Abstract
Background and Objectives:
Brushing the teeth with fibrous husk of Cocos nucifera (coconut) is a common oral hygiene practice among people of rural areas of South India. However, the probable antimicrobial properties of this plant material against common oral pathogens have not been proved scientifically. Therefore, the present study was designed.
Materials and Methods:
Alcoholic extract of the husk of Cocos nucifera was prepared and the antimicrobial properties against common oral pathogens like cariogenic bacteria, periodontal pathogens, and candidal organisms were performed by the Agar Well Diffusion Method. The results obtained were then subjected to statistical analysis using One-Way Analysis of Variance (ANOVA) and the Tukey's Honestly Significant Difference (HSD).
Results:
The alcoholic extract of Cocos nucifera showed a significant concentration-dependent antimicrobial activity, expressed as a zone of inhibition with respect to all tested organisms except Actinomyces species. The inhibitory effect was more significant, with a majority of cariogenic organisms and Candida, with a zone of inhibition ranging from 4.6 mm to 16.3 mm. However, the effect was lesser with Cocos nucifera compared to chlorhexidine. Minimum inhibitory concentration (MIC) ranged from 50 mg/ml to 75 mg/ml.
Conclusion:
Cocos nucifera has a significant inhibitory action against common oral pathogens, indicating the presence of highly effective antimicrobial compounds. Therefore, it is proved that its use can contribute to oral health to a great extent. Identification of these active compounds provides the scope for incorporating it into a modern oral care system, so as to control oral diseases.
Keywords: Antimicrobial activity, coconut husk, Cocos nucifera, Oral pathogens
INTRODUCTION
Oral health is essential to the general well-being of an individual and relates to the quality of life. There is a considerable evidence linking poor oral health to systemic conditions particularly diabetes mellitus, cardiovascular diseases, rheumatoid arthritis, etc. The link between oral diseases and the activities of the microbial species that form part of the microbiota of the oral cavity is well established.[1] Dental caries and periodontal diseases are two major oral health problems and are a major cause for the loss of teeth. Dental caries is caused by acidogenic and aciduric Gram-positive bacteria, primarily the mutans Streptococci, Lactobacilli, and Actinomycetes, which metabolize sucrose to organic acids, mainly lactic acid, which dissolves the calcium phosphate in the teeth, causing decalcification and eventual decay.[2] Similarly periodontal diseases have been linked to anaerobic Gram-negative bacteria such as Porphyromonas gingivalis, the Actinobacillus species, Prevotella species, and the Fusobacterium species.[3] The prevention strategy of these oral diseases and efforts to improve the oral health mainly involve approaches that reduce the oral microbial load.
For thousands of years, medicinal plants have been used as traditional treatments for numerous human diseases in many parts of the world. The natural products derived from medicinal plants have proven to be an abundant source of biologically active compounds, making them effective source for alternative medicines. Numerous traditional medicinal plants have been evaluated for their potential application in the prevention or treatment of oral diseases particularly those of microbial origin. A number of studies have investigated the activity of plant extracts and products against specific oral pathogens.[4] Antibacterial chew sticks such as meswak and neem, which are plant-based alternatives for oral health, have been successfully promoted and they have been advocated by health agencies.[5] An ethnomedicinal survey conducted by the authors has identified the rich knowledge of people in the rural areas of the Dakshina Kannada district of Karnataka, India, on indigenous plant materials for oral health and diseases. One of the common traditional practices identified was the use of the husk of Cocos nucifera (coconut) as herbal ‘chewing sticks’ instead of plastic bristle brushes, for daily cleaning of teeth.[6]
The coconut (Cocos nucifera Linn.) belongs to the Arecaceae family, is native to the coastal areas of Southeast Asia and is a major crop of the Dakshina Kannada region. A thorough review of literature has revealed few studies on the antibacterial activity of the husk of C. nucifera against the Vibrio species[7] and S. aureus,[8] and also antileishmanial,[9] and antitumoral[10] properties. Earlier studies have also noted the efficient free radical scavenging properties of this plant material.[11,12]
When fibrous coconut husk is used for cleaning the teeth, it is understandable that the fibers will mechanically remove the colonized microorganisms from the tooth surface. As the earlier studies have revealed some antimicrobial properties, it is possible that an additional beneficial effect of this material, that is, the antimicrobial effect against oral pathogens, may also be contributing to improve the oral health. As there are studies on this aspect, the present study was designed to find scientific evidence on the valuable effects of traditional practices.
MATERIALS AND METHODS
Preparation of extracts
The fibrous husk of Cocos nucifera was collected from the local coconut growers. It was washed with distilled water to remove dirt, cut into smaller pieces and air dried for 21 days. The dried husk fiber was then blended using a household electric blender. One hundred grams of the plant powder was extracted in a Soxhlet apparatus with 500 ml of ethanol as the solvent and concentrated using a rotor-evaporator. The extracts thus prepared were dissolved in dimethyl sulfoxide to prepare different concentrations such as 100 mg/ml, 75 mg/ml, 50 mg/ml, and 25 mg/ml.
Collection of test organisms
The test organisms were collected from carious cavities of the affected teeth by scraping the soft caries with an excavator and from periodontal pockets using paper points. After collection, the paper points were dropped into 20 ml of brain heart infusion broth (BHI broth), which was used as the transport media. After 48 hours aerobic and anaerobic incubation samples were placed on a variety of selective and nonselective media. Colonies of different test organisms were identified by colony morphology, gram staining, catalase test, pigment production, and the aerotolerance test. The following organisms were studied S. mutans, S. salivarius, S. mitis, and L. acidophilus, Prevotella intermedia, Actinomycetes species, and Candida.
Assay for antibacterial activity
The antibacterial screening was carried out using the agar diffusion method described by Lino and Deogracious, with slight modifications.[13] Three or four isolated colonies were inoculated in the 2 ml BHI broth and incubated till the growth in the broth was equivalent to the Mac-Farland standard (0.5) as recommended by the World Health Organization (WHO). Freshly prepared inocula were swabbed all over the surface of the Muller Hinton agar plate, using a sterile cotton swab. Five wells of 6 mm diameter were bored in the medium with the help of a sterile cork-borer, having a 6 mm diameter, and were labeled properly. Fifty microliters of different concentrations of plant extract and the same volume of positive and negative controls were filled in the wells with the help of a micropipette. Fifty microliters of Chlorhexidine (0.2%) were used as the positive control and dimethyl Sulfoxide (DMSO) as the solvent control. The plates were left for some time till the extract diffused in the medium, with the lid closed, and incubated at 37°C for 24 hours. The zone of inhibition was measured using a scale. The mean and standard deviation of triplicates of various concentrations of plant extracts were calculated and compared with Chlorhexidine.
Determination of minimum inhibitory concentration and minimum bactericidal concentration
The crude extract was weighed and different concentrations were prepared by dissolving it in DMSO. To determine the MIC, various dilutions of the extracts that had antibacterial activity in the previous assay were taken into sterile test tubes. Freshly prepared nutrient broth was used as a diluent. A tube containing nutrient broth was taken as a control. Fifty microliters of the standard culture inoculum was added to each test tube, except the control tube. All tubes were incubated at 37°C for 24 hours and then examined for growth, by observing the turbidity. Ten microliters of bacterial culture from the MIC tubes, which did not show any growth was pipetted, and subcultured onto the Muller Hinton agar, and incubated at 37oC for 24 hours. After incubation, the concentration at which there was not a single colony of bacteria was taken as the minimum bactericidal concentration (MBC).[14]
Statistical analysis
The results obtained were then subjected to statistical analysis using the one-way ANOVA and post-hoc Tukey HSD.
RESULTS
The zone of inhibition created by the alcoholic extract of C. nucifera, when tested against various test organisms, is shown in Table 1 and Figures 1–5. All the tested organisms, except the Actinomyces species, exhibited a zone of inhibition when different concentrations of extracts were used, at least in 200 mg/ml. A higher concentration (200 mg/ml) of extract was necessary to inhibit Prevotella intermedia. All other organisms showed a concentration-dependent increase in the antimicrobial activity, which was statistically highly significant, except for some concentrations, which are indicated by alphabets in Table 1. The MIC and MBC are shown in Table 2.
Table 1.
Zone of inhibition in millimeters of various concentrations of ethanolic extract from coconut husk in various test organisms
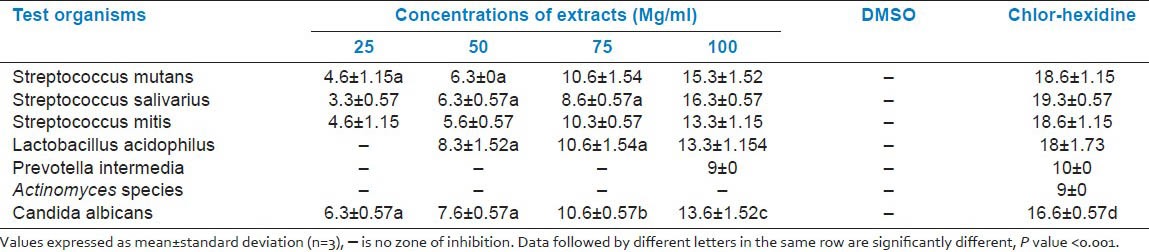
Figure 1.
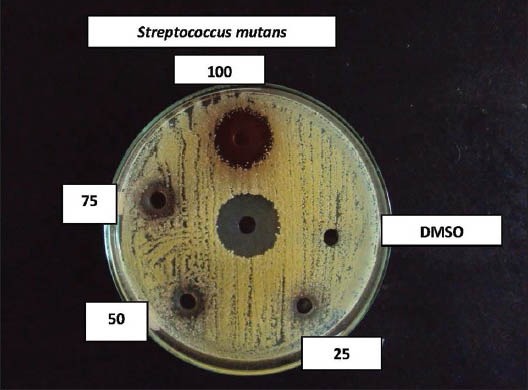
Effect of coconut husk alcoholic extract against Streptococcus mutans
Figure 5.
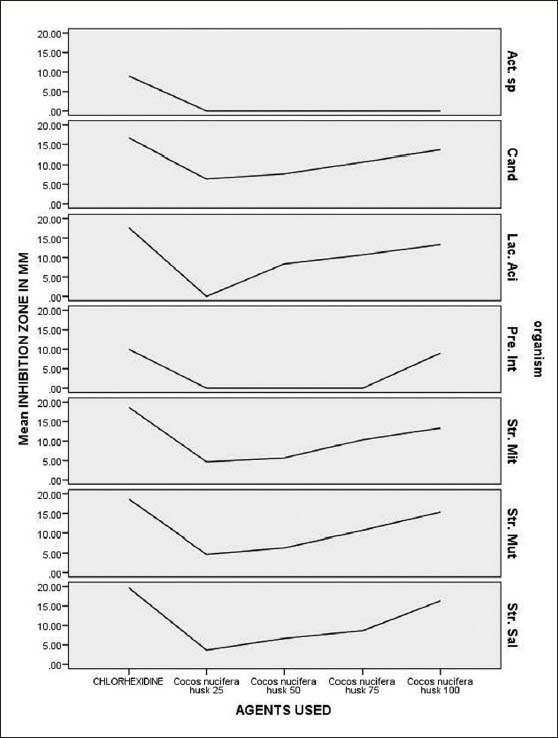
Consolidated graph showing the zone of inhibition created by the alcoholic extract of Cocos nucifera in different concentrations, with respect to different test organisms
Table 2.
MIC and MIB in milligrams per milliliter of ethanolic extracts of different plant material extracts against different test organisms
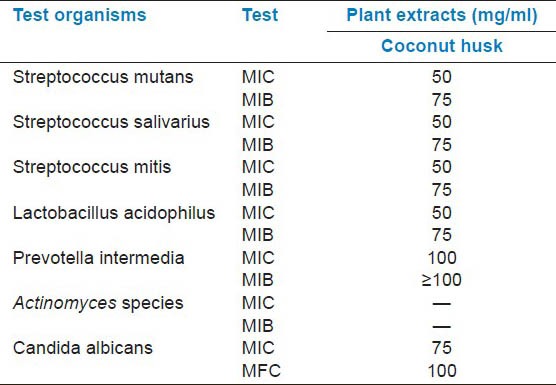
Figure 2.
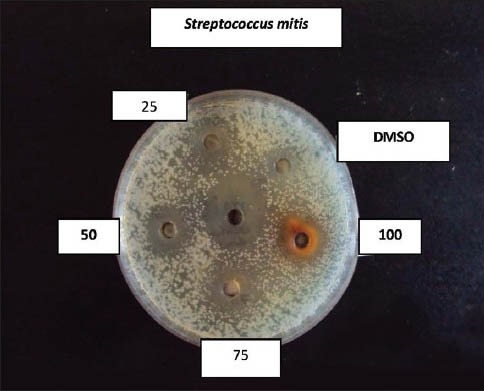
Effect of coconut husk alcoholic extract against S treptococcus mitis
Figure 3.
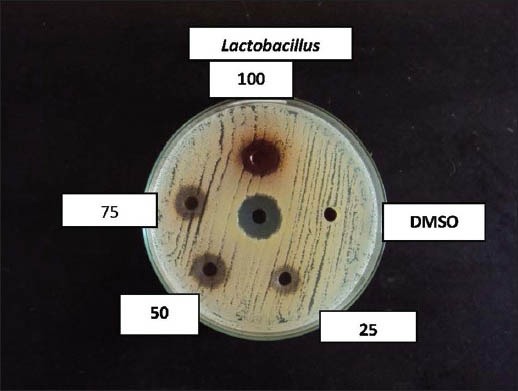
Effect of coconut husk alcoholic extract against Lactobacillus acidophilus
Figure 4.
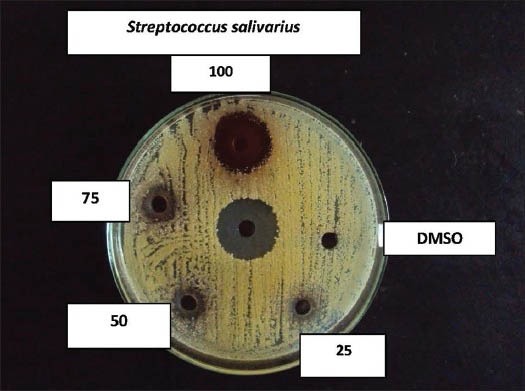
Effect of coconut husk alcoholic extract against S treptococcus salivarius
DISCUSSION
In the present study we have evaluated the antimicrobial properties of the Cocos nucifera husk, which is frequently used as a chewing stick for cleaning teeth by people residing in the rural areas of Dakshina Kannada, against the common oral pathogens. For this purpose, the effect of various concentrations of alcoholic extracts were tested on major cariogenic organisms such as S. mutans, S. mitis, and L. acidophilus, and periodontal pathogens such as Prevotella intermedia and also another oral pathogen, Candida albicans. The antimicrobial properties were expressed as inhibitory zones in the agar diffusion method. The alcoholic extract of C. nucifera, in different concentrations, showed the highest zone of inhibition against S. salivarius (16.3-6.3 mm) followed by S. mutans (15.3-6.3 mm), S. mitis (13.3-4.6 mm), and L. acidophilus (13.3-6.0 mm). Only the minimum zone of inhibition was noted with respect to Prevotella intermedia, even at the highest concentration tested, while no inhibition zone was observed with the Actinomyces species. This indicates relatively less sensitivity of these organisms.
The beneficial medicinal effects of plant materials, including the antibacterial activity, typically result from the secondary products present in the plant, although it is usually not attributed to a single compound, but to a combination of metabolites.[15] The exact mechanism by which the active components of the plant materials contribute to the antibacterial activity is not known. One of the mechanisms suggested is the hydrophobic activity, which enables them to partition the lipids of the bacterial cell membrane and mitochondria, disturbing the cell structures and rendering them more permeable. Extensive leakage from the bacterial cells or the exit of critical molecules and ions will lead to death.[16] Tannins present in the plant extracts have an astringent effect on the mucous membrane and they form a layer over the enamel, thus providing protection against dental caries.[17]
Useful antimicrobial phytochemicals can be divided into several categories, such as, Phenolics and Polyphenols; Flavones, Flavonoids, and Flavonols; Quinones, Tannins, Coumarins, Terpenoids, and Essential Oils; Alkaloids, Lectins, and Polypeptides; and Mixtures and Other Compounds. The mechanisms thought to be responsible for phenolic toxicity to microorganisms include enzyme inhibition by the oxidized compounds, possibly through reaction with the sulfhydryl groups or through more nonspecific interactions with the proteins. Quinones probably target the surface-exposed adhesions, cell-wall polypeptides, and membrane-bound enzymes in the microbial cell. Quinones may also render substrates unavailable to the microorganisms. The antimicrobial activity of flavones and flavonoids may probably be due to their ability to react with extracellular and soluble proteins and to complex with bacterial cell walls. Tannins are a group of polymeric phenolic substances and they are found in almost every plant part. One of their molecular actions is to react with proteins through the so-called nonspecific forces such as hydrogen bonding and hydrophobic effects, as well as by covalent bond formation. Coumarin was found, in vitro, to inhibit Candida albicans. As a group, coumarins have been found to stimulate macrophages. Hydroxycinnamic acids related to coumarins seem to be inhibitory to gram-positive bacteria. Terpenoids and essential oils are active against bacteria, fungi, viruses, and protozoa, and the speculated mechanism of action is to involve membrane disruption by the lipophilic compounds.[18]
The phytochemical screening of C. nucifera has reported that this plant material is rich in alkaloids, flavonoids, catechins, and epicatechin, together with condensed tannins, which confer on it potent antimicrobial properties.[19] Koschek et al. in their studies indicated that catechin, one of the compounds present in the extract of a C. nucifera plant, is capable of inhibiting tumor cell lines.[10] In addition to this they have also noted other secondary metabolites like proteins, steroids, and carbohydrates. Studies on the antimicrobial properties of tea have revealed that components in tea, particularly monomeric polyphenols, especially simple catechins such as epicatechin, epicatechin gallate, and epigallocatechin gallate, are responsible for these biological effects.[20,21,22] As the Cocos nucifera husk contains catechin and epicatechin, it can be contemplated that these components must be the primary constituents that confer the antimicrobial effects. These compounds inhibited in vitro Streptococcus mutans[23,24,25,26] and other bacteria and microorganisms, and inhibited isolated bacterial glucosyltransferases in S. mutans,[27] possibly due to the complexing activities. When the rats were fed a diet containing 0.1% tea catechins, fissure caries (caused by S. mutans) were reduced by 40%,[28] indicating the significant beneficial effect of catechin.
The inhibition produced by the plant extracts against a particular organism depends upon various extrinsic and intrinsic parameters. The variable diffusibility of the plant extract in the agar medium may also influence the zone of inhibition. In case of decreased diffusibility, the antibacterial property may not demonstrate as a zone of inhibition in proportion to its efficacy.[15] Therefore, the MIC and MBC values, the lowest concentrations of the antibacterial substance required to produce a sterile culture, have also been computed here. In this study, the MIC and MBC values ranged mainly between 50 mg/ml and 100 mg/ml. Demonstration of antibacterial activity against the test bacteria is an indication that there is a possibility of sourcing alternative antimicrobial substances from these plants for the development of newer antibacterial agents. The observed low MIC and MBC values against these bacteria means that the plant has the potential to effectively treat any ailments associated with these bacterial pathogens.
Our study has confirmed the beneficial effects of C. nucifera husk, with a high antimicrobial effect. From the results obtained, we would like to conclude that traditional oral care practices, although not proven scientifically, have time-tested valuable effects. With respect to plant materials, these beneficial effects may be related to the active chemical constituents. It is certain that dual benefits are obtained when coconut husk is used for daily cleaning of teeth, which are, the mechanical cleansing property of the fibrous component and the chemical antimicrobial properties of the active constituents. Further studies are needed to elucidate the active components of C. nucifera and their mode of action as well as their potential in combination with other plant extracts. If identified, the active compounds may be incorporated into modern oral care systems, such as, toothpastes or mouth washes. This may provide the possibility of combining the benefits of traditional and modern practices to offer maximum benefit to mankind for better oral health.
CONCLUSION
C. nucifera has significant inhibitory action against oral pathogens indicating the presence of highly effective active compounds, which can be identified and incorporated into modern oral care systems for controlling various diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–95. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Alviano DS, Alviano CS. Plant Extracts: Search for new alternatives to treat microbial diseases. Curr Pharm Biotechnol. 2009;10:106–21. doi: 10.2174/138920109787048607. [DOI] [PubMed] [Google Scholar]
- 3.Loesche W. Dental caries and periodontitis: Contrasting two infections that have medical implications. Infect Dis Clin Nth Am. 2007;21:471–502. doi: 10.1016/j.idc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases eCAM. [Last accessed on 2010 Oct 9]. doi:10.1093/ecam/nep067. Available from: http://www.ecam.oxfordjournals.org . [DOI] [PMC free article] [PubMed]
- 5.Tichy J, Novak J. Extraction, assay, and analysis of antimicrobials from plants with activity against dental pathogens. J Altern Complement Med. 1998;4:39–45. doi: 10.1089/acm.1998.4.1-39. [DOI] [PubMed] [Google Scholar]
- 6.Jose M, Sharma BB, Shantaram M. Ethnomedicinal herbs used in oral health and hygiene in coastal Dakshina Kannada. J Oral Health Comm Dent. 2011;5:107–11. [Google Scholar]
- 7.Akinyele TA, Akinpelu DA, Okoh AI. In-vitro antibacterial properties of crude aqueous and n-hexane extracts of the husk of Cocos nucifera. Molecules. 2011;16:2135–8. doi: 10.3390/molecules16032135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esquenazi D, Wigg MD, Miranda MM, Rodrigues HM, Tostes JB, Rozental Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn (Palmae) husk fibre extract. Res Microbial. 2002;153:647–52. doi: 10.1016/s0923-2508(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca-Filho RR, Rodrigues, Alviano DS, Santos AL, Soares RM, Alviano CS. Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocosnucifera Linn. (Palmae) Res Microbiol. 2004;155:136–43. doi: 10.1016/j.resmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Koschek PR, Alviano, Gattass CR. The husk fiber of Cocos nucifera (Palmae) is a source of antineoplastic activity. Braz J Med Biol Res. 2007;40:1339–43. doi: 10.1590/s0100-879x2006005000153. [DOI] [PubMed] [Google Scholar]
- 11.Alviano DS, Rodrigues KF, Leitao SG, Rodrigues ML, Matheus ME, Fernandez PD. Antinocioceptive and free radical scavenging activities of Cocos nucifera L (Palmae) husk fibre aqueous extract. J Ethnopharmacol. 2004;92:269–72. doi: 10.1016/j.jep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Jose M, Varghese I, Shantaram M. In vitro antioxidant activities and phytochemical analysis of five selected plant materials used for oral health and hygiene among people of Dakshina Kannada. Int J Appl Biol Pharm Technol. 2011;2:95–103. [Google Scholar]
- 13.Lino A, Deogracios O. The in-vitro antibacterial activity of Annonasenegalensis, Securidaccalongipendiculata and Steanotaeniaaraliacea. Afri Health Sci. 2006;6:31–5. doi: 10.5555/afhs.2006.6.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajaiyeoba EO, Onocha PA, Nwozo SO, Sama W. Antimicrobial and cytotoxicity evaluation of Buchholziacoriacea stem bark. Fitoterapia. 2003;74:706–9. doi: 10.1016/s0367-326x(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 15.Parekh J, Jadeja D, Chanda S. Efficacy of Aqueous and Methanol Extracts of Some Medicinal Plants for Potential Antibacterial Activity. Turk J Biol. 2005;29:203–10. [Google Scholar]
- 16.Sikkema J, De Bont AM, Poolman BM. Interaction of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8. [PubMed] [Google Scholar]
- 17.Prashanth GM, Chandu GN. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis, Streptococcus sanguis: An in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 18.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alviano DS, Antoniolli, Alviano CS, Farias LM. In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Arch Oral Biol. 2008;53:545–52. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton-Miller JM. Anti-cariogenic properties of tea (Camelliasinensis) J Med Microbiol. 2001;50:299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Matsumoto M, Tanaka T, Maeda M, Nakai M, Hamada S, et al. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res. 2004;38:2–8. doi: 10.1159/000073913. [DOI] [PubMed] [Google Scholar]
- 22.Batista O, Duarte A, Nascimento J, Simones MF. Structure and antimicrobial activity of diterpenes from the roots of Plectranthus hereroensis. J Nat Prod. 1994;57:858–61. doi: 10.1021/np50108a031. [DOI] [PubMed] [Google Scholar]
- 23.Sakanaka S, Kim M, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53:2307–11. [Google Scholar]
- 24.Toda M, Okubo S, Ohnishi R, Shimamura T. Antibacterial and bactericidal activities of Japanese green tea. Jpn J Bacteriol. 1989;45:561–6. doi: 10.3412/jsb.44.669. [DOI] [PubMed] [Google Scholar]
- 25.Sakanaka S, Shimura N, Aizawa M, Kim M, Yamamoto T. Preventive effect of green tea polyphenols against dental caries in conventional rats. Biosci Biotechnol Biochem. 1992;56:592–94. doi: 10.1271/bbb.56.592. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya H, Sato M, Iinuma M, Yokoyama J, Ohyama M, Tanaka T, et al. Inhibition of the growth of cariogenic bacteria in vitro by plant flavanones. Experientia. 1994;50:846–9. doi: 10.1007/BF01956469. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara K, Kawabata S, Ono H, Ogura K, Tanaka T, Ooshima T, et al. Inhibitory effect of oolong tea polyphenols on glucosyltransferases of mutans streptococci. Appl Environ Microbiol. 1993;59:968–73. doi: 10.1128/aem.59.4.968-973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooshima T, Minami T, Aono W, Izumitani A, Sobue S, Fujiwara T, et al. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutans streptococci. Caries Res. 1993;27:124–9. doi: 10.1159/000261529. [DOI] [PubMed] [Google Scholar]


