Demyelinating injuries of the human brain are generally characterized by limited remyelination, and recurrent episodes of demyelination are especially bereft of recovery potential. Yet these same myelin-deficient foci often retain ample numbers of bipotential oligodendrocyte-astrocyte glial progenitor cells (OPCs), as well as seemingly arrested pre-myelinogenic oligodendroglia1–3. This paradoxical failure in remyelination despite persistently available progenitors has been the subject of a number of recent studies designed to explore its mechanistic basis, as part of a broader effort to improve remyelination competence from endogenous progenitor cells. While a number of studies have made headway towards identifying triggers for oligodendrocyte differentiation and myelination from endogenous progenitors4,5, none have cracked the fundamental problem of addressing why the injury environment is so often non-permissive for myelinogenesis from these cells. Do disease-associated changes in the local gliovascular niche present a non-permissive environment for oligoneogenesis? Do the resident OPCs senesce under the pressures of sustained mobilization? Do they differentiate instead as reactive astrocytes rather than oligodendrocytes? Do they self-renew but undergo a discrete block in differentiation or myelinogenic competence? While evidence has arisen in support of each of these possibilities, the latter possibility - that OPC self-renewal might be effectively commandeered to prevent terminal differentiation - has become of interest in recent studies of pathways whose manipulation has permitted the prolongation of OPC self-renewal. Perhaps foremost among these pathways of interest has been that of Wnt-signaled βcatenin-dependent transcription, whose role in OPC expansion, and in tumorigenesis from OPCs when dysregulated, has been the topic of a number of recent studies6–10.
In this issue of Nature Neuroscience, Fancy and colleagues 11 studied the role of Wnt-dependent βcatenin signaling in neonatal white matter injury, using a transgenic mouse model of constitutively dysregulated βcatenin signaling. In this model, APC, an inhibitor of βcatenin availability and thus repressor of βcatenin-dependent TCF1-induction of Wnt target genes, is conditionally deleted in Olig2-expressing OPCs. The OPCs thereby generated exhibited up-regulation of a host of Wnt-regulated transcripts. Not surprisingly, many of these overlapped with Wnt-dependent transcripts already recognized as overexpressed in many colon cancers, which can be associated with disinhibited Wnt-driven gene expression due to APC mutation or deletion. But whereas the disinhibition of Wnt signaling associated with APC loss-of-function leads to carcinoma in colonic epithelial cells 12,13, in OPCs neither neoplastic expansion nor malignant transformation were noted. To the contrary, Fancy et al report that APC-null OPCs - despite their up-regulated expression of a host of Wnt-regulated transcripts - exhibited no change in expansion kinetics, but rather maturation arrest and lost myelination competence. Moreover, their up-regulation of the βcatenin binding partner LEF1 resulted in a feed-forward self-induction of LEF1 transcription, which raised the effective gain, or “tone” of Wnt signaling in these OPCs, thereby stably potentiating βcatenin-dependent signaling in a self-perpetuating fashion.
Fancy and colleagues then assessed the downstream targets of this process, recognizing that these molecules might provide a potpourri of valuable targets for modulating OPC differentiation and myelination, and thus might offer feasible targets for rational drug discovery. Focusing on the transcript most highly differentially expressed by APC-null OPCs, the SP/KLF-family transcription factor SP5, they found that SP5 expression rose sharply with the overexpression of Wnt-regulated transcripts, and that its overexpression was sufficient to suppress myelin basic protein expression by OPCs. Accordingly, SP5-null animals manifested potentiated oligodendrocytic differentiation and precocious myelination following demyelinating injury, suggesting the value of SP5 as a target for functional ablation in settings of dysregulated OPC self-renewal and arrested differentiation.
Yet many aspects of OPC biology differ fundamentally between mice and humans, so much so that one cannot assume that Wnt-regulated processes yield analogous cellular outcomes in murine and human OPCs14. To address this concern, Fancy et al. assessed the differential expression of these Wnt-regulated transcripts in tissue samples from infants with severe hypoxic-ischemic encephalopathy. Perinatal hypoxic-ischemia is a major cause of cerebral palsy (CP), and while most presentations of CP involve white matter loss, the contribution of frank OPC loss, as opposed to dysfunction and maturational arrest, to its etiology has been controversial 2,15. Fancy et al. found that resident OPCs within the white matter lesions of these children shared remarkable molecular similarities with those derived from APC-null mice: They had no detectable APC, and hence manifested de-repressed Wnt signaling, with high levels of LEF1 and SP5, as well as other Wnt-driven, APC-deficient colon cancer-associated transcripts, that included ETS2, DUSP4 and RNF43. Lest these expression patterns be considered transient, the authors described bipolar RNF43+ OPCs in a 12 year-old with CP, whose perinatal hypoxic-ischemic event had presumably yielded not only a disabling degree of white matter loss, but a functionally insufficient OPC population whose maturational arrest had in no way diminished with the passage of time.
It is the sustained nature of that maturational arrest that presents the greatest problems for those interested in mobilizing OPCs for therapeutic purposes. Can a transcription factor-based regulatory network sustain such an exquisitely balanced state of maturational arrest by a nominally self-renewing progenitor cell population without additional epigenetic changes to ensure the new status quo? If such changes do occur, is simple antagonism of Wnt-dependent pathways and downstream activators, such as SP5, sufficient to reinitiate oligodendrocytic differentiation and myelination? Fancy et al. do not answer these questions, but they certainly do provide us a window into how the virtual suspended animation of arrested, functionally-compromised oligodendrocyte progenitors might be reversed. At the same time, the authors provide us a model system within which the distinctions between those events involved in homeostatic self-renewal may be delinked from those involved in mitotic expansion, and the latter from oncogenesis and malignant transformation. As such, the worth of this wonderful paper lies not only in its insights into the pathophysiology of hypoxic-ischemic white matter injury, but also in its identification of processes by which we may now attempt to manipulate the mitotic expansion and myelination by endogenous progenitor cells across the entire spectrum of adult as well as pediatric myelin disorders, and how we might do so without the risk of concurrent oncogenesis. Rare indeed is it that such richness is offered in such a small package.
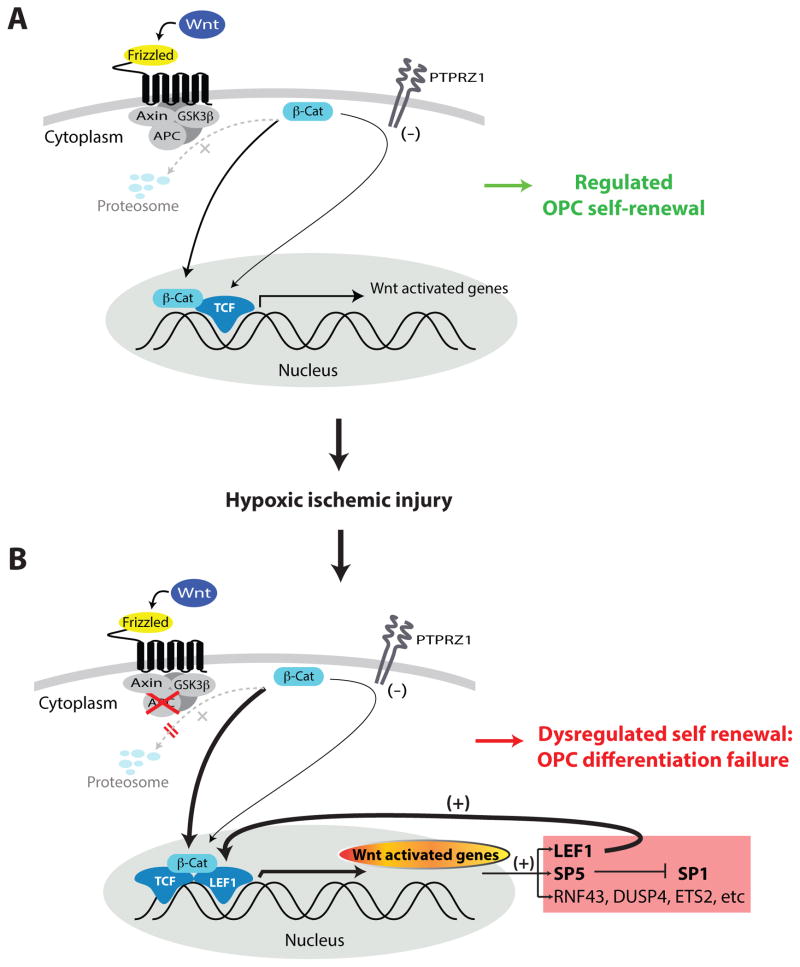
Fig. 1. Neonatal hypoxic-ischemic injury can yield dysregulated Wnt/βcatenin signaling and sustained maturational arrest by oligodendrocyte progenitor cells.
A, The homeostatic self-renewal of OPCs in healthy forebrain white matter is modulated through the regulated nuclear translocation of βcatenin, its binding therein to TCF-family transcription factors, and the resultant transcription of TCF-regulated target genes. Extracellular signals that modulate this process include the Wnt proteins in their binding to Frizzled-family receptors, the activation of which down-regulates GSK3β kinase activity, which would otherwise target cytoplasmic catenin for proteosomal degradation. Concurrent self-renewal signals also include the receptor tyrosine phosphatase β/ζ (PTPRZ1), which acts in concert with GSK3β to tonically suppress catenin availability (ref. 9).
B, Following hypoxic-ischemic injury to white matter OPCs, βcatenin-mediated TCF-dependent transcription increases, while expression of its negative regulator APC falls. These cells mimic the dysregulated Wnt/βcatenin-signaling noted in APC deficient colon cancers, with which they share up-regulation of a discrete set of Wnt-modulated transcriptional target genes. This tonically up-regulated gene set includes the LEF1 transcription factor, which drives its own expression in a feed-forward manner to sustain high levels of Wnt-associated gene expression. These transcripts also include the SP/KLF-family transcription factor SP5, which serves to sustain self-renewal competence, and whose knock-down yields precocious differentiation. The feed-forward nature of this dysregulated LEF over-expression appears to render OPCs refractory to terminal oligodendrocytic differentiation, instead favoring persistence in the progenitor state. However, unlike the neoplastic expansion seen in APC-mutant or deficient colon carcinomas, the sustained Wnt/βcatenin signal activation and diminished APC expression seen in OPCs following hypoxic-ischemic injury is associated with maturational arrest, and not with facilitated cell division or tumorigenesis, thus highlighting the effects of phenotype and local tissue context on the cellular outcomes of dysregulated Wnt signaling.
Contributor Information
Steven A. Goldman, Email: Steven_Goldman@URMC.Rochester.edu, Professor and Co-Director, University of Rochester Medical Center, Center for Translational Neuromedicine and the Department of Neurology, 601 Elmwood Avenue, Box 645, Rochester, NY 14642, Telephone: (585) 275-9550 Fax (585) 276-2298.
Joana Osorio, Email: Joana_Osorio@URMC.Rochester.edu, Instructor, University of Rochester Medical Center, Center for Translational Neuromedicine and the Department of Neurology, Child Neurology, 601 Elmwood Avenue, Box 645, Rochester, NY 14642, Telephone: (585) 276-6390 Fax (585) 276-2298.
References
- 1.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature Reviews Neuroscience. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 2.Segovia KN, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Annals of Neurology. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. The New England Journal of Medicine. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 4.Huang JK, et al. Myelin regeneration in multiple sclerosis: targeting endogenous stem cells. Neurotherapeutics. 2011;8:650–658. doi: 10.1007/s13311-011-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sim FJ, et al. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Annals of Neurology. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 6.Chew LJ, et al. SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. Journal of Neuroscience. 2011;31:13921–13935. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fancy SPJ, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nature Neuroscience. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang J, et al. Adenomatous polyposis coli regulates oligodendroglial development. Journal of Neuroscience. 2013;33:3113–3130. doi: 10.1523/JNEUROSCI.3467-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClain CR, Sim FJ, Goldman SA. Pleiotrophin suppression of receptor protein tyrosine phosphatase β/ζ Maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. Journal of Neuroscience. 2012;32:15066–15075. doi: 10.1523/JNEUROSCI.1320-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes & Development. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fancy SP, et al. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nature Neuroscience. 2014 doi: 10.1038/nn.3676. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Näthke I. Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nature Reviews Cancer. 2006;6:967–974. doi: 10.1038/nrc2010. [DOI] [PubMed] [Google Scholar]
- 13.Radtke F. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 14.Sim FJ, Windrem MS, Goldman SA. Fate determination of adult human glial progenitor cells. Neuron Glia Biology. 2009;5:45. doi: 10.1017/S1740925X09990317. [DOI] [PubMed] [Google Scholar]
- 15.Buser JR, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Annals of Neurology. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]