Abstract
Aims:
The evaluation of clinical and specific microbiological changes associated with chlorhexidine chip in the chronic periodontitis patients.
Materials and Methods:
A total of 30 chronic periodontitis patients (aged 20-65 years) having pocket depth of ≥5 mm in molar teeth were selected and randomly divided into following treatment groups: Group 1 - Scaling and root planning (SRP), Group 2 - SRP along with chlorhexidine chip and Group 3 - Only chlorhexidine chip. The clinical and microbial parameters were recorded at baseline and 1 and 3 months post-treatment as above. Benzoyl-DL-arginine-naphthylamide (BANA) chair side test was used for estimation of specific microbiota.
Results:
Gingival index, probing pocket depth and clinical attachment level scores in selected teeth within the groups at different time intervals was significantly (P < 0.001) different. Although, the comparison between groups for specific microbiota in selected sites at different intervals was not statistically significant at baseline and 1 month, it reached statistical significance at 3rd month post-treatment. Although significant reductions in percentage of BANA positive sites were observed in all three groups, the Group 2 had significantly greater percentage of BANA negative sites.
Conclusion:
Local drug delivery using chlorhexidine chip enhances the benefit of SRP in the treatment of chronic periodontitis.
Keywords: N Benzoyl-DL-arginine-β naphthylamide, chlorhexidine, chronic periodontitis, local drug delivery
INTRODUCTION
Periodontal disease is a complex multi-factorial disease characterized by destruction of periodontal tissues and loss of the connective tissue attachment. Plaque samples from diseased periodontal tissues reveal high percentage of Gram-negative (75%), anaerobic (90%) putative bacteria.[1,2] Higher levels of Porphyromonas gingivalis (Pg), Bacteroides forsythus and Treponema species are associated with chronic periodontitis. These pathogens produce various trypsin-like enzymes that degrade intercellular matrix of periodontal tissues.[3]
Various periodontal therapeutic approaches to purge and kill these microorganisms include invasive or non-invasive methods. Scaling and root planning (SRP) is said to only reduce the bacterial overload from tooth surface and does not necessarily eliminate all pathogens from deep pockets.[2,4,5,6] Although, administration of systemic antimicrobials are useful in treating recurrent periodontal pockets. However, the doses necessary to achieve sufficient local concentration of antimicrobials in periodontal environment might be associated with undesirable side-effects. Hence, the local administration are considered to overcome these side-effects.[2,7]
Local administrations of antimicrobial agents in periodontal pockets such as rinsing, irrigation and local injections are adopted previously. Indeed antibacterial mouthwashes are effective in controlling supragingival plaque; however, their effect on the subgingival flora is limited. To overcome these limitations, various local-delivery devices such as monolithic devices, gels and hybrids are recently developed with either sustained-release device or controlled-delivery device platforms.[1,5,8,9,10,11] By using these devices, antimicrobial agents such as tetracycline, metronidazole, doxycycline, minocycline or chlorhexidine[1,2,5,8,9,11] are administered directly into the periodontal pocket sites to inhibit periodontal pathogenic bacteria, associated inflammatory response and periodontal tissue destruction.
Chlorhexidine being a broad spectrum antibacterial and antifungal agent is a very effectiveness in treating periodontal disease.[11] Considering the cost and the non-availability of chlorhexidine chip (periochip) in many countries an attempted was made in the present study to evaluate indigenous fish collagen incorporated with chlorhexidine gluconate in chronic periodontitis patients, along with specific microbiological assessment by using a chair side benzoyl-DL-arginine-naphthylamide (BANA) test.
MATERIALS AND METHODS
A study was conducted by the Department of Periodontics, Narayana Dental College and Hospital, Nellore; Andhra Pradesh, India to evaluate the clinical and specific microbiological changes associated with chlorhexidine chip (Periocol-CG, Eucare Pharmaceuticals Pvt. Ltd., Thiruvakkam, Chennai, India) in chronic periodontitis patients. Approval was obtained from Institutional Ethical Committee prior to start of the study.
Selection criteria
Systemically healthy patients (20-65 years) with a gingival pocket depth of ≥5 mm in one or two sites in molars were selected. Patients who were willing and able to return for multiple follow up visits.
Patients suffering from systemic diseases, on any chemotherapeutic mouth rinses and oral irrigation during the past 6 months, who have received any surgical therapy 6 months prior to the start of the study, pregnant or lactating women, smokers and who are allergic to chlorhexidine were excluded from the study.
Study design
A 3 months simple randomized, clinical study was conducted comparing the effect of SRP with and without chlorhexidine chip in chronic periodontitis patients.
Criteria for grouping
A total of 30 patients were randomly divided into following three groups:
Group 1-10 patients with SRP
Group 2-10 patients SRP along with placement of chlorhexidine chip
Group 3-10 patients with placement of chlorhexidine chip alone.
The nature and design of the clinical study was explained and informed consent was obtained from all the participants.
The clinical parameters recorded in the proforma included Loe and Sillness gingival index (GI), probing pocket depth (PPD) and clinical attachment levels (CAL).
One molar site with pocket depth of ≥5mm was selected in each patient for the study. SRP was performed for Groups 1 and 2. The subgingival placement of chlorhexidine chip was done after proper isolation of the area in Groups 2 and 3. All patients were given oral hygiene instructions.
Microbiological study
BANA chair side test which is modification of BANA hydrolysis was used to estimate specific microbiota.
Working principle of BANA
An unusual enzyme produced by specific bacteria (Treponema denticola (Td), Pg or B. forsythus) capable of hydrolyzing the synthetic peptide BANA present on BANA test strips in turn reacts with embedded diazo dye to produce the permanent blue color indicating a positive test.
Procedure
(1) Subgingival plaque sample was obtained using curette and applied on to the raised reagent matrix affixed to the lower portion of the test strip. (2) Moisten the upper test strip (salmon color) with distilled water using a cotton swab. Care should be taken not to over wet. (3) Fold the BANA-zyme test strip at the given crease mark so that the lower and upper reagent strips meet. (4) Place the BANA-zyme test strip into either of the slots on the top of the processor. The heating element of the processor will start automatically when strip is inserted into the bottom of the slot, as indicated by the flashing light. When the indicator light remains on, the heating element has reached 55°C and will stay on for 5 min. The BANA-zyme test strips color development will be complete when the indicator light goes off and the bell rings. A second BANA-zyme test strip can be placed into the other slot. Each slot has its own heater and timer and is independent of the events in the other slot. After completion of a cycle it is best to allow at least 10 min cool down time between insertions of a new BANA-zyme test strip into either slot. (5) Remove the BANA-zyme test from the processor and discard the lower reagent strip that had been inoculated with plaque in a manner appropriate for contaminated material. (6) Examine the upper reagent strip for the presence of blue color. If a blue color is a detected mark the site as either weak positive or positive. (7) Recording was done for each sampled site as negative, weak positive or positive.
Both clinical and microbiological recordings were carried out at baseline and 1 and 3 months post-treatment. All recordings were subjected for statistical analysis by using Pearson Chi-square test with Yates continuity correction or Analysis of variance (ANOVA) test.
RESULTS
All patients (15 males and 15 females with mean age of 35 ± 16 years) completed the study except one patient who missed 1 and 3 months evaluation, was excluded from the study.
The age, sex and educational status were compared between the groups by using Pearson Chi-square test showing no statistical significance.
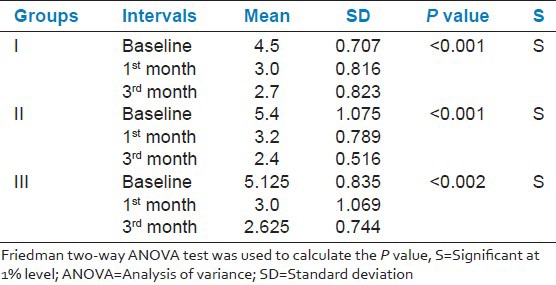
GI, PPD and CAL scores between the three groups were similar at baseline and 1 and 3 months post-therapy. However, GI within the groups at different time points was significantly (P < 0.001) different. Interestingly Group 2 showed a significant reduction (mean 0.23 ± 0.18) than Group 1 (0.300 ± 0.19) and Group 3 (0.305 ± 0.19) at the end of 3rd month [Table 1]. PPD and CAL within the groups at baseline 1 and 3 months, significantly reduced by 1% [Tables 2 and 3].
Table 1.
Comparison of gingival index for selected tooth site at baseline, 1 and 3 months within the groups
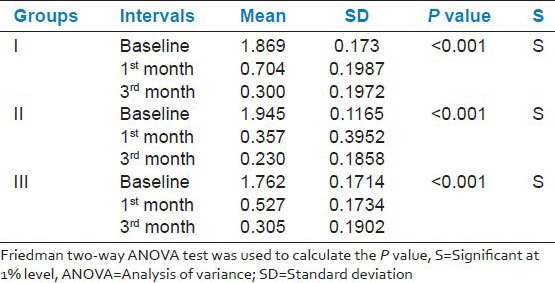
Table 2.
Comparison of probing pocket depth for selected tooth site at baseline, 1 and 3 months within the groups

Table 3.
Comparison of clinical attachment level for selected tooth site at baseline, 1 and 3 months within the groups
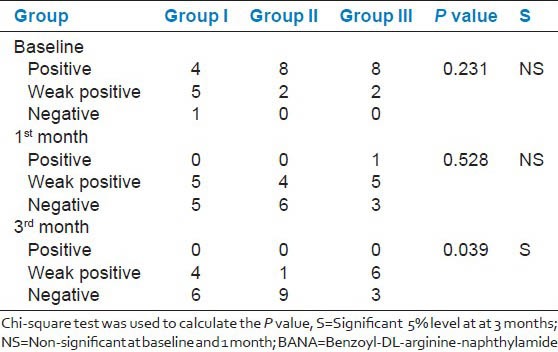
Microbiological assessment between the groups at different intervals showed no significance at baseline and 1 month; however, statistically significant difference was observed at 3rd month post-treatment. Although significant reduction in percentage of BANA positive sites was observed in all the three groups, Group 2 showed significantly greater percentage of BANA negative sites [Table 4].
Table 4.
Comparison of microbial assessment in selected tooth site by BANA test between the groups at baseline, 1 and 3 months
DISCUSSION
The study was conducted to evaluate the clinical efficiency of chlorhexidine chip and assess the specific microbial changes associated with chronic periodontitis patients. The study was conducted for 3 months period because, effects of controlled release chlorhexidine have been shown to be evident up to 11 weeks after administration of the chip and 3 months study period corresponds to typical recall interval for periodontal patients.[12,13]
Consistent with previous reports[14,15] our study did not show any superior effect of chlorhexidine chip when compared with SRP group. This could be due to the reduction in the clinical parameters with SRP, which was equivalent to that of chlorhexidine chip alone by removing subgingival biofilms and other microscopic reservoirs of bacteria residing in the subgingival environment. However, our results are contradictory to other studies[12,13] reporting superiority of chlorhexidine chip over SRP, which was attributed to a low substantivity of chlorhexidine due to its poor adherence to root surfaces and its high affinity for salivary or serum proteins and blood.
In the present clinical study, teeth selected were without recession keeping the PPD and CAL on baseline constant. In contrary, the study conducted by Jeffcoat et al.[13] included gingival recession as one of the parameters, which may have contributed to alterations in the clinical outcome of the study.
When the GI scores were compared, all the groups had greater reduction in the scores (P < 0.001) and showed highly significant differences between baseline to 3 months. No significance was observed between the groups, indicating similar inhibition of bacterial growth and gingival inflammation in both groups. These results are in consistent with previous studies.[15,16,17] In contrast, the studies by Soskolne et al.[12] and Jeffcoat et al.[13] showed better results with SRP along with chlorhexidine chip compared with SRP alone attributing it to the beneficial anti plaque properties of chlorhexidine.
Highly significant (P < 0.001) mean PPD reductions at different time intervals were observed, whereas no significance was found between the groups. This is in accordance with Grisi et al.,[15] Paolantonio et al.[18] The PPD reduction was greater in SRP plus chlorhexidine chip, in previous studies conducted by Soskolne et al.,[12] Jeffcoat et al.,[13] Grisi et al.[15] using periochip. This could be due to the additional antibacterial effects (cationic molecule binding to extra microbial complexes and negatively charged microbial cell walls altering the osmotic equilibrium), during the healing process of tissues that could have enhanced the effect of SRP.[5] It also could bind to salivary bacteria thus interfering with their adsorption to teeth.[2]
In Group 3, the mean CAL reduction was 1.8 mm, whereas in Group 2, the mean reduction was 3.0 mm and was statistically significant (P < 0.001). No statistical significance was observed when groups were compared at 3 months. This is in accordance with Heasman et al.,[14] Grisi et al.[15] and Carvalho et al.[16] In contrary Soskolne et al.[12] and Jeffcoat et al.[13] reported a significant association between SRP plus chlorhexidine on CALs and attributed to the advantage of having a minimal potential to induce resistant bacterial strains.
The microbiological assessment was performed using a chair side BANA test showing specific microbiota. Tannerella forsythus (Tf), Pg, Td and Capnocytophaga species share a common enzymatic profile and have a trypsin-like enzyme in common. The activity of this enzyme can be measured with the hydrolysis of the colorless substrate BANA; hence, all the three BANA positive species are frequently cited as potential periodontal pathogens.[3,19,20]
In our study, the percentage of BANA positive sites for the detection of number of putative periodontal bacteria were reduced significantly from baseline to 1 month and 3 months within the groups. In Group 2, the number of BANA negative sites was comparatively higher than the other two groups. This could be due to more effective control of the periodontal anaerobic microorganisms enhancing the effect of SRP. This is in accordance with previous studies.[12,18] In contrary studies conducted by Grisi et al.[15] and Daneshmand et al.[21] did not show any significant reduction in the microorganisms.
When BANA test sites were compared between the groups, it did not show significance at 1 month; however, significant (P < 0.039) differences were observed at 3 months after treatment with Group 2 better over Groups 3 and 1. These results explain that SRP alone will reduce the bacterial load at 1 month, but additional benefits are observed with chlorhexidine chip at 3 months in reducing the microbial load. In addition, inhibition of microbial proteases from potent periodontopathogens such as Pg, Tf, Td is therapeutically favorable. In contrary, it has been reported that the release of vesicles from Pg inactivate chlorhexidine and potentially induce bacterial drug resistance.[7]
CONCLUSION
Local drug delivery using chlorhexidine chip enhances the benefit of SRP in the treatment of chronic periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–43. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 2.Newmann MG, Takei HH, Klokkevold PR, Carranza FA. 10th ed. Missouri: Saunders Elsevier; 2006. Text Book of Clinical Periodontology; pp. 134–1692. [Google Scholar]
- 3.Amalfitano J, De Filippo AB, Bretz WA, Loesche WJ. The effects of incubation length and temperature on the specificity and sensitivity of the BANA (N-benzoyl-DL-arginine-naphthylamide) test. J Periodontol. 1993;64:848–52. doi: 10.1902/jop.1993.64.9.848. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–34. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanes PJ, Purvis JP. Local anti-infective therapy: Pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98. doi: 10.1902/annals.2003.8.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Kornman KS. Controlled-release local delivery antimicrobials in periodontics: Prospects for the future. J Periodontol. 1993;64:782–91. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 7.Cosyn J, Wyn I. A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2006;77:257–64. doi: 10.1902/jop.2006.050216. [DOI] [PubMed] [Google Scholar]
- 8.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 10.Quirynen M, Mongardini C, de Soete M, Pauwels M, Coucke W, van Eldere J, et al. The rôle of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. J Clin Periodontol. 2000;27:578–89. doi: 10.1034/j.1600-051x.2000.027008578.x. [DOI] [PubMed] [Google Scholar]
- 11.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 12.Soskolne WA, Heasman PA, Stabholz A, Smart GJ, Palmer M, Flashner M, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi-center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoat MK, Bray KS, Ciancio SG, Dentino AR, Fine DH, Gordon JM, et al. Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 14.Heasman PA, Heasman L, Stacey F, McCracken GI. Local delivery of chlorhexidine gluconate (PerioChip) in periodontal maintenance patients. J Clin Periodontol. 2001;28:90–5. doi: 10.1034/j.1600-051x.2001.280114.x. [DOI] [PubMed] [Google Scholar]
- 15.Grisi DC, Salvador SL, Figueiredo LC, Souza SL, Novaes AB, Grisi MF. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome. J Clin Periodontol. 2002;29:875–81. doi: 10.1034/j.1600-051x.2002.291001.x. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho J, Novak MJ, Mota LF. Evaluation of the effect of subgingival placement of chlorhexidine chips as an adjunct to scaling and root planing. J Periodontol. 2007;78:997–1001. doi: 10.1902/jop.2007.060122. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues IF, Machion L, Casati MZ, Nociti FH, Jr, de Toledo S, Sallum AW, et al. Clinical evaluation of the use of locally delivered chlorhexidine in periodontal maintenance therapy. J Periodontol. 2007;78:624–8. doi: 10.1902/jop.2007.060317. [DOI] [PubMed] [Google Scholar]
- 18.Paolantonio M, D’Angelo M, Grassi RF, Perinetti G, Piccolomini R, Pizzo G, et al. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: A multicenter study. J Periodontol. 2008;79:271–82. doi: 10.1902/jop.2008.070308. [DOI] [PubMed] [Google Scholar]
- 19.Loesche WJ, Giordano J, Hujoel PP. The utility of the BANA test for monitoring anaerobic infections due to spirochetes (Treponema denticola) in periodontal disease. J Dent Res. 1990;69:1696–702. doi: 10.1177/00220345900690101301. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt EF, Bretz WA, Hutchinson RA, Loesche WJ. Correlation of the hydrolysis of benzoyl-arginine naphthylamide (BANA) by plaque with clinical parameters and subgingival levels of spirochetes in periodontal patients. J Dent Res. 1988;67:1505–9. doi: 10.1177/00220345880670121201. [DOI] [PubMed] [Google Scholar]
- 21.Daneshmand N, Jorgensen MG, Nowzari H, Morrison JL, Slots J. Initial effect of controlled release chlorhexidine on subgingival microorganisms. J Periodontal Res. 2002;37:375–9. doi: 10.1034/j.1600-0765.2002.01003.x. [DOI] [PubMed] [Google Scholar]


