Abstract
Background:
Dental plaque being one of the most-studied biofilm communities, is particularly complex because it consists of thousands of bacterial species, and new species are still being isolated and characterized. The aim of the present study is to characterize surface topography of the biofilm formed by a new bacterial isolate, obtained from the dental caries lesion using atomic force microscopy.
Materials and Methods:
Ten clinical isolates were obtained from five teeth with carious lesions involving dentine. Quantification of the biofilm forming ability of the clinical isolates was performed using microtiter plate assay. Bacterial isolate exhibiting maximum biofilm formation was subjected to phylogenetic analysis based on 16S rRNA gene sequencing and atomic force microscopic analysis.
Results:
The bacterial strain JKAS-CD2 displayed the highest similarity to 16S rRNA gene sequences of members of the family Streptococcaceae. It shared 95.3-99.3% similarity to the type strains of genus Streptococcus and 99.9% sequence similarity to the type strain Streptococcus infantarius. Atomic Force Microscopic analysis confirmed that the sucrose dependent bacterial adhesion for stable biofilm development has increased over a time-span on the thin film of enamel. Major structural components of plaque such as clumping of colonies and slime layer were clearly visualized by surface image of JKAS-CD2 cells grown on the enamel powder coated glass surface.
Conclusion:
JKAS-CD2 emerged as an obligate biofilm forming microbe under sucrose-dependent condition; a mechanism for adherence that determines the survival and persistence of the bacteria in the oral cavity and thus implicated with the dental caries.
Keywords: Atomic force microscopy, biofilm, dental caries, S. infantarius, virulence factor
INTRODUCTION
Dental caries is the single most common biofilm dependent oral infectious disease worldwide that results from the interaction of specific bacterial and salivary constituents with dietary carbohydrates in a biofilm tightly adherent on the tooth surface.[1,2]
Dental caries is most likely the result of a polymicrobial infection caused by one or more of the over 500 bacterial species that have been identified from the human oral cavity.[3,4] It is apparent that as in many natural microbial communities, a large proportion of the microbes present in the mouth have not yet been cultured.[5] Advanced molecular methods such as polymerase chain reaction (PCR) and 16S rRNA gene sequencing analysis have revealed that the bacterial involvement in the development of dental caries is more complex than previously believed.[6]
Streptococcus mutans is known as the principal dental pathogen associated with caries; with an ability to synthesize glucan from sucrose as its most important virulent factor.[7,8] Glucan synthesis allows the bacteria to firmly attach to the tooth surface and form a biofilm; whereas the gelatinous nature of glucan retards diffusion of acid produced by the bacteria from fermentable sugars in the dental plaque.[9] This eventually leads to dissolution of the hard enamel surface of the tooth. Cell to surface interactions play an enormous role in initial cell colonization, and cell to cell protein interactions largely mediate the growth and continued existence of biofilm.[10]
The bacterial communities within a biofilm are of particular medical importance due to their increased tolerance to antibiotics.[11] Novel strategies based on detailed understanding of the biofilm formation mechanisms are therefore an important issue. Atomic force microscopy (AFM) is an advanced technology that offers great advantages in mapping of biological samples such as bacterial biofilms and observation of biofilm growth in their natural complex environment.[12,13,14,15]
The aim of present study is to characterize the surface topography of the biofilm formed by a new bacterial isolate obtained from dental caries lesion, using AFM.
MATERIALS AND METHODS
Sample collection
Ethical approval for the study was granted by the Internal Research and Review Board (IRB), Ethical Clearance (EC), Biosafety and Animal Welfare Committee at Madurai Kamaraj University, Madurai. Five adult patients elected to have extractions for unrestored teeth (due to chronic periodontitis) that presented with large coronal dentine caries lesions which, by macroscopic examination, had not penetrated to the underlying vital pulp tissue; participated with their informed consent. The samples were excavated with sterile, spoon excavator and collected at a level that represented the middle of the dentine lesion. Five dentinal caries samples were taken and transported to the laboratory and subjected to further analysis.
Cultural analysis
Lesion samples were serially diluted in sterile MilliQ water and inoculated on Brain Heart Infusion (BHI) agar medium (HiMedia) supplemented with 5% sucrose, incubated at 37°C in 5% CO2 for 18-36 h. Ten clinical isolates were obtained from five dentinal caries samples and were designated as JKAS-CD1 to JKAS-CD10 [CD: Culture Dependent]. The isolates were differentiated based on their colony morphology, hemolysis on blood agar medium with and without 5% sucrose and biochemical characteristics.
Biofilm assay
The clinical isolates were grown on BHI broth with and without 5% sucrose, incubated overnight in microtiter plates at 37°C under 5% CO2. The biofilm development was determined spectrophotometrically by measuring the absorbance at 590 nm. Experiments were performed in triplicates and the heat-killed Escherichia coli cells were used as control. The clinical isolate showing maximum biofilm formation was selected for further study.
Molecular analysis
Genomic DNA was extracted from the selected clinical isolate utilizing Hopwood et al.[16] protocol with minor modifications; integrity of the isolated DNA was analysed on 0.8% agarose gel.
Using a universal primer set [27F 5’- AGA GTT TGA TCC TGG CTC AG-3’ and 1492R 5’-GGT TAC CTT GTT ACG ACT T-3’] synthesized commercially [Sigma Genosys, India]; PCR amplification of 16S rRNA gene was performed in a total volume of 50 μL reaction mixture containing 1X reaction buffer (10 mM Tris [pH 8.3], 50 mM KCl, 1.5 mM MgCl2), 200 μM dNTPs, 0.05 units Taq DNA polymerase [Sigma, USA], 0.5 μM of each primer and 1 ng template DNA. The thermal cycling conditions were: 5 min at 94°C for initial denaturation; 31 cycles of 30 s at 95°C, 1 min 30 s at 54°C, 2 min at 72°C, and a final extension for 5 min at 72°C. The amplification reaction was performed with a thermal cycler (MyCycler, Bio-Rad, USA) and the PCR amplicons were resolved by electrophoresis in 1% (w/v) agarose gel to confirm the expected size (1500 bp) of the product.
The PCR product was purified by QIAquick PCR purification kit (Qiagen, USA) and cloned into pGEM-T Easy Vector (Promega, USA). The recombinant plasmid was isolated from positive clones, purified with QIAprep plasmid purification kit (Qiagen, USA) and sequenced with Applied Biosystems 3730XL DNA Analyzer using BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA).
Phylogenetic analysis
Phylogenetic analysis was performed by RDP-II database,[17] CLUSTALW, by the neighbor-joining method. The phylogenetic tree was analyzed using PHYLIP 3.68,[18] with DNADIST, NEIGHBOR using bootstrapping over 1000 replicates, and maximum-likelihood analysis was performed with DNAML of PHYLIP 3.68. The 16S rRNA gene sequence of the selected isolate was deposited in the GenBank nucleotide database under the accession number (JX548342).
Sample preparation for AFM analysis
Enamel was powdered by polishing action from caries unaffected region of extracted teeth using sterile, fine diamond bur. Enamel powder was dissolved in 500 μL MilliQ water and sterilized by autoclaving.
To visualize the biofilm formation of the selected isolate by AFM; the glass cover slips were cleaned by sonication and immersed in piranha solution (Conc. H2SO4 and 30% H2O2 in a ratio of 3:1) for 15 min followed by washing thrice with sterile MilliQ water and drying in the vacuum desiccator. Cover slips were uniformly coated with 20 μL sterile enamel powder solution with necessary nutrients supplied as 20 μL BHI broth.
The experimental samples were designed as follow:
Enamel + BHI broth + Selected isolate bacterial cells, incubated at 37°C for 6, 12 and 24 h
Enamel + BHI broth + Selected isolate bacterial cells supplemented with 5% sucrose, incubated at 37°C for 6, 12 and 24 h.
Bacterial cells at logarithmic growth phase, grown in BHI broth were centrifuged at 5000 rpm for 10 min at 4°C and the cell pellets were washed thrice with distilled water and re-suspended up to 10-3 dilution in sterile distilled water. The experimental samples were inoculated with 5 μL of 10-3 diluted bacterial cells and incubated at 37°C. After incubation, the cover slips were gently washed thrice with sterile distilled water to remove the unbound cells from the surface and air-dried for 12 h.
To observe bacterial cells of selected isolate by AFM, 5 μL of 10-3 diluted bacterial cells were deposited onto a freshly cleaned glass cover slips and air dried for 12 h. AFM analysis of the bacterial cells and its biofilm topography on the enamel powder coated glass surface was performed using an A100-SGS Atomic Force Microscope (A.P.E Research, Italy). Images were taken at tapping mode in air at room temperature. The silicon etched Ultrasharp™ cantilever (MikroMasch, USA) with a spring constant of 40 Nm-1 and 10 nm radius was used to obtain non-contact images at 1 Hz scan rate. The surface and roughness of AFM images were analyzed using the image acquisition software.
RESULTS
The clinical isolates inoculated on BHI agar medium exhibited different colony morphological features. The results of hemolysis revealed that JKAS-CD1 JKAS-CD3 to CD6, JKAS-CD9 and JKAS-CD10 exhibited Gamma hemolysis both on blood agar medium with and without sucrose.
JKAS-CD2 and JKAS-CD8 exhibited Alpha-hemolysis both on blood agar medium with and without sucrose; while JKAS-CD7 exhibited Alpha-hemolysis on blood agar medium with sucrose and Gamma hemolysis on blood agar without sucrose. JKAS-CD2, CD7, CD8 and CD6 displayed more biofilm forming ability when compared to others in the decreasing order [Figure 1]. Based on these results, the clinical isolate JKAS-CD2 was selected for the phylogenetic analysis and subjected to AFM for the topographic analysis of the biofilm formed by the same.
Figure 1.
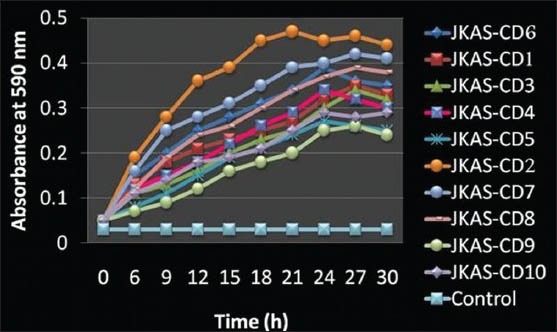
Biofilm formation by the clinical isolates. [The absorbance of the eluted stain measured at 590 nm up to 30 h at 3 h interval. The values were depicted as mean values of independent triplicate experiments and the error bars represent the standard error of the mean for each absorbance value]
Phylogenetic analysis of JKAS-CD2
The size of the 16S rRNA gene sequence of carious lesion derived bacterial isolate JKAS-CD2 was 1508 bp with the G + C content of 52.38%. Nucleotide similarity searches revealed that the bacterial isolate belongs to the phylum Firmicutes and also displayed the highest similarity to 16S rRNA gene sequences of members of the family Streptococcaceae. Phylogenetic analysis of 16S rRNA gene sequences of JKAS-CD2 showed that it shared 95.3% to 99.3% similarity to the type strains of recognized species of the genus Streptococcus and its closely related type strain was Streptococcus infantarius with 99.9% sequence similarity [Figure 2].
Figure 2.

Phylogenetic tree view of 16S rRNA gene of bacterial isolate JKAS-CD2 based on Neighbor-joining method. Bootstrap values > 50% were shown at each node and expressed as percentages of 1000 replications. Lactobacillus lactis T was used as out-group. [Scale bar represents 0.01 substitutions per nucleotide]
Atomic Force Microscopic analysis of biofilm
AFM image analysis indicated that the enamel coating was almost uniform over the glass surface [Figure 3a and b] and JKAS-CD2 cells exhibited colony morphology as individual and small chains in cocci form (0.25-0.4 μm diameter) [Figure 4a and b].
Figure 3.
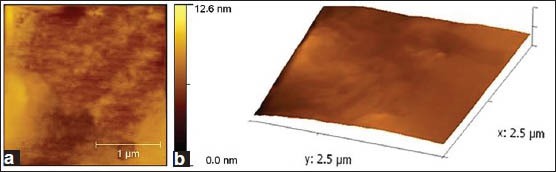
(a) 2-Dimensional atomic force microscopy images depicting the surface characteristics of enamel powder suspension coated glass cover slip. (b) 3-Dimensional AFM images depicting the surface characteristics of enamel powder suspension coated glass cover slip
Figure 4.
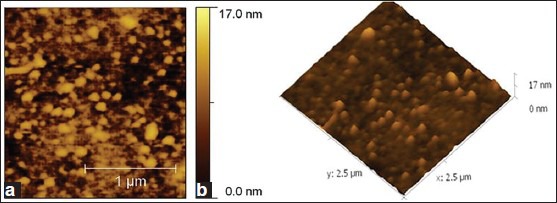
(a) 2-Dimensional atomic force microscopy images depicting colony morphological characteristics of JKAS-CD2 (Bacterial cells seen as individual and small chains in cocci form). (b) 3-Dimensional AFM images depicting colony morphological characteristics of JKAS-CD2 (Bacterial cells seen as individual and small chains in cocci form)
After 6 h incubation, sucrose dependent biofilm formation on the glass cover slip coated with enamel was observed as initial small clusters of colonies; in comparison to scarcely distributed cells on the surface of the enamel under sucrose independent conditions. Glucan production was observed at places with dense population of bacteria. The number of colony forming units was more in case of sucrose mediated biofilm with significant distribution of larger clusters of cells.
Under sucrose dependent conditions, the microbial colonies adhered and were seen embedded in the glucan, as the biofilm attained 12 h growth [Figure 5a and b]. While, more number of bacterial colonies appeared to be exposed outside in sucrose independent biofilm. Further, the shape of JKAS-CD2 cells did not undergo any significant difference as the biofilm attained maturity under both sucrose dependent and sucrose independent conditions. Following 24 h incubation, the texture and roughness of the biofilm formed by JKAS-CD2 under sucrose dependent condition (biofilm topography height 104 nm) [Figure 6a and b] was ~ 3 fold higher than that formed under sucrose independent condition (biofilm topography height 37 nm) [Figure 7a and b].
Figure 5.
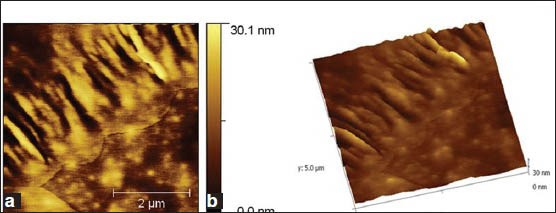
(a) 2-Dimensional atomic force microscopy images of sucrose dependent biofilm formed by JKAS-CD2 cells. Following 12 h incubation, the microbial colonies adhered and seen embedded in the glucan produced.(b) 3-Dimensional AFM images of sucrose dependent biofilm formed by JKAS-CD2 cells. Following 12 h incubation, the microbial colonies adhered and seen embedded in the glucan produced
Figure 6.
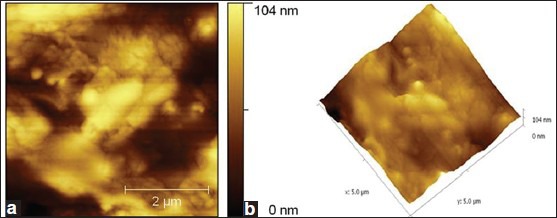
(a) 2-Dimensional atomic force microscopy images showing sucrose-dependent-biofilm formed by JKAS-CD2 cells following 24 h incubation [Biofilm topography height: 104 nm] (b) 3-Dimensional AFM images showing sucrose-dependent-biofilm formed by JKAS-CD2 cells following 24 h incubation [Biofilm topography height: 104 nm]
Figure 7.
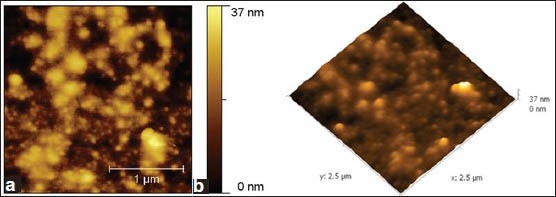
(a) 2-Dimensional atomic force microscopy images showing sucrose-independent-biofilm formed by JKAS-CD2 cells following 24 h incubation [Biofilm topography height: 37 nm]. (b) 3-Dimensional AFM images showing sucrose-independent-biofilm formed by JKAS-CD2 cells following 24 h incubation [Biofilm topography height: 37 nm]
DISCUSSION
With the advent of molecular methods based on the recent well established technique of PCR, cloning, and sequencing of 16S rRNA genes, many hitherto unknown aspects of the oral microbiome have been revealed. Another aspect of this pioneering method is that, as the 16S rRNA gene is approximately 1550 bases in length, it could be surmised that more sensitive and specific information on taxonomical lineage and bacterial diversity might be obtained through analyzing longer lengths of sequences in comparison to the bacterial identification based on the sequence data of relatively shorter segments of 300-400 bases.[4]
Molecular methods involving sequencing have proved invaluable in the taxonomy to determine phylogenetic relationship between the known and the newly recognized microorganisms aiding in taxonomic rearrangement and the creation of new species, thus further increasing the microbial diversity.[19]
16S rRNA gene sequence analysis revealed that JKAS-CD2 formed a distinct subclade closer to the Streptococcus infantarius, indicating that this isolate is closely related at the 16S rRNA gene sequence level but not to any previously described taxa of other species of Streptococcus represented in the phylogenetic tree. This sequence belongs to a strain isolated from the infant's feces. The other closely related lineages of Streptococcus clustered in the phylogenetic tree were reported from dairy products, human blood and endocarditis, suggesting that the closely related lineages of JKAS-CD2 are present in different environment. The nearest type strain S. infantarius subsp. infantarius exhibits no exopolysaccharide (EPS) production on 5% sucrose medium; shows acid production from lactose, maltose and sucrose and is urease negative.[20]
In spite of the 16S rRNA gene sequence identity to the nearest type strain S. infantarius subsp. infantarius, the JKAS-CD2 exhibited phenotypic differences such as EPS production on 5% sucrose medium, but lack acid production from lactose and maltose. Therefore, the isolate JKAS-CD2 obtained from the dentinal caries lesion seems to be distinct and diverged significantly from the members of the S. infantarius, and thus could represent a new species of the genus Streptococcus.
S. mutans can metabolize the major form of cariogenic food sucrose to produce α (1 → 3) and α (1 → 6) glucosidic polymers and forms a plaque on the surfaces such as glass, plastic and tooth enamel.[21] The selected bacterial isolate JKAS-CD2 also utilized sucrose to synthesize the EPS to form glucan aided enhanced biofilm slime layer. Therefore, the isolate exhibited the virulent factor responsible for the dental caries. As in the case for many other Streptococci bacteria inhabiting the oral cavity such as S. gordonii, S. mitis, S. mutans and S. sanguis,[22] the isolate JKAS-CD2 emerged as an obligate plaque forming microbe under sucrose-dependent condition.
Biofilm formation leads to increased tolerance to antibiotics and antimicrobial peptides and more persistent infections due to their special physiology and physical matrix barrier.[23] Therefore, assessment of biofilm formation capabilities is important. The microtiter plate assay, being a rapid and simple method, can be used to screen difference in biofilm formation between bacterial strains.[24] In view of this advantage, the microtiter plate assay was used in the present study.
Bacteria can be imaged either under dry conditions or in aqueous solution using AFM. The advantage of imaging dry samples is a better resolution than for bacteria examined in aqueous media.[25] In the present study, the bacterial morphology and surface topography of the formed biofilm was imaged under dry conditions. AFM images were collected at a very slow scan rate of 1 Hz, in order to obtain the details and to avoid damaging the tip. ‘Tapping mode’ imaging was selected for the procedure, as it is a more sophisticated type of measurement. Such imaging is recommended for more sensitive samples, which could be damaged during the tip-rastering process. Another advantage of ‘tapping mode’ imaging is the possibility to collect height, amplitude, and phase type data.[26]
In the present study, the topography of slime layer exhibited fully embedded bacterial colonies and suggests that such a biofilm prevents antimicrobials and xenobiotics from gaining access to the cells inside the biofilm, thus protecting the bacterial cells. Further, the results observed by spectroscopic absorbance of bound crystal violet to the bacterial cells on polystyrene surface over the time course experiment was in close agreement with biofilm topography observed by AFM imaging.
As adherence is a key ecological determinant for oral bacteria to survive and persist,[27] microorganisms that cannot form a biofilm are unable to adhere to a surface and washed out easily by the salivary flow. The biofilm forming ability of the isolate JKAS-CD2, as per the results of microtiter plate assay and AFM analysis holds the mechanism for its adherence to glass surface coated with tooth enamel even after washing.
However, although mutans streptococci are strongly implicated with caries, the association is not unique.[2] Indeed in such circumstances, some acidogenic, non-mutans streptococci are implicated with the disease, as seen in the present study.
CONCLUSION
An understanding of the basis of bacterial community behavior points to therapeutic targets that may provide a means for the control of biofilm infections.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13:126–31. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- 2.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–94. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 3.Moore WE, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, Palcanis KG, et al. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985;48:507–19. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner A, Maiden MF, Paster BJ, Dewhirst FE. The impact of 16S ribosomal RNA-based phylogeny on the taxonomy of oral bacteria. Periodontol 2000. 1994;5:26–51. doi: 10.1111/j.1600-0757.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 6.Davey ME, O’toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–84. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuramitsu HK. Virulence factors of mutans streptococci: Role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–76. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 10.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Ann Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 12.Touhami A, Jericho MH, Beveridge TJ. Atomic force microscopy of cell growth and division in Staphylococcus aureus. J Bacteriol. 2004;186:3286–95. doi: 10.1128/JB.186.11.3286-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre G, Kulakauskas S, Chapot-Chartier MP, Navet B, Deghorain M, Bernard E, et al. Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat Commun. 2010;1:27. doi: 10.1038/ncomms1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaer-Zammaretti P, Ubbink J. Imaging of lactic acid bacteria with AFM-elasticity and adhesion maps and their relationship to biological and structural data. Ultramicroscopy. 2003;97:199–208. doi: 10.1016/S0304-3991(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 15.Qin Z, Zhang J, Hu Y, Chi Q, Mortensen NP, Qu D, et al. Organic compounds inhibiting S. epidermidis adhesion and biofilm formation. Ultramicroscopy. 2009;109:881–8. doi: 10.1016/j.ultramic.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, et al. Norwich, UK: The John Innes Foundation; 1985. Genetic Manipulation of Streptomyces-A Laboratory Manual. [Google Scholar]
- 17.Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, et al. The Ribosomal Database Project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 2003;31:442–3. doi: 10.1093/nar/gkg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felsenstein J. Seattle: Department of Genome Sciences and Department of Biology, University of Washington; 2008. PHYLIP-Phylogeny Inference Package (version 3.68) [Google Scholar]
- 19.Tanner AC, Izard J. Etiology of oral disease in view of microbial complexity. Oral Biosci Med. 2005;213:209–13. [Google Scholar]
- 20.Schlegel L, Grimont F, Collins MD, Régnault B, Grimont PA, Bouvet A. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp infantarius subsp. nov. and Streptococcus infantarius subsp coli subsp. nov., isolated from humans and food. Int J Syst Evol Microbiol. 2000;50:1425–34. doi: 10.1099/00207713-50-4-1425. [DOI] [PubMed] [Google Scholar]
- 21.Kreth J, Hagerman E, Tam K, Merritt J, Wong DT, Wu BM, et al. Quantitative analyses of Streptococcus mutans biofilms with quartz crystal microbalance, microjet impingement and confocal microscopy. Biofilms. 2004;1:277–84. doi: 10.1017/S1479050504001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemos JA, Abranches J, Burne RA. Responses of cariogenic streptococci to environmental stresses. Curr Issues Mol Biol. 2005;7:95–107. [PubMed] [Google Scholar]
- 23.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–13. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 24.Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68:2950–8. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Zhang J, Ulstrup J. Investigation of Streptococcus mutans biofilm growth on modified Au (111)-surfaces using AFM and electrochemistry. J Electroanal Chem (Lausanne Switz) 2011;656:41–9. [Google Scholar]
- 26.Batina N, Renugopalakrishnan V, Lavín PN, Hernandez Guerrero JC, Morales M, Garduño-Juárez R. An atomic force microscopic study of the ultrastructure of dental enamel afflicted with amelogenesis imperfecta. J Biomater Sci Poly Ed. 2002;13:337–48. doi: 10.1163/156856202320176565. [DOI] [PubMed] [Google Scholar]
- 27.Lamont RJ, Jenkinson HF. Adhesion as an ecological determinant in the oral cavity. In: Kuramitsu HK, Ellen RP, editors. Oral Bacterial Ecology: The Molecular Basis. Horizon: Wymondham, UK; [Google Scholar]


