Abstract
Background:
Hypertension is one of the most common complication during pregnancy. It contributes significantly to maternal and perinatal morbidity and mortality. This study was designed to investigate the morphological and histopathological changes in placenta from pregnancies complicated with hypertension.
Objectives:
To study the morbid changes in placenta in cases of pregnancy-induced hypertension (PIH) and to correlate the findings with birth weight of new born babies in comparison with normotensive mothers.
Materials and Methods:
The study was done on 100 placentas, out of which 50 were collected from normotensive mothers and the remaining 50 from PIH cases. All the placentas were studied morphologically and histologically. The birth weight of neonates was recorded.
Results:
In the present study it was observed that weight and dimensions of placenta was less in study group when compared with control group. The mean neonatal birth weight was more in normal pregnancy and feto-placental weight ratio was significantly high in hypertensive group. Histopathological study showed significant number of syncitial knots, areas of fibrinoid necrosis, hyalinization, calcification, and medial coat proliferation of medium sized blood vessels in hypertensive group.
Conclusion:
PIH significantly affects the placenta by reducing its weight and dimensions. These changes may cause placental insufficiency as a result of compromised utero-placental blood flow. Therefore has an adverse affect on the neonatal birth weight. PIH has definite influence on morphology, histology of placenta, and thus affects the growth of the fetus.
Keywords: Neonatal birth weight, placenta, pregnancy-induced hypertension
INTRODUCTION
The intrauterine existence of fetus is dependent on one vital organ “The Placenta”. Placenta is essential for maintenance of pregnancy and for promoting normal growth and development of fetus.[1] It is the most accurate record of the infant's prenatal experience.[2] It forms the morphological record of anatomical condition, intrauterine events and intrapartum events of gestation. Pregnancy-induced hypertension (PIH) is the leading cause of maternal mortality and is an important factor in fetal wastage. Pregnancy complications like hypertension are reflected in placenta in a significant way both macroscopically and microscopically. Several studies have shown that utero-placental blood flow is decreased in PIH due to maternal vasospasm.[3] This leads to constriction of fetal stem arteries and has been associated with the changes seen in the placenta of preeclamptic women.[4] Maternal vasospasm leads to fetal hypoxia and accordingly it may lead to fetal distress and fetal death.[5] Present study has been undertaken to record the data on the morphology, morphometry, and histology of placenta from mothers with PIH and correlate the findings with the birth weight of the new born babies. This study was done to find out the morbid changes of the placenta of hypertensive mothers in comparison to normotensive mothers. As placenta is the mirror of maternal and fetal status, it reflects the changes due to maternal hypertension.
MATERIALS AND METHODS
The study was done in the Department of Anatomy in collaboration with the Department of Obstetrics and Gynaecology, S.V.S Medical College, a tertiary care hospital, Mahabubnagar, Andhra Pradesh, during the period from October 2009 to October 2011. The study was approved by Institutional Ethics Committee.
The study was done in 100 placentas, which were collected from Obstetrics and Gynecology department. Out of the 100 placentas collected, 50 placentas were from uncomplicated full term deliveries and served as control group. Another 50 placentas were collected from preeclamptic and eclamptic cases and served as study group.
The age of the women varied from 20 to 35 years. The cases were divided into three groups, namely, normal, preeclampsia, and eclampsia group.
Collection and examination of placenta
The placenta with attached membranes and umbilical cord was collected soon after delivery, washed in running tap water, labeled, and then fixed with 10% formalin for 4-6 weeks. Gross and microscopic examination of the placenta was carried out. The size, shape, weight, thickness at centre, number of cotyledons, and site of insertion of umbilical cord were noted down. The birth weights of newborn babies were documented and feto-placental weight ratio was calculated. Histo-pathological study of placenta was done and the slides were studied under light microscope.
The data collected from morphological and morphometric studies were recorded. Descriptive statistics was used to analyze the data. They were represented as Mean ± SD (standard deviation). The statistical significance between the means of the control group and study groups were analyzed by using Students unpaired “t” test. A P value of <0.05 was considered statistically significant. Statistical analysis was done by using GraphPad Quick Calcs software.
RESULTS
From the study, it was observed that the weight of the placenta was less in preeclampsia and eclampsia when compared with normal placenta. The majority of placenta were oval (73%) followed by round placenta (27%) [Table 1]. The insertion of the umbilical cord central in 18% of cases and eccentric in 82% of cases. The number of cotyledons varied from 18 to 23 in all groups.
Table 1.
Comparison of morphological features of placenta in different groups

The mean neonatal birth weight was more in normal pregnancy (3.14 kg). It was less in preeclamptic and eclamptic cases (<2.5 kg). The feto-placental weight ratio was significantly higher in the hypertensive group than in the control group [Table 1].
Histological study of placenta showed significant number of syncytial knots, fibrinoid necrosis, areas of calcification and hyalinisation and areas of medial coat proliferation of medium sized blood vessels in the hypertensive group, whereas the control group showed normal histological features.
DISCUSSION
In the present study, the weights of the placenta in study groups were below 500 g. The least weight recorded being 200 g. In the control group, majority of the placenta weighed more than 500 g, the heaviest being 650 g.
The observations correlate well with the previous studies done by various workers. Mallik et al. reported five cases of toxemia with placental weight less than 300 g.[6] Nobis and Das, in their study, observed that the placental weight in toxemic cases varies from 279 to 407 g.[7] Bhatia et al, in their study, have shown reduced placental weight in severe toxemia, the lowest recorded was 280 g.[8] The change in placental weight observed in the present study between the control group and study groups were statistically significant [Table 1].
A significant increase in syncytial knot formation in placental villi indicates the disturbance in the hormonal factors, which may probably lead to altered blood flow. According to Robertson, the cause of reduction in blood flow is due to vasculopathies of spiral arteries, which in turn causes reduction in the weight of placenta. It has been recorded that maternal utero-placental blood flow is decreased in preeclampsia because of maternal vasospasm. Reduced maternal utero-placental blood flow indirectly leads to constriction of fetal stem arteries.[9]
The preeclamptic women will have a lower mean gestation, so the proportion of fetal capillaries will be lower. The capillaries become larger as the gestation proceeds. This relative increase in fetal capillary volume with decrease in proportion of connective tissue will lead to smaller parenchymal volume leading to decrease in placental weight.[9]
In the present study, the average diameter of placenta in control group was 18.62 cm, in preeclamptic cases it was 17.33 cm, and in case of eclamptic cases it was 16.24 cm. The study by Mallik et al. reported that the mean diameter of placenta was 17.54 cm.[10] Cibils reported that placenta from PIH cases were smaller than normal indicating an underlying pathological process interfering with the normal growth of placenta.[10]
The majority of cases showed eccentric insertion (82%) and few showed central insertion (18%) in both the control and study groups. Whereas in the earlier studies by Nobis and Das, the pattern of cord insertion was central in 44.19%, eccentric in 42.17%, and battledore in 1.26%.[7]
The neonatal weight was significantly less in study groups when compared with control group (P < 0.01). The average weight was 3.14 kg in normal pregnancies, 2.44 kg in preeclampsia, and 2.29 kg in eclampsia cases. An earlier study by Palaskar had shown the reduced mean weight of the neonates in cases of PIH.[11]
The arrangement of intracotyledon vasculature is altered in hypertension resulting in low birth weight of the babies.[12] Heavy proteinuria increases the incidence of low birth weight babies in preeclampsia.[13] Also the reduction in the villous population will interfere with fetal nutrition and growth, leading to decrease in neonatal weight.[14]
In the present study, the average feto-placental weight ratio in normal pregnancy was 5.72 ± 0.93, 6.35 ± 2.05 in preeclamptic cases, and 6.44 ± 2.02 in eclamptic cases. The values corelate with the earlier study by Majumdar et al.[12]
In the study group, the histology revealed various structural changes such as significant number of syncytial knots, areas of fibrinoid necrosis, areas of medial coat proliferation of medium sized blood vessels, areas of calcification, and areas of hyalinization [Figure 1]. A significant increase in syncytial knot formation in placental villi indicates the disturbance in the hormonal factors, which may probably lead to altered morphometry of placenta resulting in PIH in the mother and to low birth weight babies. Microscopic findings of localized fibrinoid necrosis, medial coat proliferation of arteries, and hyalinization depict the mosaicism of placenta and probably the aftermath of hypertension.[15] Again the mosaicism of the placenta probably leads to placental insufficiency and ultimately to fetal growth retardation, thus creating a vicious cycle.[16]
Figure 1.
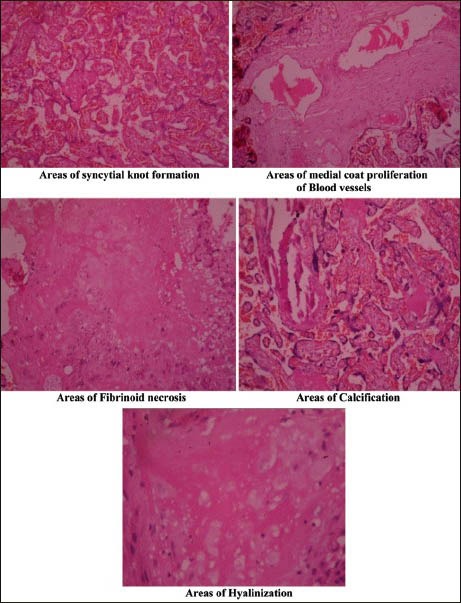
Histological changes in placenta in cases of preeclampsia and eclampsia
CONCLUSION
PIH significantly affects the placenta by reducing its weight and dimensions. However, it does not have any effect on placental shape, umbilical cord insertion, and number of cotyledons on maternal surface. It induces histological changes such as, areas of syncytial knot formation, fibrinoid necrosis, calcified areas, hyalinised areas, and areas of medial coat proliferation of the blood vessels. These changes compromise utero-placental blood flow and significantly reduce the neonatal birth weight.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Udainia A, Jain ML. Morphological study of placenta in pregnancy induced hypertension with its clinical relevance. J Anat Soc India. 2001;50:24–7. [Google Scholar]
- 2.Benirshke K. The placenta: How to examine it and what you can learn. Contemp Obstet Gynaecol. 1981;17:117–9. [Google Scholar]
- 3.Bewly S, Cooper D, Campbell S. Doppler investigation of utero-placental blood flow resistance in the second trimester. A screening study for pre-eclampsia and intra-uterine growth retardation. Br J Obst Gynaecol. 1991;98:871–9. doi: 10.1111/j.1471-0528.1991.tb13508.x. [DOI] [PubMed] [Google Scholar]
- 4.Stock MK, Anderson DF, Phernetton TM, McLaughlin MK, Rankin JH. Vascular response of the maternal placental vasculature. J Dev Physiol. 1980;2:239–46. [PubMed] [Google Scholar]
- 5.Thomson AM, Billewickz, Hytten FE. Placenta in relation to birth weight. J Obstet Gynecol Br CW. 1969;76:865–72. doi: 10.1111/j.1471-0528.1969.tb15722.x. [DOI] [PubMed] [Google Scholar]
- 6.Bazaz G, Mirchandani JJ, Chitra S. Placenta in intrauterine growth retardation. J Obstet Gynecol India. 1979;29:805–10. [PubMed] [Google Scholar]
- 7.Nobis P, Das U. Placental morphology in hypertensive pregnancy. J Obstet Gynecol. 1991;41:166–9. [Google Scholar]
- 8.Bhatia A, Sharma SD, Jalnawalla SF, Sagreiya K. A comparative study of placental and foetal outcome. Indian J Pathol Microbiol. 1981;24:277–83. [PubMed] [Google Scholar]
- 9.Boyd PA, Scott A. Quantitative structural studies on human placenta associated with preeclampsia essential hypertension and intrauterine growth retardation. Br J Obstet Gynaecol. 1985;92:714–21. doi: 10.1111/j.1471-0528.1985.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 10.Cibils LA. The placenta and newborn infant with hypertension conditions. Am J Obstet Gynaecol. 1974;118:256–70. doi: 10.1016/0002-9378(74)90557-2. [DOI] [PubMed] [Google Scholar]
- 11.Palaskar A, Choudhary KR, Mayadeo NM. Foeto placental weight relationship in normal pregnancy, pre-eclampsia and eclampsia. Bombay Hosp J. 2001;43:361–3. [Google Scholar]
- 12.Majumdar S, Dasguptha H, Bhattacharya K, Bhattacharya A. A study of placenta in normal and hypertensive pregnancies. J Anat Soc India. 2005;54:34–8. [Google Scholar]
- 13.Chakravorthy AP. Foetal and placental weight changes on normal pregnancy and pre-eclampsia. J Obstet Gynaecol Br Commonw. 1967;74:247–53. doi: 10.1111/j.1471-0528.1967.tb14868.x. [DOI] [PubMed] [Google Scholar]
- 14.Fox H. The morphological basis of placental insufficiency. J Obstet Gynaecol India. 1975;25:441–50. [Google Scholar]
- 15.Teasdale F. Gestational changes in functional structure of the human placenta in relation to foetal growth. Am J Obstet. 1980;137:560–2. doi: 10.1016/0002-9378(80)90696-1. [DOI] [PubMed] [Google Scholar]
- 16.Zacutti A, Borruto F, Bottacci G, Giannoni ML, Manzin A, Pallini M, et al. Umbilical blood flow and placental pathology. Clin Exp Obstet Gynaecol. 1992;19:63–9. [PubMed] [Google Scholar]


