stat3 activation in the colonic epithelial compartment in ETBF-colonized C57Bl/6 mice (Fig. 2A; Table 2). Similar results were observed by IHC in ETBF-colonized C57BL/6 mice from 3 to 8 months compared with sham or NTBF-colonized control mice (Table 4). The patchy Stat3 activation detected in ETBF-colonized mice was without predominant localization in the proximal or distal colon at each time point examined.
TABLE 4.
Evaluation of Late Stat3 Activation in the Colons of ETBF-colonized C57Bl/6 Mice Compared with Controls
| Sham |
NTBF |
ETBF |
||||
|---|---|---|---|---|---|---|
| Time Point | ICs | CECs | ICs | CECs | ICs | CECs |
| 1 mo | ||||||
| Mean ± SEMa | 0.5 ± 0.29 | 0.0 | 0.80 ± .25 | 0.2 ± 0.2 | 1.6 ± 0.3 | 1.7 ± 0.353 |
| Range | 0–1 | N/A | 0–2 | 0–2 | 0–3 | –3 |
| N | 4 | 4 | 10 | 10 | 14 | |
| 3–4 mo | ||||||
| Mean ± SEM | 0.1 | 0.0 | N/A | N/A | 1.5 ± 0.19 | 1.75 ± 0.31 |
| Range | 0–1 | N/A | — | — | 1–2 | –3 |
| N | 2 | 2 | — | — | 8 | |
| 8 mo | ||||||
| Mean ± SEM | N/A | N/A | 0.0 | 0.0 | 1.6 ± 0.26 | 1.5 ± 0.31 |
| Range | — | — | N/A | N/A | 0–3 | 0–3 |
| N | — | — | 3 | 3 | 7 | |
SEM standard error of mean, for sample sizes of 2, individual values are shown.
ICs, mucosal immune cells, CECs, colonic epithelial cells; N/A, not applicable.
FIGURE 5.
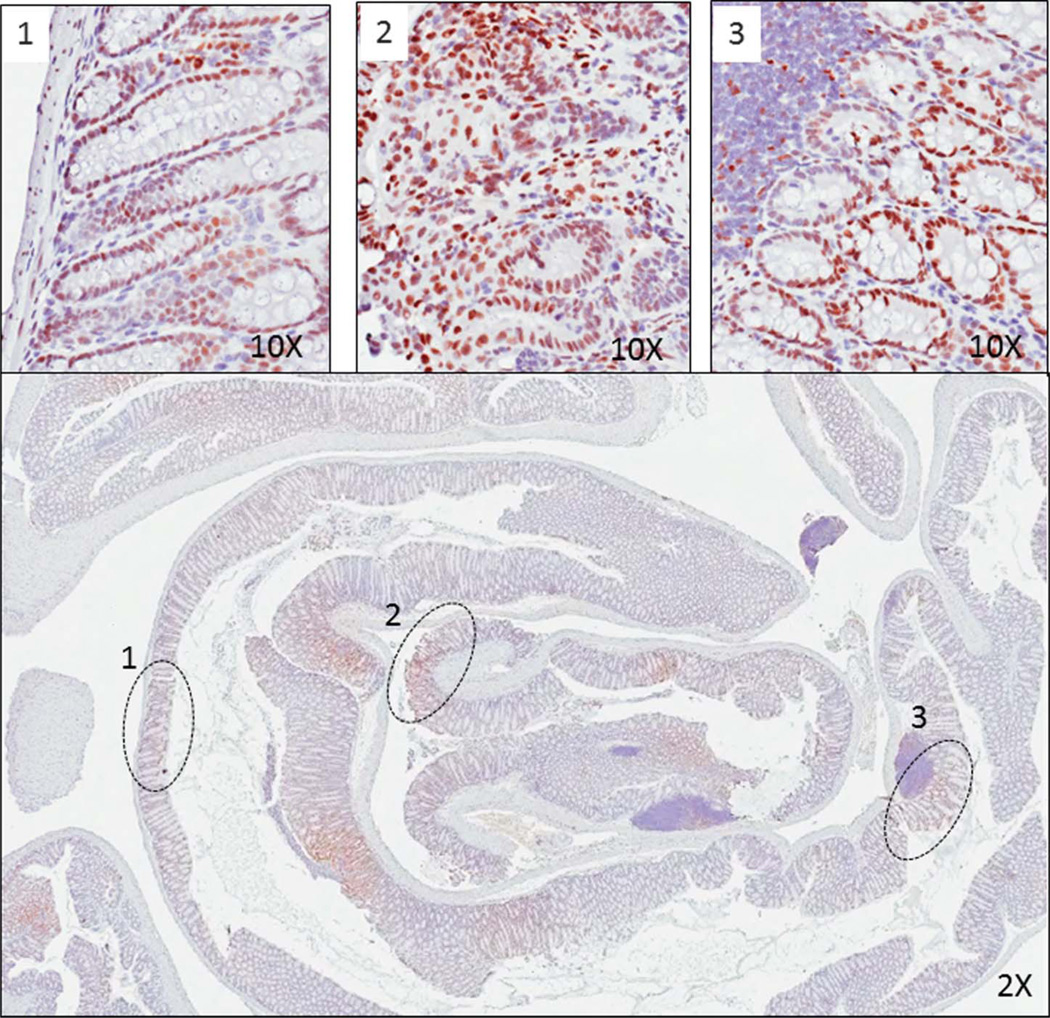
ETBF-induced Stat3 activation in the colon at 1 month by IHC. ETBF induces patchy Stat3 immune and epithelial cell activation in both the proximal and distal colon, which persists for up to 18 months. Representative slide from 1 month after inoculation. Low power (×2) view illustrates patchy areas of positive staining, with high power (×10) insets demonstrating immune and epithelial cell staining at (1) distal colon, (2) midcolon, and (3) proximal colon.
We have previously reported that ETBF chronically colonizes and induces asymptomatic persistent colitis lasting at least 15 months in C57Bl/6 mice maintained on antibiotic-containing water throughout the experiments.20 To test if continuous antibiotic exposure modified ETBF-induced colon Stat3 activation, ETBF-infected C57Bl/6 mice treated with preinoculation or continuous antibiotics (see Materials and Methods) were evaluated for colonic Stat3 activation at 3 months. Both groups of mice had similar levels of pStat3 by IHC (mean ± standard error of the mean, immune cells and colonic epithelial cells: continuous antibiotics, 1.5 ± 0.19 and 1.75 ± 0.31, respectively, n = 8; preinoculation antibiotics: 1.33 ± 0.17 and 1.83 ± 0.24, respectively, n = 9), suggesting that antibiotic exposure did not modify this response to ETBF colonization. Although ETBF colonization was different in the 2 groups of mice (continuous antibiotics, 9.47 × 109 versus no continuous antibiotics, 1.71 × 10; P = 0.005; 1 experiment: n = 10 mice per group), the overall colonization in both groups was very high, and the differences were likely inconsequential.
We previously reported that flow cytometry analysis of the isolated intraepithelial lymphocytes and lamina propria lymphocyte populations showed a brisk Th17 response at 1 week of ETBF colonization.25
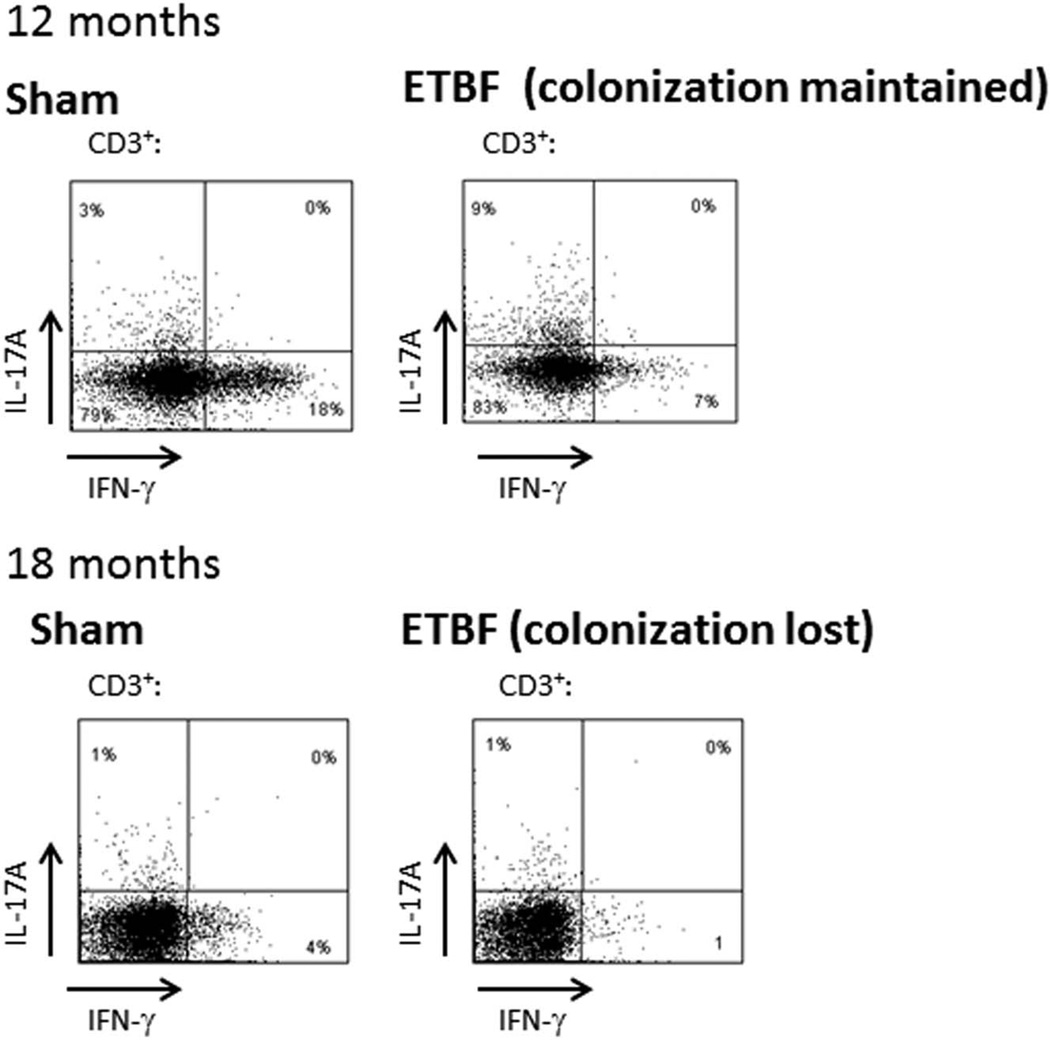
This predominant colonic Th17 immune response was present at 2 months (data not shown) and persisted at 12 months of ETBF colonization in the absence of continuous antibiotic exposure (Fig. 6). However, at 18 months, ETBF fecal colonization was lost, and the mucosal Th17 response was similar to sham mice (Fig. 6).
FIGURE 6.
ETBF colonic mucosal immune profile at 12 and 18 months by flow cytometry analysis. Intracellular staining for IL-17 and IFN-γ in CD3+ mucosal immune cells of sham and ETBF-colonized C57BL/6 mice from mucosal immune cells isolated from the distal colon at 12 and 18 months. At 12 months, ETBF mice were colonized with 108 ETBF/g stool, and at 18 months, ETBF colonization was lost; n = 3 (12 mo); n = 2 mice/group (18 months).
BFT Does Not Activate Stat3 In Vitro
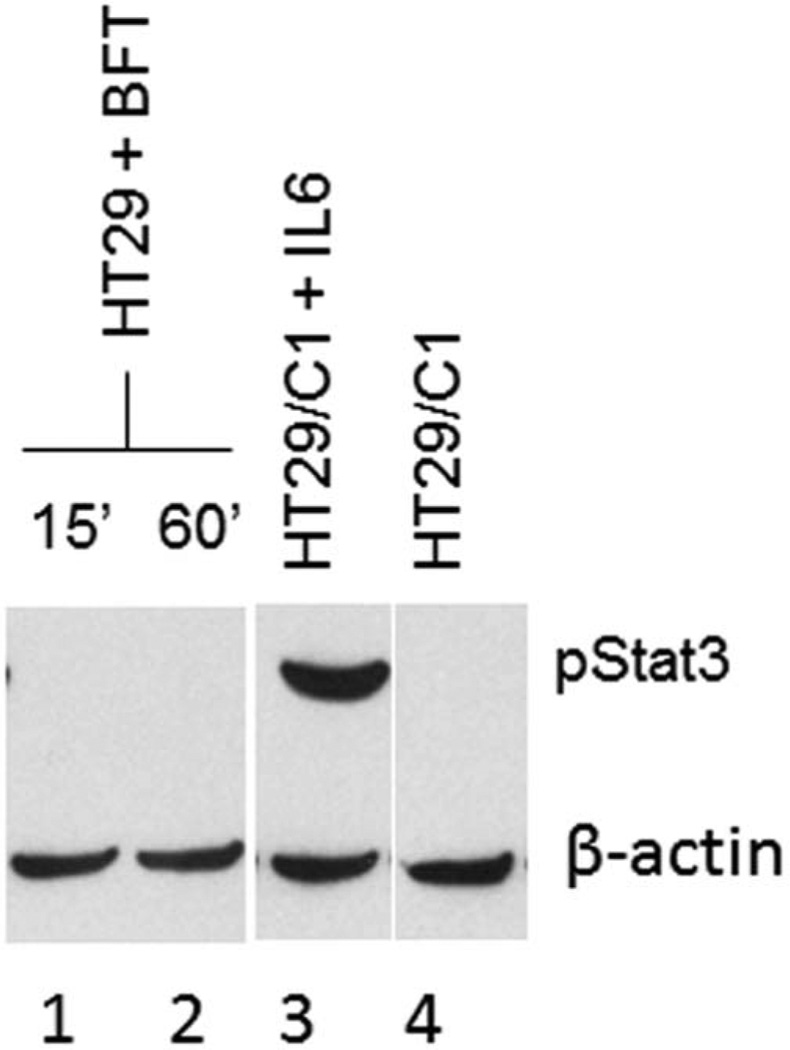
To begin to determine the role of BFT in ETBF-induced activation of Stat3, we treated subconfluent HT29/C1 cells (n = 5 experiments; data pooled) or polarized T84 cells (n = 1 experiment) with BFT (5 nM) for varying periods and assessed the activation of Stat3 by Western blot. Both cell lines exhibit biologic responses to BFT that are maximal at 100 ng/mL (5 nM) of BFT.29 Treatment of both cell lines with IL-6 (20 ng/mL for 20 min), a known inducer of Stat3 transcription, led to Stat3 activation detected by Western blot in these cell lines. In contrast, pStat3 was not detected in BFT-treated HT29/C1 cells at time points from 20 minutes to 72 hours or in polarized T84 cells treated with apical and basolateral BFT for 24 hours (Fig. 7). These results suggested that BFT does not directly induce Stat3 activation at least in continuous human colonic epithelial cells in vitro.
FIGURE 7.
Immunoblot for pStat3 of HT29/C1 cells treated with BFT. HT29/C1 cells treated with BFT for 15 and 60 minutes (lanes 1 and 2) do not show Stat3 activation. Stat3 activation was observed in cells treated with IL-6 (positive control, lane 3). Untreated cells serve as a negative control (lane 4). Similar results were obtained with cells treated up to 72 hours with BFT (results not shown).
DISCUSSION
Our results demonstrate that in C57BL/6 mice, ETBF, but not NTBF, induces extremely rapid then persistent Stat3 activation in the colonic immune and epithelial cells. Our time course analyses reveal, unexpectedly, that Stat3 activation presents first in the colonic mucosal immune cells followed by Stat3 activation in the colonic epithelial cells, all occurring in less than 24 hours. Our previous data identified that the BFT receptor is limited to epithelial cells and that colitis is ablated in C57Bl/6 mice colonized with a wild-type ETBF strain in which BFT is deleted.20,28 Herein, our in vitro data reveal that BFT did not directly activate Stat3 in human continuous colonic epithelial cell lines. These cell lines are used to define the biology of BFT and have correlated well over time with in vivo findings.15
Initial immune cell Stat3 activation (16 h) occurred before the detection of a significant increase in permeability (24 h) (Fig. 2C). Together, these data suggest a model by which ETBF colonization initiates a signaling relay; namely, ETBF, through BFT, briskly signals through the colonic epithelial cells to the mucosal immune compartment, resulting in the activation of Stat3 in a subset of immune cells that, in turn, may induce subsequent Stat3 activation in the colonic epithelial cell compartment. Our use of the C57BL/6Stat3ΔIEC mice revealed that the ETBF-induced initial Stat3 activation in the mucosal immune cell is not dependent on the Stat3 signaling in the colonic epithelial cells. Despite the absence of T and B cells, Rag-1 mice developed epithelial pStat3 activation, suggesting that the innate immune response alone can provide the signals that activate epithelial cell Stat3 after ETBF colonization. This type of early, rapid submucosal signaling from an intraluminal bacterial toxin has previously been reported, for example, with Clostridium difficile toxin A and is consistent with the concept that intestinal physiology and pathophysiology is, in part, dictated by, as yet, poorly understood and complex signaling occurring between the luminal microbiota, the epithelial compartment, and the enteric immune and nervous systems.30
ETBF does increase mucosal permeability between 1 and 3 days after colonization, coinciding with peak mucosal Stat3 activation (immune and epithelial cells), suggesting that other colonic bacteria may contribute to this mucosal response via barrier dysfunction. In addition consistent with in vitro studies, BFT, likely, also contributes to barrier dysfunction through its ability to induce E-cadherin cleavage.26,28,31,32 Further studies are needed to define the critical colonic epithelial cell signaling mediators induced by ETBF, resulting in subepithelial immune cell Stat3 activation, the initial responsive immune subset(s), and the mediators from the submucosa triggering subsequent colonic epithelial cell Stat3 activation.
Our data show that long-term ETBF colonization continues to be associated with patchy, but persistent and closely associated, colonic and immune cell Stat3 activation. This is in sharp contrast to our controls, sham-treated or NTBF-colonized mice, that exhibit quite limited to virtually no Stat3 activation in the colonic immune and epithelial cell compartments. Notably, despite the sporadic colonic distribution of pStat3-positive immune or epithelial cells detected by IHC in chronic ETBF colonization, the overall colonic immune response in ETBF-colonized mice at 12 months remained Th17 dominant (Fig. 6). The critical link between ETBF, colonic Stat3 signaling, the Th17 mucosal immune response, and colitis is emphasized by the loss of these parameters when ETBF colonization is no longer detectable (Fig. 6; data not shown).
In contrast, only transient Stat3 activation with subtle small intestinal cell hyperplasia was detected in ETBF-inoculated mice. These results are likely explained by our understanding that neither B. fragilis nor ETBF establish stable colonization of the small intestine.20
Nonetheless, the data suggest that only transient exposure to ETBF is sufficient to trigger, at least limited, changes in epithelial cell biology, even in the small intestine.33 We suspect that this finding, in part, results from the coprophagic behavior of mice. Despite the rapid development by 1 week of splenomegaly in ETBF-colonized mice, indicating a systemic immune response to ETBF colonization, Stat3 activation was not identified in the spleen or liver, and furthermore, the splenic immune response is predominantly Th1 in character.25
Recent data indicate that the Stat3 pathway may be an important target in the treatment of IBD. In addition to a few murine colitis models, Stat3 is activated in patients with Crohn’s disease and ulcerative colitis.31,34–36 Genome-wide association studies have identified genetic variations in Stat3 as one of the genes that confers risk for IBD.1 A recent study demonstrated that certain Stat3 risk alleles are associated with increased activation of Stat3 in patients with IBD and induction of pathways regulating leukocytes in affected colons.37 Janus kinases (JAK) are tyrosine kinases essential for the signaling pathways of various cytokines. Stat proteins are phosphorylated (activated) by JAKs, leading to downstream cytokine production. An oral JAK inhibitor has been shown effective in the treatment of moderate-to-severe ulcerative colitis.36 This points to the potential need for further investigation of possible IBD treatments through the JAK/Stat signaling pathways and emphasizes the importance of murine colitis models to study these pathways.
Similarly, downstream effects of activated Stat3, IL-17, and Th17 immune responses are increasingly recognized as important in the pathogenesis of IBD. Th17 cells, as well as IL-17a and IL-17f, are found at high levels in the diseased intestinal mucosa from some patients with IBD, suggesting the importance of these mediators in IBD pathogenesis.38,39 The Th17 family of cytokines is a target for autoimmune disease drug development, and these drugs are currently being studied in IBD patients.40 Specifically, clinical trials using anti-IL17 antibody for the treatment of Crohn’s disease are underway.41 Likewise, Th17 cells and IL-17 are important to the pathogenesis of many murine colitis models. Specifically, mice treated with trinitrobenzenesulfonic acid or with Citrobacter rodentium–associated or Helicobacter hepaticus–associated colitis have elevated levels of IL-17 detected in their intestinal mucosa.42–44 Mice deficient in the IL-17A receptor are significantly protected against trinitrobenzenesulfonic acid colitis.45 However, the contribution of IL-17 to colonic health or disease is nuanced. For example, anti–IL-17A antibody administration to mice with dextran sodium sulfate–induced colitis exacerbates dextran sodium sulfate–associated intestinal inflammation, but IL-17A–knockout mice treated with dextran sodium sulfate do not develop as severe colitis as litter mate control mice.46 In the ETBF model of colitis, IL-17 is a critical mediator of colonic inflammation. Consistent with this, the loss of chronic ETBF colonization ameliorates both histologic colitis and the colon Th17 immune response.
In IBD, long-standing intestinal inflammation is associated with an increased risk of dysplasia and cancer. Several small studies have demonstrated that IBD patients with dysplasia have higher levels of activated Stat3 (pStat3) as compared with those without dysplasia, and the degree of Stat3 activation increases along the continuum of dysplasia to colitis-associated cancer.36,47,48
In humans, ETBF is associated with inflammatory diarrhea and, in the few studies conducted to date, active IBD and colorectal cancer.17 Available data further suggest that ETBF is often acquired at a young age and that chronic colonization may ensue. In fact, in a small study, 40% of evaluated adults were identified as asymptomatically colonized with ETBF. However, no longitudinal human studies have defined the impact of ETBF colonization on colonic function or immune responses. Thus, we postulate that chronic ETBF colonization with subclinical colitis and induction of persistent foci of Stat3 activation and IL-17 induction serve as foci for the initiation of colon tumor formation in humans. This putative time line is consistent with the acknowledged long lead time until human colorectal cancer is clinically detected (10–40 yr) and with data demonstrating that Stat3 not only is absolutely required for the generation of Th17 cells but also serves as a critical regulatory protein by which inflammatory and oncogenic signals converge.49,50 In murine models, both immune and epithelial Stat3 activation contributes to oncogenesis. Consistent with the hypothesis that ETBF may contribute to colon tumor initiation, ETBF is oncogenic in the multiple intestinal neoplasia mouse bearing an Apc mutation, a model of the familial adenomatous polyposis syndrome that inexorably is associated with intestinal tumor formation.25
In summary, murine ETBF infection rapidly activates Stat3 first in a subset of the mucosal immune cells followed by the colonic epithelial cells, coinciding with increased mucosal permeability. Mucosal permeability increased by 24 hours and persisted at 3 days in ETBF mice, concurrent with the onset of histologic changes in the colon epithelium induced by ETBF. These data support the hypothesis that early epithelial cell mediators triggered by initial interactions of colonic epithelial cells with ETBF (likely through BFT) trigger early immune Stat3 activation, whereas peak mucosal Stat3 activation (immune and epithelial cells) occurs when other colonic bacteria may contribute to the immune response due to barrier dysfunction. Chronic ETBF colonization results in patchy colonic Stat3 activation and Th17 predominant colitis. Our results support the concept that specific members of the microbiota can contribute to long-term chronic intestinal inflammation in IBD patients with implications for their development of dysplasia or colitis-associated cancer. Future multidisciplinary studies should define the association of ETBF or other inflammatory microbiota members, Stat3 activation, and intestinal Th17 immune responses in the disease course and dysplasia associated with IBD.
ACKNOWLEDGMENTS
Supported by K08 DK 087856 (to E.C.W.) and American College of Surgeons Career Development Award (to E.C.W.), the Crohn’s & Colitis Foundation through a Senior Investigator Award (to C.L.S.) and a Research Fellowship Award (to K-J.R.), RO1 DK45496, DK080817 and CA151325 (to C.L.S.), Institutional Training for Pediatricians 5 T32 HD44355 (to G Dover), NRSA Individual Training Grant F32 DK079509 (to S.R.) and K08 DK089076-01 (to S.R.).
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 4.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Vijay-Kumar M, Gewirtz AT. Role of flagellin in Crohn’s disease: emblematic of the progress and enigmas in understanding inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:789–795. doi: 10.1002/ibd.20734. [DOI] [PubMed] [Google Scholar]
- 6.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 8.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Franke A, Balschun T, Karlsen TH, et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713–715. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 10.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 11.Swidsinski A, Weber J, Loening-Baucke V, et al. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitomersky NL, Coyne MJ, Comstock LE. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect Immun. 2011;79:2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 14.Bennion RS, Baron EJ, Thompson JE, Jr., et al. The bacteriology of gangrenous and perforated appendicitis—revisited. Ann Surg. 1990;211:165–171. doi: 10.1097/00000658-199002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349–369. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sears CL, Islam S, Saha A, et al. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin Infect Dis. 2008;47:797–803. doi: 10.1086/591130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prindiville TP, Sheikh RA, Cohen SH, et al. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 19.Basset C, Holton J, Bazeos A, et al. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–1432. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 20.Rhee KJ, Wu S, Wu X, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabizadeh S, Rhee KJ, Wu S, et al. Enterotoxigenic bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007;13:1475–1483. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerdeno-Tarraga AM, Patrick S, Crossman LC, et al. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science. 2005;307:1463–1465. doi: 10.1126/science.1107008. [DOI] [PubMed] [Google Scholar]
- 23.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 24.Yu CL, Meyer DJ, Campbell GS, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers FG, Koshy SS, Saidi RF, et al. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 cells) in vitro. Infect Immun. 1997;65:3561–3570. doi: 10.1128/iai.65.9.3561-3570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Shin J, Zhang G, et al. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect Immun. 2006;74:5382–5390. doi: 10.1128/IAI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Morin PJ, Maouyo D, et al. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 30.Pothoulakis C, Castagliuolo I, LaMont JT, et al. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovato P, Brender C, Agnholt J, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J Biol Chem. 2003;278:16777–16781. doi: 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Powell J, Mathioudakis N, et al. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004;72:5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Rhee KJ, Zhang M, et al. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944–1952. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudter J, Weigmann B, Bartsch B, et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 35.Carey R, Jurickova I, Ballard E, et al. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, de Haar C, Chen M, et al. Disease-related expression of the IL6/ STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 37.Willson TA, Kuhn BR, Jurickova I, et al. STAT3 genotypic variation and cellular STAT3 activation and colon leukocyte recruitment in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:32–43. doi: 10.1097/MPG.0b013e318246be78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58:1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 41.Fitzpatrick LR. Novel pharmacological approaches for inflammatory bowel disease: targeting key intracellular pathways and the IL-23/IL-17 axis. Int J Inflam. 2012;2012:389404. doi: 10.1155/2012/389404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factorbeta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 44.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Zheng M, Bindas J, et al. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 46.Ito R, Kita M, Shin-Ya M, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Morikawa T, Baba Y, Yamauchi M, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452–1462. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wick EC, Leblanc RE, Ortega G, et al. Shift from pStat6 to pStat3 predominance is associated with inflammatory bowel disease-associated dysplasia. Inflamm Bowel Dis. 2012;18:1267–1274. doi: 10.1002/ibd.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kortylewski M, Xin H, Kujawski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]