The ability to reprogram differentiated cells to pluripotency has revolutionized stem cell research and provides the foundation for new approaches to model human disease and possible stem cell-based therapies for degenerative diseases. To fully exploit this potential it will be necessary to develop simpler and more efficient ways to generate induced pluripotent stem (iPS) cells. Anokye-Danso and colleagues present a novel strategy for generating human and mouse iPS cells that is quicker and 100-fold more efficient than conventional protocols1. Remarkably, unlike all previous reports that use transcription factors, their approach uses only microRNAs (miRNAs)2. So how do these miRNAs accomplish this amazing feat? Subramanyam et al., shed some light on this question and identify a set of miRNA target genes that when depleted can facilitate iPS cell formation3. Together these studies highlight the utility of miRNAs in both iPS generation as well as dissecting the mechanisms and pathways underlying cell reprogramming.
The groundbreaking findings that differentiated can be reprogrammed to iPS cells by enforced expression of a few defined factors (Oct3/4, Sox2, Klf4, and c-Myc (OSKM)) overcomes many of the technical, ethical, and political obstacles in human embryonic stem cell (ESC) research and regenerative medicine4. iPS cells represent an alternative source of pluripotent cells for possible therapeutic applications. However, the initial reprogramming efficiencies were low (less than 0.1%) and the original cocktail of reprogramming factors includes oncogenes that can lead to tumorigenesis4. Also, retroviral infection of the target cells causes multiple potentially harmful integrations of the transgenes into the host genome. Since then, efforts to refine and develop alternative approaches include the use of non-integrating systems to deliver the reprogramming genes, and the replacement of individual factors with small molecules5. However, common to all of these is the use of at least one transcription factor, usually Oct3/42.
Though the ability of transcription factors to reprogram cells is well established, the role of miRNAs in this process is much less well understood. miRNAs represent a large family of regulatory RNAs that posttranscriptionally repress the expression of large sets of target genes and are essential for normal development and ESC biology6. Importantly, ESCs express a unique a set of miRNAs with the majority transcribed from two genomic loci, the miR-302 cluster (that contains five miRNAs – miR-302a/b/c/d and miR-367) and the miR-290 cluster in mice (miR-290, miR-291a, miR-292, miR-291b, miR-293, miR-294, and miR-295) or the miR-371-373 cluster in humans (miR-371, miR-372 and miR-373). ESC-specific miRNAs share a very similar ‘Seed’ sequence and therefore likely regulate overlapping sets of target genes. These miRNAs are required rapid ESC proliferation and cell-cycle progression6. Previous studies indicated that the miRNA pathway could be exploited for cell reprogramming7,8. Introduction of individual members of the miR-290 cluster or miR-302 was found to enhance cell reprogramming by OSK, but not by OSKM8. More recently however, it was demonstrated that miRNAs enhance reprogramming of MEFs with either OSKM or OSK. Furthermore, antagonizing these miRNAs with antisense oligonucleotides conversely inhibited reprogramming9.
An exciting new study demands close attention because instead of using any of the standard OSKM transcription factors the authors simply express a single primary miRNA transcript (the miR-302/367 cluster) to reprogram mouse and human somatic cells1. Importantly the resulting iPS cells exhibit gene expression and functional properties characteristic of fully reprogrammed pluripotent cells. Remarkably, the reprogramming, mediated by miR-302/367, is 100-fold more efficient than with OSKM, with ~10% of fibroblasts forming iPS colonies. Furthermore, the temporal kinetics of reprogramming may also be accelerated. Though this approach works for both mouse and human cells, valporic acid (VPA) was required for reprogramming mouse cells and was administered in conjunction with the miRNA-expressing virus. Interestingly, reprogramming of human cells did not require VPA and the miRNAs alone were sufficient for efficient reprogramming. So what is the difference for the species-specific requirement for VPA? It seems that a key barrier to reprogramming is the histone deacetylase, HDAC2, which is targeted for destruction by VPA and is expressed at considerably higher levels in mouse compared to human fibroblasts. Accordingly, reprogramming of mouse HDAC2−/− MEFs did not require VPA and miR-302/367 alone could efficiently reprogram these cells.
What is the mechanism behind miRNA-mediated cell reprogramming? miRNAs commonly target a large group of mRNAs simultaneously, and the combinatorial regulation of more than a handful of genes is likely required for cell reprogramming. The demonstration that ESC miRNAs enhance reprogramming, strongly suggests that these miRNAs are functioning to repress expression of genes that would otherwise work to maintain the differentiated cell state and may therefore represent barriers to reprogramming. Using this rationale, the Blelloch laboratory began to address the mechanism by which ESC miRNAs enhance reprogramming3. Using synthetic miRNA mimics, they find that introduction of miR-302b or miR-372 can enhance both OSKM- and OSK-mediated reprogramming of human fibroblasts. Next, using a published data set they chose a group of around thirty potential miR-302/miR-372 target genes for further analysis7. From this list they focus on a subset of twelve genes that respond to miRNA expression in the context of reprogramming. Importantly, they use siRNAs to individually deplete each of these putative miRNA targets and measure the effect on reprogramming. They show that knockdown of three of these genes (RBl2, CDC2L, and RHOC) enhances reprogramming both with OSKM and with OSK. They find that individual depletion of an additional three genes (SMARCC2, MBD2, and MECP2) enhances reprogramming in one of the two conditions (i.e. either OSKM or OSK). Since the gene encoding the TGFß receptor, TGFBR2, was among the selected gene set they use a small molecule inhibitor to confirm the involvement of this signaling pathway. They find that TGFBR2 mRNA is directly repressed by these miRNAs. Mouse Tgfbr2 is also regulated by similar miRNAs and siRNA-mediated knockdown of Tgfbr2 enhances reprogramming of MEFs9. The evolutionarily conserved requirement for the TGFß receptor further supports data that a mesenchymal-epithelial transition (MET) accompanies human cell reprogramming as it does in mouse. Importantly, since inhibition of any single gene led to relatively modest enhancement in cell reprogramming compared to the effects of the miRNA itself, as well as the increased efficiency observed by the simultaneous inhibition of more than one pathway, support the notion that miRNAs promote reprogramming by targeting multiple genes in several different downstream pathways (Figure 1).
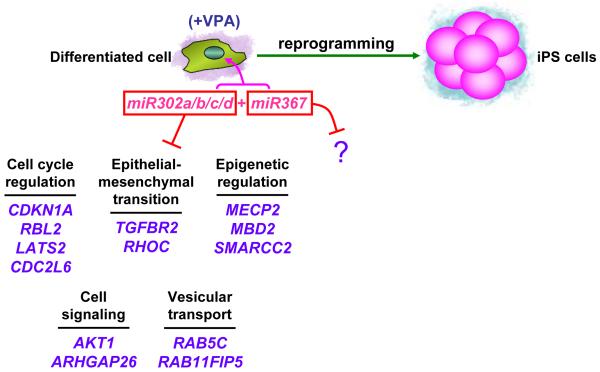
Figure 1. How do miRNAs reprogram mouse and human cells to pluripotency?
Differentiated cells can be reprogrammed to iPS cells by expressing the miR-302/367 cluster of miRNAs. For mouse cells valproic acid (VPA) is additionally required. Multiple downstream target genes and pathways have been identified for miR-302. miR-367 is essential for reprogramming and Oct4 activation however targets of this miRNA remain unknown.
These studies illuminate some of the genes that are targeted by the ESC-specific miRNAs in the context of reprogramming and demonstrate the remarkable power of miRNAs to efficiently reprogram cells to pluripotency1,3,8,9. Both miR-302 and miR-367 are required for iPS cell formation1. What genes are repressed by miR-367 to promote reprogramming? This missing piece of the puzzle remains to be addressed. However, a highly related miRNA, miR-92b, represses expression of Cdkn1c (p57) and is important for cell cycle progression of human ESCs10. Therefore it is likely that miR-367 facilitates reprogramming at least in part by modulating cell proliferation.
An obvious next experiment is to try and replace the viral delivery of the miRNA transgene. Since Morrisey’s lab showed that miR-367 is essential for miRNA-mediated reprogramming it will be interesting to see whether adding this synthetic miRNA to those used in the Blelloch study will enable the generation of iPS cells without the need for viruses or genome integration1,3. miRNAs may prove particularly useful in this regard since they are small and easily synthesized. Moreover, they are relatively easily transfected into cells and do not stimulate an innate immune response in the host cell. Furthermore, once incorporated into the relevant ribonucleoprotein complexes they are relatively stable with a reported half-life of several days in cells. These properties, together with the breakthrough proof-of-concept experiments, identify miRNAs as perhaps the ideal mode for reprogramming human cells and may help unleash the therapeutic potential of iPS cells.
Footnotes
Competing financial interests:
Authors declare no competing financial interests
References:
- 1.Anokye-Danso F, et al. Highly Efficient miRNA-Mediated Reprogramming of Mouse and Human Somatic Cells to Pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 3.Subramanyam D, et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011 doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez NJ, Gregory RI. MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell. 2010;7:31–35. doi: 10.1016/j.stem.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengupta S, et al. MicroRNA 92b controls the G1/S checkpoint gene p57 in human embryonic stem cells. Stem Cells. 2009;27:1524–1528. doi: 10.1002/stem.84. [DOI] [PubMed] [Google Scholar]