Abstract
Background:
Metallo-beta-lactamase (MBL) mediated resistance to carbapenems is an emerging threat in Pseudomonas aeruginosa (PA) nosocomial infections. Limited data on role of Imipenem resistant MBL positive PA (IR-MBLP-PA) and IR-MBL negative-PA (IR-MBLN-PA) infections on mortality and morbidity initiated the present study.
Objectives:
The aim of this study is to determine the role of IR-MBLP-PA and IR-MBLN-PA infections on mortality and morbidity.
Materials and Methods:
Prospective observational study of 1 year with 110 PA nosocomial infections was conducted with Imipenem + ethylene-diamine-tetra-acetic acid combined disc test for MBL detection. Role of IR-MBLP-PA and IR-MBLN-PA infections on the outcome and morbidity were assessed in terms of crude mortality rate, Charlson's comorbidity score and mean duration of stay in intensive care unit (ICU) until cure and until death, number of episodes of complications and underlying disease. Results were analyzed by z test for proportions and Student t-test.
Results:
Relatively high crude mortality was observed among IR-MBLP-PA infections than IR-MBLN-PA (42.86% [6/14] vs. 20% [2/10], Z = 0.69, P = 0.49 NS). Ventilator-associated pneumonia was the underlying disease and a confounding factor in all deaths due to IR-MBLP-PA infections. IR-MBLP-PA infections resulted in rapid downhill course to death with short mean duration of stay in ICU until death than IR-MBLN-PA infections (3.167 ± 0.98 days vs. 16 ± 2.82, P < 0.001 highly significant [HS]) with more number of complications (5.85 ± 1.65 vs. 3.7 ± 1.31, P < 0.001 HS). With the exception of previous Imipenem therapy, association of higher Charlson's comorbidity score, severe underlying diseases, multidrug and pandrug resistance and pre-disposing risk factors with IR-MBLP-PA infections was not statistically significant.
Conclusions:
Higher mortality in IR-MBLP-PA than in IR-MBLN-PA was not significant indicating IR as an important predictor of mortality than MBL production. Higher morbidity and increased virulence was observed with certainty in IR-MBLP-PA infections.
Keywords: Imipenem resistant metallo-beta-lactamase negative Pseudomonas aeruginosa, imipenem resistant metallo-beta-lactamase positive Pseudomonas aeruginosa, imipenem sensitive Pseudomonas aeruginosa, pre-disposing risk factors
INTRODUCTION
Pseudomonas aeruginosa (PA) is an important nosocomial pathogen mainly in intensive care units (ICUs) being responsible for different types of nosocomial infections with increasingly limited therapeutic options due to multi drug resistance (MDR) and pan-drug resistance (PDR) resulting in higher mortality and morbidity.[1] Imipenem, a carbapenem antibiotic, is a last resort for multi and PDR PA infections because of its broad-spectrum antimicrobial activity and stability against most common beta lactamases.[2] However, resistance to carbapenems is increasingly being reported due to decreased outer membrane permeability, increased efflux systems, alteration of penicillin binding proteins and recently, carbapenem hydrolyzing enzymes-carbapenemases.[3]
Acquired metallo-betalactamases (MBLs: IMP and VIM-Verona-integron-encoded Metallo-beta-lactamases), a class B carbapenemases have recently emerged globally as an important mechanism of imipenem resistance (IR) since the first report from Japan in 1991. This is the most worrisome resistance mechanism especially among PA due to its ability to hydrolyze with the exception of Aztreonam all betalactam antibiotics, including carbapenems and failure of serine beta lactamase inhibitors like Clavalunic acid to inhibit MBLs.[2] Several non-molecular screening tests are done on IR PA isolates for detection of MBL production viz., Imipenem + ethylene-diamine-tetra-acetic acid (EDTA) combined disc test, imipenem-EDTA double disc synergy test, EDTA disc potentiation test and MBL-E test. IR PA isolates are selected for MBL detection since only small number of Imipenem sensitive (IS) isolates produce MBLs.[4]
A higher mortality and increased virulence of MBL positive PA (MBLP-PA) isolates in nosocomial infections is reported from very few studies conducted so far, with the majority of the studies across the world highlighting only the incidence and prevalence of MBLP-PA infections.[5,6,7,8,9] Systematic studies on morbidity due to IR-MBLP-PA and IR-MBL negative-PA (IR-MBLN-PA) nosocomial infections are scarce in the literature.[5,6,7,8,9]
Increasing IR PA infections at our hospital and limited information on incidence and influence of IR-MBLP-PA and IR-MBLN-PA nosocomial infections on mortality and morbidity in patients with multiple pre-disposing risk factors necessitated the present study.
MATERIALS AND METHODS
A prospective observational study of consecutive patients with PA nosocomial infections was performed at a tertiary care hospital for a period of 1 year with prior approval from Institutional Ethical Committee to determine the influence of IR-MBLP-PA and IR-MBLN-PA on mortality and morbidity. Polymicrobial infections and infections not fulfilling Centre for Disease Control criteria for nosocomial infections were excluded.[10]
Different specimens from patients were collected and processed according to standard laboratory procedures.[11] Data regarding prognostic factors and pre-disposing risk factors were collected from medical records, computer database and in most of the cases in consultation with treating doctors. Susceptibility to Amikacin, Ciprofloxacin, Netilmycin, Gentamicin, Tobramycin, Piperacillin, Piperacillin-Tazobactam, Cefotaxime, Ceftazidime, Cefoperazone, Cefoperazone-Sulbactam and Imipenem was determined by Kirby-Bauer's disc diffusion method according to Clinical and Laboratory Standards Institute guidelines.[12] Aztreonam, Polymyxin-B and Colistin were tested only against IR-MBLP-PA isolates.
PA isolates resistant to imipenem were subjected to the screening test for MBL production by Imipenem + EDTA combined disc test as described previously by Yong et al.[13] Isolates with enhancement of zone size of more than or equal to 7 mm between Imipenem + EDTA disc compared with Imipenem disc alone were considered as IR-MBLP-PA others were considered as IR-MBLN-PA. MBLN ATCC (27853) standard strain of PA was used as a negative control.
Severity of patient's condition was assessed by Charlson's comorbidity score, mean duration of stay in ICU until cure and until death, number of episodes of complications related to infections, pre-disposing risk factors and length of hospital stay prior to isolation of IR-MBLP-PA isolate. Statistical analysis was carried out by z test for proportions and Student t-test by using the Statistical Package for the Social Sciences (SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, IL, USA) Windows version 13.0.
RESULTS
Of the 523 patients presenting with PA, 110 isolates from nosocomial infections were included in the present study after excluding 283, 60 and 70 isolates from community acquired infections, contaminants or colonizers and polymicrobial infections respectively.
Incidence of IR PA, IR-MBLP-PA isolates and IR-MBLN-PA isolates was 21.82% (24/110), 12.73% (14/110) and 8.91% (10/110). Out of 24 IR isolates, 58.33% (14/24) were IR-MBLP-PA and 41.67% (10/24) were IR-MBLN-PA [Table 1].
Table 1.
Hospital area wise distribution of IR-MBLP-PA isolates

In the present study, 42.86% (6/14) of patients with IR-MBLP-PA infections suffering from ventilator associated pneumonia (VAP), rapidly progressed to death with a mean duration of stay until death of 3.167 ± 0.98 days. However, 57.14% (8/14) of patients with other infections improved with a mean duration of stay in ICU of 21 ± 4.95 days. Patients with IR-MBLP-PA infections suffered more number of episodes of complications related to infection. Neither IR-MBLP-PA nor IR-MBLN-PA isolates were observed in neonatal ICU (NICU) [Table 2].
Table 2.
Distribution of prognostic factors in IR-MBLP-PA and IR-MBLN-PA patients

Relatively high crude mortality was observed among IR-MBLP-PA infections than IR-MBLN-PA (42.86% [6/14] vs. 20% [2/10], Z = 0.69, P = 0.49 NS). However, overall in-hospital mortality of patients with PA infections was 13.64% (15/110): 42.86% (6/14) for IR-MBLP-PA infections and 8.14% (7/86) for IS-PA infections (P < 0.03, Z = 2.07 S).
Nearly, 80% (8/10) patients with IR-MBLN-PA isolates recovered faster with a mean duration of stay in ICU of 6.125 ± 2.1 days compared with 21 ± 4.95 days in IR-MBLP-PA isolates (P < 0.001 highly significant [HS]). Patients with IR-MBLN-PA infections had less number of complications related to infection compared with IR-MBLP-PA infections (3.7 ± 1.31 vs. 5.85 ± 1.65 P < 0.001 HS). Nearly, 80% (8/10) of IR-MBLN-PA infections presented with good prognosis with improvement versus 57.14% (8/14) of IR-MBLP-PA infections (P > 0.05 NS).
Antimicrobial therapy with Imipenem during previous 3 weeks prior to the isolation of IR PA (78.57% vs. 30%, P < 0.047 S) and higher Charlson's co-morbidity scores (4 ± 1.4 vs.3 ± 0.8, t = 2.4, P = 0.02 S) were more frequently associated with IR-MBLP-PA than IR-MBLN-PA infections respectively. Pre-disposing risk factors were more commonly associated with IR-MBLP-PA than IR-MBLN-PA infections (statistically not significant) [Table 3].
Table 3.
Distribution of predisposing risk factors in IR-MBLP-PA and IR-MBLN-PA infections

Aztreonam and Polymyxin B were the drugs with least resistance, 14.29% and 0% respectively [Table 4].
Table 4.
Resistance rates of IR-MBLP-PA and IR-MBLN-PA isolates to different antibiotics

A total of six distinct antibiotic resistance strains were observed in IR-MBLP-PA isolates. Strain 1 was the most common (resistant to all drugs except Polymyxin B, Colistin and Aztreonam) causing 42.86% (6/14) infections [Table 5].
Table 5.
Antibiogram types of 14 IR-MBLP-PA isolates

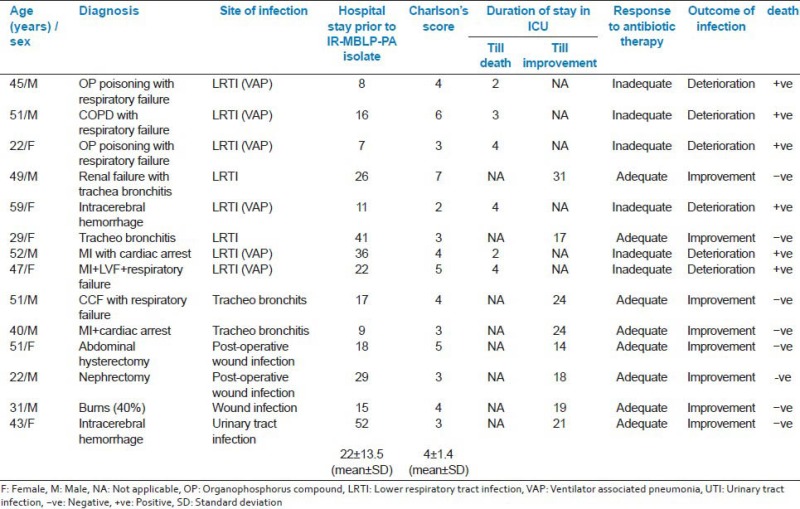
Characteristics of the fourteen patients with IR-MBLP-PA infections is presented with the response to antibiotic treatment, length of hospital stay prior to isolation of IR-MBLP-PA isolation, Charlson's comorbidity score in Appendix 1.
DISCUSSION
The present study, first of its kind to the best of our knowledge, was conducted to determine the influence of IR-MBLP-PA and IR-MBLN-PA nosocomial infections on mortality and morbidity in patients with multiple pre-disposing risk factors and serious underlying diseases often with multidrug and PDR strains.
The study revealed no significant difference in the mortality between IR-MBLP-PA and IR-MBLN-PA infections even after the objective assessment of confounding predisposing risk factors by appropriate statistical tests. Neither increased mortality rate nor decreased survival rate in IR-MBLP-PA infections were significant compared with IR-MBLN-PA infections. However, the study revealed with certainty, higher mortality in IR PA than in IS-PA infections, indicating IR as the most important factor affecting mortality and morbidity than MBL production.
Higher virulence of IR-MBLP-PA isolates observed in terms of rapid downhill course to death with a short mean duration of stay in ICU until death and higher frequency of complications related to infections was significantly associated with IR-MBLP-PA than IR-MBLN-PA infections. Significantly higher crude mortality in MBL-PA patients than non-MBL patients (5.8% [4/69] vs. 1.2% [3/247], odds ratio [OR] 5.0, 95% confidence interval [CI]; 1.09-22.9; P = 0.02) was reported by Hirakata et al. and Laupland et al. (25% [23/93] vs. 13% [10/80], RR 1.98 [95% CI; 1.0-3.90]; P = 0.05).[6,7] Higher mortality rates and higher frequency of infections in such group were reported to be due to higher virulence of MBL producing PA infections.[5,6,7] Zavascki et al. reported MBL production in PA as an independent risk factor for mortality by Cox model with multivariate analysis for confounding factors.[5] However, all the above studies used genotypic methods for detection of MBL production, the phenotypic expression of which is doubtful in a clinical situation and the possibility of multi and PDR contributing to mortality could not be ruled out.[14] Higher virulence of IR-MBLP-PA resulting in higher mortality in the present study and other studies need to be confirmed by further large scale, multicentric, prospective studies by nullifying confounding factors by suitable statistical tests along with phenotypic and genotypic studies to characterize virulence factors of IR-MBLP-PA. Studies on virulence factors are absolutely necessary to declare IR-MBLP-PA as more virulent than IR-MBLN-PA.
Imipenem + EDTA combined disc test, a non-molecular sensitive screening test, clearly discriminating positive and negative results for MBL detection was used in the present study with its limitation of missing small number of IS-PA with MBL production. Though polymerase chain reaction is highly sensitive and specific test, is limited by high-cost and non-availability at all hospitals.[8]
What emerges clearly with certainty is higher crude mortality in IR PA infections compared with IS-PA infections (33.3% [8/24] vs. 8.14% [7/86] P < 0.01 S]). Irrespective of MBL production, IR PA with multi and PDR isolates and multiple pre-disposing risk factors resulted in higher mortality highlighting IR, irrespective of mechanism of resistance as an important cause of mortality.
Most of the studies across the world highlight the impact of IR-MBLP-PA infections only on mortality, which in reality is the tip of the iceberg.[5,6,7] The present study reports a higher morbidity with certainty, in terms of prolonged ICU stay (21 ± 4.95 days vs. 6.125 ± 2.1, t = 7.87, P < 0.001 S) in survivors, higher Charlson's comorbidity scores and higher incidence of the number of complications related to IR-MBLP-PA infections significantly adding to the cost of treatment and prolonged stay in the hospital. Similar studies are not available for comparison after review of the literature.
The most important confounding factor in determining the impact of IR-MBLP-PA and IR-MBLN-PA infections on mortality and morbidity was VAP, which by itself, irrespective of the causative agent, carries a high mortality and morbidity. All deaths due to IR-MBLP-PA infections (6/14) and one of the two deaths (2/10) among IR-MBLN-PA infections were due to VAP. This highlights the importance of serious disease like VAP as the most important predictor of mortality than MBL production.
In the present study, although with a higher virulence, 57.14% of IR-MBLP-PA infections did not result in mortality and on the contrary a patient with 50% burns with IR-MBLN-PA rapidly progressed to death. Survival of two patients with IR-MBLP-PA nosocomial tracheobronchitis, two from post-operative wound infections and one each from burns and urinary tract infection (UTI) signifies the role of less severe underlying disease as an important predictor of good prognosis. However, findings of the present study underscore the role of severity of underlying diseases like VAP and burns wound infections contributing significantly to mortality and morbidity than less severe infections like UTI and ventilator associated tracheobronchitis with a good prognosis.
Higher mortality and morbidity in IR-MBLP-PA infections in the present study were directly associated with more frequent isolation of PDR isolates (strain 1) among IR-MBLP-PA compared with IR-MBLN-PA (6 vs. 2) and MDR isolates (14 vs. 4) complicating the selection of antibiotic for treatment in all cases of IR-MBLP-PA infections. Paterson reported that all the MBL-PA isolates and 52% of non-MBL-PA isolates were MDR while 11% of MBL-PA isolates and 8% of non-MBL-PA isolates were PDR.[1,5] However, in the present study association of MDR and PDR with IR-MBLP-PA than with IR-MBLN-PA was statistically not significant, highlighting IR with different mechanisms of its resistance as the most important factor affecting mortality and morbidity and not MBL production. Clinicians were practically left with few options for treating patients with pan and MDR infections irrespective of MBL production resulting in higher mortality and morbidity.
In the present study, previous antibiotic therapy with imipenem was significantly associated with IR-MBLP-PA than IR-MBLN-PA isolates (78.57% vs. 30%, P < 0.04 S) resulting in the acquisition of IR-MBLP-PA. This is probably due to the fact that antimicrobial resistance increases the likelihood of an inadequate initial antibiotic regimen and of increased morbidity and mortality from inadequate initial treatment. As result, the mere possibility of infections due to antimicrobial-resistant pathogens necessitates broad spectrum initial empirical antimicrobial therapy, usually with a combination of drugs including Imipenem. This increases the cost of treatment, the occurrence of adverse drug effects and ironically, the local prevalence of antimicrobial resistance.[16]
Carmeli et al. reported emergence of resistance to imipenem in eight patients, seven of whom were treated with imipenem.[15] Clinical emergence of resistant PA has been described during the imipenem therapy, ranging from 14% to 53% limiting future therapeutic choices and associated with increased mortality, morbidity and higher costs.[15,16]
Pre-disposing risk factors [Table 3] and higher Charlson's comorbidity scores [Table 2] resulting in higher mortality, morbidity, emergence and/or acquisition of IR-MBLP-PA isolates, were more commonly associated with IR-MBLP-PA than IR-MBLN-PA infections, but the association was not statistically significant [Table 2].
MBL producing PA isolates are known for rapid clonal spread within and outside the hospital, into the community and intercontinentally, crossing geographical barriers than its influence on mortality and morbidity.[5,6] Distribution of IR-MBLP-PA infections was not uniform in ICUs of our hospital. Five of the strain 1, IR-MBLP-PA isolates were found in medical ICU (MICU) and intensive cardiac care unit (ICCU), contributing to the majority of deaths due to IR-MBLP-PA infections. Most of the cases (71.42%) were from MICU and ICCU, where imipenem was used heavily for empirical and definitive antimicrobial treatment. A single case of UTI with IR-MBLP-PA (strain 1) was reported from general ward, but he was a patient recently shifted from MICU. Absence of IR-PA and IR-MBLP-PA infections from NICU was a direct result of strict infection control practices during last 3 years due to increasing mortality among infants with neonatal septicemia. Burden of IR-MBLP-PA infections was found to be just short of endemicity with a strong potential to spread within different ICUs.
Although no significant difference in mortality between IR-MBLP-PA and IR-MBLN-PA was observed in the present study, occurrence of IR-MBLP-PA isolate in a localized hospital environment poses not only therapeutic problem, but more importantly serious concern for infection control because of its ability of rapid clonal spread within and outside the hospital.[14] However, from the present study it is quite evident that mortality due to IR-MBLP-PA infections is more or less same as that of IR-MBLN-PA except for the potential spread of IR-MBLP-PA resulting in more number of infections in a localized hospital area. More important than the role of IR-MBLP-PA on mortality and morbidity, these can transfer MBL genes through plasmids to Enterobacteriaceae, probably within a clinical environment. Rapid emergence and clonal spread of MBL positive PA in hospital has been reported by several studies.[3,7,14]
Mortality and morbidity in IR-MBLP-PA and IR-MBLN-PA infections was associated with severe underlying disease, previous antibiotic therapy with imipenem, MDR and PDR isolates in patients with multiple predisposing risk factors and increased Charlson's comorbidity scores. Impact of IR-MBLP-PA and IR-MBLN-PA infections on mortality and morbidity in an index patient could be assessed only in the background of these factors prevailing in a patient at the time of isolation.
Limitations of the present study
Findings of present study are an underestimation of burden of IR-MBLP-PA infections since polymicrobial PA infections were excluded from the study.
Detection of IS- MBL positive PA, though usually in small numbers was not done.
CONCLUSION
Difference in the attributable mortality in IR-MBLP-PA and IR-MBLN-PA was not statistically significant indicating IR as the most important predictor of prognosis and not MBL production. However, further large scale, multicentric prospective studies to determine the clinical significance of impact on mortality are needed.
Higher attributable mortality in IR-MBLP-PA infections, is partially mediated by production of MBLs, severity of underlying disease, pre-disposing risk factors, MDR, PDR, higher Charlson's co-morbidity scores and probably increased virulence.
Higher morbidity with certainty was observed in IR-MBLP-PA than IR-MBLN-PA infections.
IR PA infections significantly causes were higher mortality than IS-PA infections.
ACKNOWLEDGMENT
We duly acknowledge the statistical analysis performed by Mrs. Rajashree Patil, Asst. Prof. and Statistician, Department of Community Medicine. SSIMS and RC, Davangere.
Appendix 1: Clinical characteristics of fourteen patients with IR-MBLP-PA infections
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yogeesha KV, Jayasimha VL, Basavarajappa KG, Arun K, Raghu KK, Niranjan HP. A comparative study of ventilator associated pneumonia and ventilator associated tracheobronchitis: Incidence, outcome and risk factors. Biosci Biotech Res Asia. 2011;8:195–203. [Google Scholar]
- 2.Wright GD, Sutherland AD. New strategies for combating multidrug-resistant bacteria. Trends Mol Med. 2007;13:260–7. doi: 10.1016/j.molmed.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002;8:321–31. doi: 10.1046/j.1469-0691.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- 4.Behera B, Mathur P, Das A, Kapil A, Sharma V. An evaluation of four different phenotypic techniques for detection of metallo-beta-lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol. 2008;26:233–7. doi: 10.4103/0255-0857.39587. [DOI] [PubMed] [Google Scholar]
- 5.Zavascki AP, Barth AL, Gonçalves AL, Moro AL, Fernandes JF, Martins AF, et al. The influence of metallo-beta-lactamase production on mortality in nosocomial Pseudomonas aeruginosa infections. J Antimicrob Chemother. 2006;58:387–92. doi: 10.1093/jac/dkl239. [DOI] [PubMed] [Google Scholar]
- 6.Hirakata Y, Yamaguchi T, Nakano M, Izumikawa K, Mine M, Aoki S, et al. Clinical and bacteriological characteristics of IMP-type metallo-beta-lactamase-producing Pseudomonas aeruginosa. Clin Infect Dis. 2003;37:26–32. doi: 10.1086/375594. [DOI] [PubMed] [Google Scholar]
- 7.Laupland KB, Parkins MD, Church DL, Gregson DB, Louie TJ, Conly JM, et al. Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary health region: Importance of metallo-beta-lactamase (MBL)-producing strains. J Infect Dis. 2005;192:1606–12. doi: 10.1086/444469. [DOI] [PubMed] [Google Scholar]
- 8.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to carbapenems in a tertiary care hospital in north India. Indian J Med Res. 2006;124:95–8. [PubMed] [Google Scholar]
- 9.Hemalatha V, Sekar U, Kamat V. Detection of metallo betalactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res. 2005;122:148–52. [PubMed] [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 11.Govan JR. Pseudomonas aeruginosa. In: Collee G, Barrie PM, Andrew PF, Anthony S, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 2006. pp. 413–24. [Google Scholar]
- 12.Performance Standards for Antimicrobial Susceptibility Testing. Suppl 27. Vol. 17. Wayne, PA: Clinical Laboratory Standards; 2007. Clinical and Laboratory Standards Institute. [Google Scholar]
- 13.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798–801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18:306–25. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: Comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43:1379–82. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park DR. Antimicrobial treatment of ventilator-associated pneumonia. Respir Care. 2005;50:932–52. [PubMed] [Google Scholar]


