Abstract
Context:
Diabetes has emerged as pandemic health problem and its prevalence is increasing at an alarming rate.
Aims:
The aim of the following study was carried out to understand the prevalence of diabetes mellitus, its associated risk factors and related co-morbidities amongst persons residing in rural hilly terrain of Upper Siang district of Arunachal Pradesh, India.
Settings and Design:
Population based cross-sectional study on 1370 participants in the field practice area of a private Nursing School in Yingkion in Upper Siang district of Arunachal Pradesh, India was conducted during April 2009-March, 2010.
Materials and Methods:
The clinico-social data related to diabetes was gathered by personal interview. Body weight, height, waist circumference and blood pressure were measured and blood glucose was estimated in fasting venous blood samples.
Results:
Diabetes mellitus was observed among 19.78% of the participants with additional 12.04% patients with impaired glucose tolerance (IGT). Diabetes mellitus was most prevalent among 50-59 years age group (32.10%). Hypertension was observed among participants with diabetes and IGT was 65.13% (hypertensive diabetics) and 53.94% (diabetics only). Mean body mass index and associated family history was added risk factors in prevalence of diabetes.
Conclusions:
Effective primary prevention strategies are to be intensified among the high-risk population groups to promote awareness through behavior change communication.
Keywords: Body mass index, diabetes mellitus, hypertension, impaired glucose tolerance
INTRODUCTION
World Health Organization (WHO) has declared that the incidence of diabetes is increasing rapidly world-wide to become a major public health concern.[1] All age global diabetes prevalence of diabetes was estimated to be 2.8% in 2000 and is predicted to be 4.4% in 2030; currently, 190 million people around the world suffer from diabetes mellitus with over 330 million predicted to have the condition by 2025 and 366 million by the year 2030.[2] It is predicted that the developing countries will contribute 77.6% of the total number of diabetic patients in the world by the year 2030.[3,4] Diabetes in urban Indians is reaching an epidemic scale. The prevalence of type 2 diabetes mellitus in Asian Indians ranges from 2.7% in rural Indians to 14% in urban India.[5,6,7,8,9] This growing prevalence is of great concern because of high morbidity and mortality and the cost associated with the treatment of the complications of diabetes.[10] Diabetes has noteworthy involvement with hypertension, overweight and obesity and other long-term vascular complications as the main cause of morbidity and mortality. Recognizing the magnitude of the growing regional problem, many countries have taken active measures by establishing National Diabetes Control Programs.[11,12,13,14,15] There are no studies regarding the prevalence of Diabetes Mellitus among the tribal population living in the remote hilly terrain of this part of the country. Therefore, this study was undertaken to assess the magnitude of the problem of diabetes and its association with hypertension, obesity and positive family history.
MATERIALS AND METHODS
This cross-sectional study was done among the population in the field practice area of a private Nursing school in Damro village, which is the largest village in upper Siang and it is 62 km from Yingkiong, the District Head Quarter of Upper Siang, Arunachal Pradesh, India. The study was done from April 2009 to March 2010. 62.47/1000 prevalence rate of Diabetes in India (urban and rural) among adults (20 year and above) was calculated by the experts at WHO.[16] Considering this prevalence of diabetes with 5% alpha error, 20% absolute allowable error, 1370 sample size (male = 685, female = 685) was included in the study.
All eligible individuals were identified from the electoral roll of the election commission of India in the population of the field practice area and randomly sampled. We included all participants aged 20 years and above in the study who agreed to blood glucose estimation. The participants with co-morbidities viz. febrile illness, suffering from any other hormonal disorders, benign or malignant disorders, diabetic ketoacidosis, renal failure, transplant rejection, central nervous system disorders and other chronic diseases, were excluded from the study.
The data collection tool used for the study was an interview schedule that was developed at the institute with assistance from the faculty members and other experts. This pre-designed and pre-tested questionnaire contained questions relating to the socio-demographic information of the participants. By initial translation, back-translation, re-translation followed by the pilot study the questionnaire was custom-made for the study. The pilot study was carried out among comparable population following which some of the questions from the interview schedule were modified.
The study conformed to the Helsinki declaration. Institution ethical committee approved the study. The health workers motivated the families to participate in the study along with the scope of future intervention, if necessary. All the participants were explained about the purpose of the study and were ensured strict confidentiality and academic use only. Informed consent was taken from each participant. Participants were given the options to withdraw themselves from the study whenever they wished. A maximum of five visits were arranged for those who could not be contacted during the first visit. The data on family and personal characteristics were recorded by personal interview for age, sex, personal past history of diabetes mellitus and/or hypertension. Venous blood samples were taken after 10-12 h fast and were examined in the biochemistry lab of Yingkiong District Hospital, Arunachal Pradesh, India.
The diagnosis of diabetes mellitus was based on WHO criteria. A fasting plasma glucose (FPG) level = 7.0 mmol/l or = 126 mg/dl after a minimum 12 h fast, or a 2 h post-glucose level (oral glucose tolerance test [OGTT] or 2 h OGTT) = 11.1 mmol/l or = 200 mg/dl on more than one occasion, with symptoms of diabetes. Impaired glucose tolerance (IGT) was defined as an FPG level of 100 mg/dl (5.6 mmol/l) but <126 mg/dl (7.0 mmol/l).[13]
An average of three blood pressure readings measured thrice at an interval of 15 min was taken with participants in a sitting position using the mercury sphygmomanometer. The average of three measurements of Korotkoff phase I was considered as systolic blood pressure (SBP) and the average of three values of phase IV was recorded for diastolic blood pressure and estimation of fasting and post-prandial blood glucose levels was performed using the Star Plus 21 semi-auto analyzer of Rapid Diagnostic Company. Blood samples were obtained in the morning hours and assayed for serum glucose. Hypertension was defined according to the new criteria (SBP of 140 mmHg or over, or a diastolic of 90 mmHg or over).[14]
Body weight was measured (to the nearest 0.5 kg) in the standing motionless on the weighing scale with feet 15 cm apart and weight equally distributed on each leg without shoes and using minimum of clothing. Height was recorded (to the nearest 0.5 cm) by stadiometer in bare footed standing position, with closed feet, holding their breath in full inspiration, back and heels against the upright bar of height scale, head upright in Frankfort horizontal plane “look straight ahead.” Waist circumference was measured by flexible non-stretchable measuring tape in standing. Weights and heights were used to calculate body mass index (BMI) using the formula: BMI = weight in kilograms per height in meters. The participants were classified on BMI: <25 kg/m2 as normal, 25-29 kg/m2 as overweight and 30 kg/m2 and over as obese.[17]
The number and rates of participants with diabetes and IGT among different age groups and gender was analyzed. Old case of diabetes was considered on the basis of being under treatment as per their previous reports and prescriptions. The results were expressed in percentages represented by tables and statistically analyzed using Chi-Square test.
RESULTS
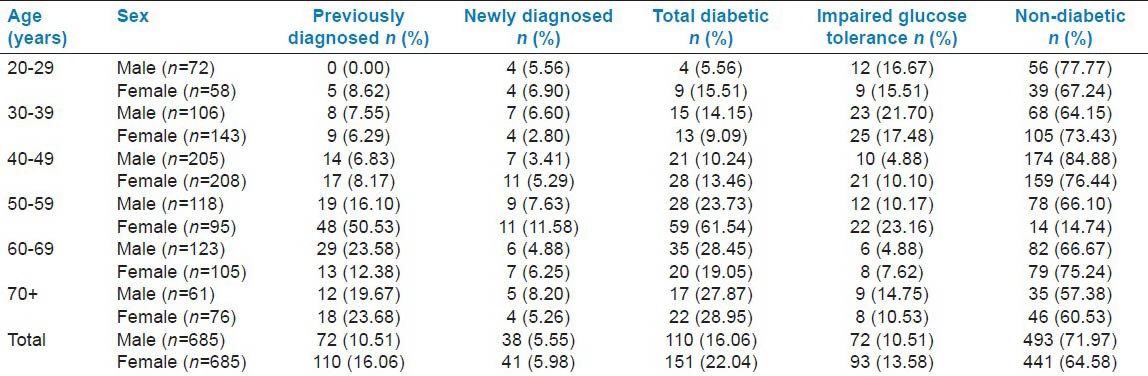
Out of a total of 1370 study participants in our study population, higher than normal blood glucose level was observed amongst 13.28% (Males: 10.51%; Females: 16.06%) previously diagnosed cases. The newly diagnosed cases of diabetes were observed to be 5.77% (Males: 5.55%; Females: 5.98%). The total percentage of new and old cases of diabetes mellitus was 19.78% (Males: 16.06%; Females: 22.04%). Majority of the participants (97%) were diagnosed with diabetes after 40 years of age. It was observed that frequency of diabetic cases was highest in the 50-59 years age group (with prevalence of 32.10% in males and 39.07% in females). The magnitude of the problem of IGT was 12.04%, constituting 10.51% males and 13.58% females. It was also noted that the frequency of IGT was highest (29.09%) among the 30-39 years age group. Among female participants also highest frequency of IGT was noted in this age group (26.88%) [Table 1].
Table 1.
Distribution of diabetics and subjects with impaired glucose tolerance
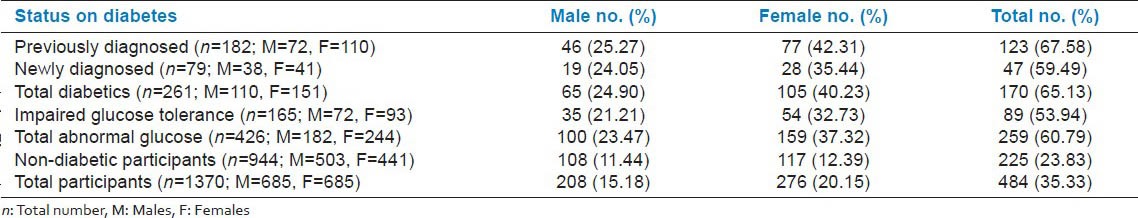
The frequency of hypertension was 65.13% among all diabetic participants and 53.94% among those with IGT; this difference was statistically significant (P = 0.0275). In our study population, only 23.43% of the non-diabetic persons had hypertension. Compared with participants having abnormal glucose the difference of hypertension was found to be extremely significant (P < 0.0001). We did not find any difference in sexes in hypertension among diabetics or with IGT (P = 0.9966). However, compared with non-diabetic females, those with abnormal glucose tolerance had significantly higher magnitude of hypertension than male counterpart (P = 0.0467) [Table 2].
Table 2.
Hypertension as co-morbidity among the study participants
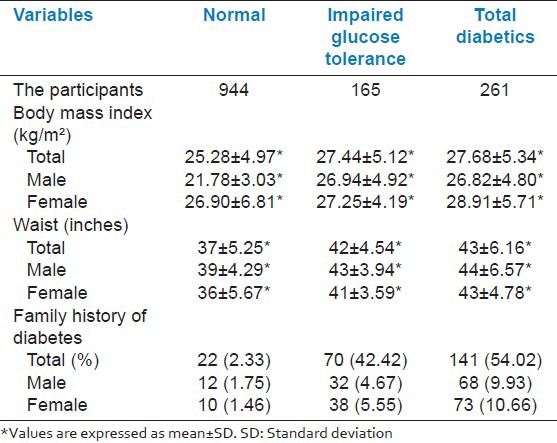
A higher than normal BMI was found in participants with both diabetes and IGT. A significant higher BMI was observed in male diabetics and IGT compared to non-diabetics (P < 0.001). However the difference of BMI was not significant between diabetics and IGT among males. In case of female diabetics the difference of BMI was observed highly significant compared to non-diabetic (P < 0.001). However no difference in BMI was observed in IGT participants with normal and diabetic condition. A significant higher waist circumference was observed in male diabetics and IGT compared to non-diabetics. (P < 0.001), in male participants this difference of waist was not significant between diabetics and IGT. In case of female participants with diabetes and IGT, the difference of waist circumference was observed highly significant compared to non-diabetic (P < 0.001). It was seen that people having a family history of diabetes were more affected (54.02% diabetics had a family history of diabetes while 42.42% of IGT had the same) than non-diabetic people having least (2.33%) family history of diabetes. This difference was extremely significant (P < 0.0001) [Table 3].
Table 3.
Anthropometric measurements and family history of the participants
DISCUSSION
A population based study on magnitude of problem of diabetes was carried out in Damro village of Upper Siang district in Arunachal Pradesh which is a very remote area bordering China. The result revealed 19.78% prevalence of diabetes among the study participants. This study corroborated with the findings of other studies.[1] Men and women were almost equally affected with diabetes and it was similar to results from other surveys,[1] however IGT was observed to be more in women concurrent to other studies.[3,18] Interestingly high association of diabetes with risk factors of diabetes, such as age, obesity, family history and hypertension was observed, which is in agreement with previous reports.[19,20,21] Studies in India and abroad have shown that Indians develop diabetes at relatively young ages, at least 10-15 years earlier than the western population.[22] Previous studies from India and abroad have shown that more than 50% of patients with diabetes develop the disorder before the age of 50 years. This might be due to increased intake of high energy foods and an increase in psychosocial stress that have proportionately increased risk of the disease even in relatively young adults between 25 and 35 years of age.
Significantly higher blood pressure (systolic and diastolic) was observed in diabetics compared to non-diabetic participants in the present study. The prevalence of hypertension among diabetic participants was more than twice that of non-diabetic participants, which is similar to previous reports.[23,24]
Obesity was observed to be high among the participants with abnormal glucose metabolism (Both diabetic and IGT), compared to non-diabetic participants, which correlates with the findings of other studies.[25,26] The results of this study showed that increasing of waist circumference had a significant association with Diabetes mellitus. These findings are consistent with previous studies,[27,28] where abdominal fat distributions was associated with Diabetes mellitus, independent of overall adiposity.[29,30] Studies in India and abroad have shown that central obesity was more strongly associated with glucose intolerance than generalized obesity.[31] In other words, Asian Indians have a higher degree of central adiposity for a given BMI i.e., Asian Indians have a greater amount of intra-abdominal fat[32,33] and thicker truncal skinfolds.[34] Weight gain during adult life is an important risk factor for the development of hypertension and diabetes mellitus.[35] The risk is greatest in those who gain weight during the third and fourth decades of life.[36] South Asians and Asian Indians have been consistently shown to be less physically active when compared with other ethnic groups.[37,38,39]
Participants having a family history of diabetes were at the highest risk in their productive years of life. People having a family history of diabetes mellitus should adapt healthy life-styles from an early age to prevent or retard the development as a positive association between family history and diabetes mellitus onset is observed. The results from previous studies in Sweden[40] and China[41] provide evidence that changes in lifestyles is effective in preventing diabetes. Either aerobic or resistance training alone improves glycemic control in type 2 diabetes, but the improvements are greatest with combined aerobic and resistance training than either alone.[42]
A population-based cross-sectional survey was conducted in 2004 in a rural community north of Dhaka city in Bangladesh. The prevalence of IGT and diabetes were 2.0, and 7.0%, respectively and were more prevalent in females. Age showed a significant positive relationship with increasing levels of glucose intolerance. BMI, waist circumference and waist-to-hip ratio were higher in the glucose-intolerant group than in the normal glucose tolerance group. The researchers concluded that it is very important to detect the larger number of subjects with glucose intolerance to prevent diabetes.[43]
CONCLUSION
Promotion of awareness of the disease is needed in order to improve the competency of the health care team and to utilize the existing screening programs to detect more of the unknown cases. National Non-Communicable disease Control Program in general and National Diabetes Control program in particular should be seriously implemented by Government of India with the same priority as given to the other National Programs for control of Communicable diseases. Under this National Program, if screening for diabetes coupled with suitable disease risk reduction programs are made; it will decrease the mortality and morbidity associated with this devastating disease and increases the standard of health. Life-style changes should also be given top priority through behavior change communication program.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Alwan A. The WHO Eastern Mediterranean programme on diabetes prevention and control. Bulletin of the Arab Group for Study of Diabetes. 1993;2:38–40. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Azizi F, Guoya MM, Vazirian P, Dolatshati P, Habbibian S. Screening for type 2 diabetes in the Iranian national programme: A preliminary report. East Mediterr Health J. 2003;9:1122–7. [PubMed] [Google Scholar]
- 4.Hussain A, Vaaler S, Sayeed MA, Mahtab H, Ali SM, Khan AK. Type 2 diabetes and impaired fasting blood glucose in rural Bangladesh: A population-based study. Eur J Public Health. 2007;17:291–6. doi: 10.1093/eurpub/ckl235. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Gupta R, Sarna M, Rastogi S, Gupta VP, Kothari K. Prevalence of diabetes, impaired fasting glucose and insulin resistance syndrome in an urban Indian population. Diabetes Res Clin Pract. 2003;61:69–76. doi: 10.1016/s0168-8227(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M. Rising prevalence of NIDDM in an urban population in India. Diabetologia. 1997;40:232–7. doi: 10.1007/s001250050668. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 8.Knight TM, Smith Z, Whittles A, Sahota P, Lockton JA, Hogg G, et al. Insulin resistance, diabetes, and risk markers for ischaemic heart disease in Asian men and non-Asian in Bradford. Br Heart J. 1992;67:343–50. doi: 10.1136/hrt.67.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons D. Ethnic comparisons in diabetes and insulin levels. Asia Pac J Clin Nutr. 1995;4:346–8. [PubMed] [Google Scholar]
- 10.WHO Consultation. Diagnosis and classification of Diabetes Mellitus. Part 1. Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Report No. 99.2. [Google Scholar]
- 11.Lathrop GM, Terwilliger JD, Weeks DE. Multifactorial inheritance and genetic analysis of multifactorial disease. In: Rimoin DK, Conner JM, Pyeritz RE, editors. Emer's and Rimoin's Principles and Practice of Medical Genetics. 3rd ed. New York: Churchill Livingstone; 1996. pp. 333–46. [Google Scholar]
- 12.DeFronzo RA. Pathogenesis of type 2 diabetes: Metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:177–9. [Google Scholar]
- 13.American Diabetes Association. Report of the expert committee on diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–10. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 14.Fuller H, Stevens LK. Prevalence of hypertension among diabetic patients and its relation to vascular risk. Diabetes Hypertension Study Group. J Hum Hypertens. 1991;5:237–43. [PubMed] [Google Scholar]
- 15.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 16. [Last retrieved on 2009 Apr 02]. Available from: http://www.whoindia.org/SCN/AssBOD/06-Diabetes.pdf .
- 17.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–53. [PubMed] [Google Scholar]
- 18.National Food and Nutrition Institute Warsaw and Nutrition Unit WHO Regional Office for Europe Copenhagen. Measuring Obesity — Classification and Description of Anthropometric Data- Report on a WHO Consultation of the Epidemiology of Obesity. EUR/ICP/NUT 125 0612v- Warsaw 21-23. 1987 Oct [Google Scholar]
- 19.King H, Rewers M WHO Ad Hoc Diabetes Reporting Group. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care. 1993;16:157–77. doi: 10.2337/diacare.16.1.157. [DOI] [PubMed] [Google Scholar]
- 20.Sekikawa A, Tominaga M, Takahashi K, Eguchi H, Igarashi M, Ohnuma H, et al. Prevalence of diabetes and impaired glucose tolerance in Funagata area, Japan. Diabetes Care. 1993;16:570–4. doi: 10.2337/diacare.16.4.570. [DOI] [PubMed] [Google Scholar]
- 21.Bierman EL, Bagdade JD, Porte D., Jr Obesity and diabetes: The odd couple. Am J Clin Nutr. 1968;21:1434–7. doi: 10.1093/ajcn/21.12.1434. [DOI] [PubMed] [Google Scholar]
- 22.Vol. 1. Bethesda: US Department of Health, Education and Welfare Publication; 1975. Report on the National Commission on Diabetes; p. 1021. No. 76. [Google Scholar]
- 23.Swai AB, Lutale J, McLarty DG. Diabetes in tropical Africa: A prospective study, 1981-7. I. Characteristics of newly presenting patients in Dar es Salaam, Tanzania, 1981-7. BMJ. 1990;300:1103–6. doi: 10.1136/bmj.300.6732.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurba FI. Ahmed G Prevalence of diabetes mellitus among Bahrainis attending primary health care centres. East.Mediterr.Health J. 1996;2:274–82. [Google Scholar]
- 25.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 26.Report of a WHO Study Group. Technical Report Series, No. 727. Geneva: World Health Organization; 1985. Diabetes Mellitus; p. 113. [PubMed] [Google Scholar]
- 27.Hajian-Tilaki KO, Heidari B. Prevalence of obesity, central obesity and the associated factors in urban population aged 20-70 years, in the north of Iran: A population-based study and regression approach. Obes Rev. 2007;8:3–10. doi: 10.1111/j.1467-789X.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–9. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 29.Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Can G. Determinants and definition of abdominal obesity as related to risk of diabetes, metabolic syndrome and coronary disease in Turkish men: A prospective cohort study. Atherosclerosis. 2007;191:182–90. doi: 10.1016/j.atherosclerosis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Botton J, Heude B, Kettaneh A, Borys JM, Lommez A, Bresson JL, et al. Cardiovascular risk factor levels and their relationships with overweight and fat distribution in children: The Fleurbaix Laventie Ville Santé II study. Metabolism. 2007;56:614–22. doi: 10.1016/j.metabol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelgikar KM, Hockaday TD, Yajnik CS. Central rather than generalized obesity is related to hyperglycaemia in Asian Indian subjects. Diabet Med. 1991;8:712–7. doi: 10.1111/j.1464-5491.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 32.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–44. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 33.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–71. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 34.Kalhan R, Puthawala K, Agarwal S, Amini SB, Kalhan SC. Altered lipid profile, leptin, insulin, and anthropometry in offspring of South Asian immigrants in the United States. Metabolism. 2001;50:1197–202. doi: 10.1053/meta.2001.26704. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, Brand N, Skinner JJ, Jr, Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med. 1967;67:48–59. doi: 10.7326/0003-4819-67-1-48. [DOI] [PubMed] [Google Scholar]
- 36.Chiang BN, Perlman LV, Epstein FH. Overweight and hypertension. A review. Circulation. 1969;39:403–21. doi: 10.1161/01.cir.39.3.403. [DOI] [PubMed] [Google Scholar]
- 37.Williams R, Bhopal R, Hunt K. Coronary risk in a British Punjabi population: Comparative profile of non-biochemical factors. Int J Epidemiol. 1994;23:28–37. doi: 10.1093/ije/23.1.28. [DOI] [PubMed] [Google Scholar]
- 38.Lip GY, Luscombe C, McCarry M, Malik I, Beevers G. Ethnic differences in public health awareness, health perceptions and physical exercise: Implications for heart disease prevention. Ethn Health. 1996;1:47–53. doi: 10.1080/13557858.1996.9961769. [DOI] [PubMed] [Google Scholar]
- 39.Hughes K, Lun KC, Yeo PP. Cardiovascular diseases in Chinese, Malays, and Indians in Singapore. I. Differences in mortality. J Epidemiol Community Health. 1990;44:24–8. doi: 10.1136/jech.44.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson KF, Lindgärde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study. Diabetologia. 1991;34:891–8. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 41.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 42.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann Intern Med. 2007;147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 43.Rahim MA, Khan AK, Ali SM, Nahar Q, Shaheen A, Hussain A. Glucose tolerance in a rural population of Bangladesh. Int J Diabetes Dev Ctries. 2008;28:45–50. doi: 10.4103/0973-3930.43098. [DOI] [PMC free article] [PubMed] [Google Scholar]


