Abstract
Background:
Colorectal carcinoma (CRC) is the fourth most commonly diagnosed malignant disease worldwide, with over 1 million new cases and approximately 5,00,000 deaths each year.
Aims and Objectives:
This prospective observational study was done to study the clinicopathological characteristics of CRC including mucin stains and correlate the preoperative serum carcinoembryonic antigen (CEA) and cancer antigen (CA)-125 levels with the prognosis.
Materials and Methods:
A total of 90 CRCs were included from December 2010 to June 2013. Detailed history and relevant clinical/radiological findings were noted in all clinically and/or radiologically suspected cases of CRC. Preoperative blood samples were collected for serum CEA and CA-125 level estimation. The mucin expression was evaluated with special stains.
Results:
The combined Alcian blue-periodic acid Schiff (PAS) staining was positive for both stains in 68.88% cases indicating that both neutral and acidic mucins are increased in CRC. High preoperative serum CEA levels were seen in 82.22% cases, whereas preoperative serum CA-125 levels showed an increase in 20% cases. Higher levels of these tumor markers corresponded with higher TNM stage.
Conclusions:
Mucin evaluation in CRCs remains one of the valuable methods as mucinous variants correlate with worse prognosis. Preoperative serum CEA level assessment is an indispensible adjunct to the diagnosis and prognosis of CRC. However, preoperative serum CA-125 level measurement is not an efficient tool for prognostication in CRC and should not be recommended for routine use.
Keywords: Colorectal carcinoma, CEA, CA-125, mucin stains
INTRODUCTION
Colorectal cancer (CRC) is the fourth most commonly diagnosed malignant disease worldwide, with over 1 million new cases and approximately 5,00,000 deaths each year.[1]
Incidence and deaths from this cancer are generally increasing, most of all in the developed world and urban areas of the developing world. Age adjusted incidence rate (AAR) per 100,000 person years of colorectal cancer in various population based cancer registries in Kolkata in 2005 was 3.1 for males and 3.2 for females. Mucinous adenocarcinoma is a subtype of colorectal adenocarcinoma with more than 50% of the lesion composed of mucin and is characterized by pools of extracellular mucin that contain malignant epithelium as acinar structures, strips of cells, or single cells. The prognosis of CRC patients is mainly dependent on pathological, clinical, and biological factors. Tumor stage is generally considered the strongest prognostic factor in CRC.
Measurement of blood tumor markers is the most widely used and convenient method for the diagnosis of CRC.
MATERIALS AND METHODS
A total number of 90 patients of CRCs were studied for over a period of 30 months from December 2010 to June 2013 in the Pathology Department in collaboration with the Department of General Surgery and the Department of Biochemistry, Medical College, Kolkata. The detailed history and relevant clinical/radiological findings were noted in all clinically and/or radiologically suspected cases of CRC. Preoperative blood samples were collected from these patients for serum carcinoembryonic antigen (CEA) and cancer antigen (CA)-125 level estimation. The cases were selected after histopathological confirmation of carcinoma.
The results of serum levels of CEA and CA-125 were recorded only for the histopathologically confirmed cases of CRC. Periodic acid Schiff (PAS) and Alcian blue stains were used as special stains to differentiate neutral and acidic mucin. The combined Alcian blue-PAS staining was used to show the presence of both neutral and acidic mucins simultaneously.
RESULTS
In our study the age interval of patients was from 23 to 78 years, the median age being 53 years. Most (82.22%) of the cases occurred in patients above 40 years of age. The male:female ratio was 1.4:1. There were 52 (57.77%) male patients and 38 (42.22 %) female patients. A positive family history was seen in 8.88% cases.
Seventy-two out of 90 cases (80%) were of adenocarcinoma type [Figure 1]. Out of 90 cases, 10 (11.11%) were of mucinous type, 4.44% were adenosquamous type, while basaloid and signet ring types were 2.22% each.
Figure 1.
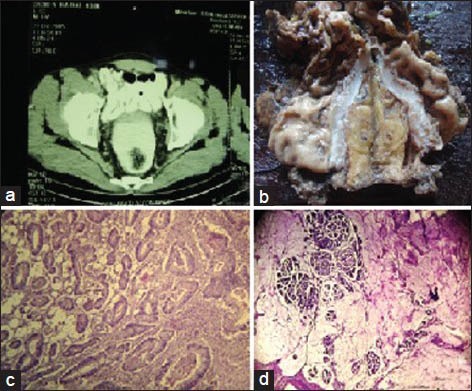
(a) Contrast enhanced computed tomography (CECT) of whole abdomen (cross-sectional view) showing colorectal carcinoma. (b) Abdominoperineal resection (APR) specimen showing colorectal growth. (c) Well-differentiated adenocarcinoma of colon with invasion of serosa (hematoxylin and eosin (H and E), ×100). (d) Mucinous adenocarcinoma of colon (H and E, ×100)
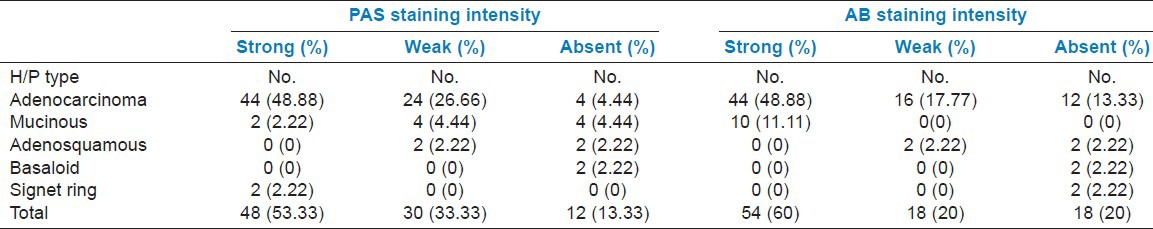
Table 1 shows the clinical presentation and the relation with smoking and alcohol consumption. Most (40%) of the carcinomas had a right colonic location followed by rectum with 24.44% of cases. Left colon accounted for 20% of the cases, whereas the rectosigmoid region accounted for 15.55% cases. Adenocarcinoma and mucinous variants had a right colonic predilection. Fifty-one percent cases presented with TNM stage III at diagnosis, 26.66% were stage II, 17.77 % were stage I, and 4.44 % were stage IV tumors. The mucinous variants mostly presented with TNM stage III at diagnosis. Table 2 shows the relation of TNM stage with preoperative serum CEA and CA-125 levels. Table 3 shows the PAS and Alcian blue staining intensity of the different histopathological variants in our study.
Table 1.
Clinical presentation, smoking, and alcohol consumption among the different histopathological (H/P) variants of colorectal carcinoma
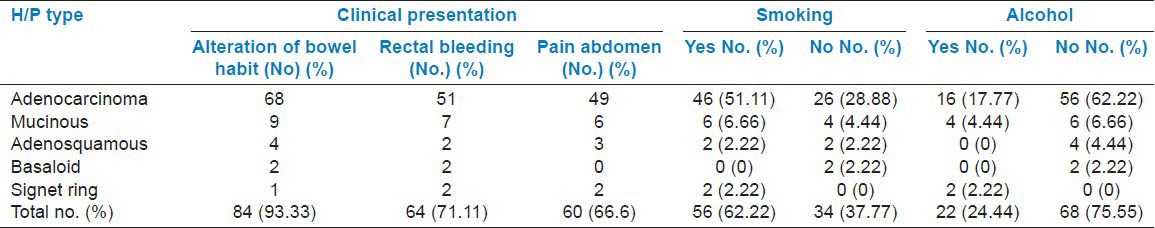
Table 2.
The relation of TNM stage with preoperative serum carcinoembryonic antigen (CEA) and cancer antigen (CA)-125 levels
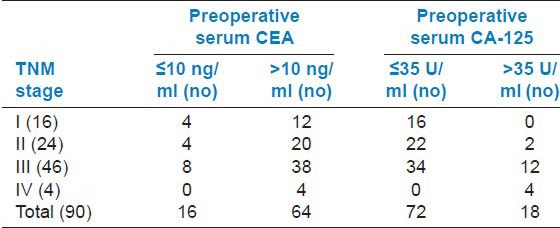
Table 3.
PAS and Alcian blue (AB) staining intensity of the different histopathological (H/P) variants in the study
The combined Alcian blue-PAS staining was positive for both stains in 68.88 % cases, positive for only one stain in 28.88 % cases, and negative for both stains in 2.22% cases indicating that both neutral and acidic mucins are increased in CRC [Figure 2].
Figure 2.
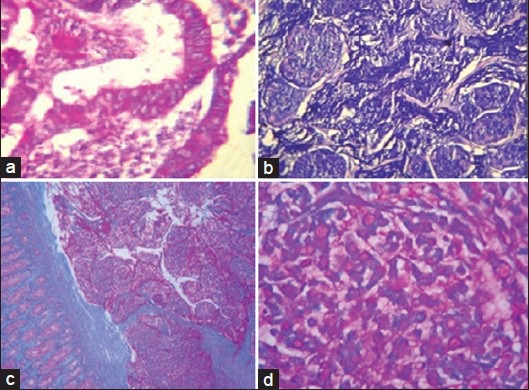
(a) Adenocarcinoma colon showing malignant cells with neutral mucins (periodic acid Schiff (PAS), ×400). (b) Mucinous carcinoma showing pools of acidic mucin (Alcian blue, ×400). (c) Mucinous carcinoma showing acidic and neutral mucins (combined Alcian blue and PAS, ×100). (d) Signet ring cell carcinoma showing neutral mucin (combined Alcian blue and PAS, ×400)
DISCUSSION
Sun et al., performed a retrospective cohort study on 1,422 patients with histologically proven CRC in which 55.4% were males and 44.6% were females,[2] the values being close to those in our study. In various studies, mucinous tumors have accounted for roughly 5-15% of cases; while signet-ring cell tumors were rarer, accounting for about 1% of cases.[3,4] In our study, 11.11% cases were of mucinous type, the age range being 27-42 years and most of these cases occurred in males. This variant showed predilection for patients younger than 40 years of age. The youngest cases in our study, 23- and 25-year-old male patients, presented with signet-ring cell morphology accounting for 2.22% of total number of cases. The study by Song Wu et al., similarly showed that patients with mucinous tumors were statistically younger than those with nonmucinous tumors, and patients with signet-ring cell tumors were statistically younger than those with either mucinous or nonmucinous tumors.[5]
Perea et al., studied 45 patients with CRC aged 45 or younger and found that early onset CRC is characterized by advanced stage at diagnosis, right colon location, low-grade of differentiation, mucin production, and presence of polyps.[6] Hereditary forms represented at least 21% of the early onset CRC cases. In our study, eight cases had a positive family history accounting for 8.88 % of total cases. Among these cases, four had mucinous histology. The other four cases were adenocarcinomas with metastases. All of these cases had a right colon location and presented with higher TNM stage: Four were stage IIIC tumors and four were stage IV tumors.
Chew et al., evaluated 2,764 patients with sporadic colorectal cancer.[7] The age and site related findings were similar to our study. Mucinous adenocarcinomas and signet ring cell carcinoma tended to occur in patients aged ≤50 years and were more commonly right-sided than ordinary adenocarcinomas.
Chao et al., found long-term cigarette smoking to be a risk factor of CRC in both men and women.[8] In our study, 62.22% cases were smokers including 18 women smokers. Though, many epidemiological studies have reported a positive association between alcohol consumption and CRC occurrence; in our study, only 24.44%% of the patients had history of alcohol consumption.
Nabi et al., studied 100 colectomy/hemicolectomy specimens among which, 59 were nonmucinous adenocarcinomas, of which four were in Dukes’ stage A, 51 were in Dukes’ stage B, and four were in Dukes’ stage C.[9] In 30 cases of mucinous carcinomas, 18 were in Dukes’ stage B and 12 were in Dukes’ stage C. All the 11 signet ring cell carcinomas were in Dukes’ stage C. Conclusively, it was established that mucin secreting and signet ring cell adenocarcinomas of colon and rectum are high grade tumors and presented at an advanced stage. In our study, most of the mucinous variants and both of the signet ring cell variants presented with stage C at diagnosis.
Contrary to findings of the previous studies and our study, a recent study by Langner et al., showed that mucinous adenocarcinomas and/or adenocarcinomas with mucinous component do not differ from conventional adenocarcinomas with respect to prognosis and histological predictors of outcome.[10] Hence, recording of mucinous differentiation may be used as an indicator of mismatch repair deficiency, but not for prognostic stratification.
Ionilă et al., investigated 149 CRC patients over a 5-year period.[11] For histochemical investigation, stains such as mucicarmine, PAS + Alcian blue and high iron diamine + Alcian blue. The predominant cases were those with acidic mucins, especially in pure mucinous adenocarcinomas (>90%), while those with mixtures of acidic and neutral mucins were present in 62% of the cases. This study showed the prevalence of sialomucins over sulfomucins (68%), particularly in pure mucinous adenocarcinomas (77%). Zakout and Ezeldeen studied 100 samples, 50 were colorectal cancer samples (cases) and 50 were normal (controls), all were stained with Alcian blue method.[12] It was observed that acid mucin is increased in 30 (60%) of the cases compared to only six (12%) of controls (P < 0.0001). However, complete absence of acid mucin was detected among 10 (20%) of cases compared to none among controls. Similarly, in our study the Alcian blue staining intensity was strong in 60% cases, weak in 20% cases, and absent in 20% cases indicating that acidic mucins are increased in CRC. Thus, secretion of increased amounts of acid mucin in most cases should be considered as a valuable histological finding.
Our study indicated that mucin histochemistry was an indirect evidence of carcinoma as we found that both neutral and acidic mucins are increased in CRCs. All the mucinous adenocarcinomas in our study showed strong Alcian blue staining intensity, thus highlighting the fact that acidic mucins were predominant in these variants. Four cases among these weakly stained for PAS stain, two showed strong PAS positivity, while the remaining four were negative. The signet ring variants were strongly PAS positive. As our study lacked controls from normal colon mucosa and colon mucosa in benign pathological processes, these changes might not be specific for carcinoma. However, in comparison to the previous studies, our study also showed that malignancy was associated with an alteration in mucin histochemistry as evidenced by increased amount of neutral mucin secretion which is otherwise present in small amount in normal colon mucosa. The basaloid variants in our study were negative for both the mucin stains pointing out that this variant is associated with mucin depletion. The adenosquamous variants showed mucin stain positivity only in the glandular (adeno) component, the squamous component not staining for mucins.
Fifty-four out of 90 cases (82.22%) in our study had preoperative serum CEA levels >10 ng/ml. Recently, Gara et al., in their study, found preoperative serum CEA to be a reliable predictor factor for recurrence in patients with CRC.[13] They claimed that CEA might be used in staging system and for therapeutic orientation in patients undergoing curative resection of CRC. The relapse-free survival was significantly higher in patients with CEA <5 ng/ml compared to CEA ≥ 5 ng/ml. They observed significant differences in relapse-free survival between patients with CEA <5 ng/ml and those with CEA 5 ng/ml among patients classified as Dukes’ stage B and C. However, there was no significant difference in relapse-free survival among those classified as Dukes’ stage D.
Sun et al., found that preoperative serum albumin level, CEA level and age could affect postoperative outcome of CRC patients undergoing surgical treatment.[2]
Of these factors, preoperative serum CEA level is the only significant prognostic factor for patients with stage II and III CRCs. They found that high serum CEA levels were associated with poor survival in CRC patients, and the possible reason might result from increased tumor volume leading to a higher incidence of postoperative metastasis. CRC patients with serum CEA level <5 ng/ml (P < 0.001) had significantly greater cancer-specific survival rates than those with serum CEA levels ≥ 5 ng/ml.
The cases with preoperative serum CEA levels >10 ng/ml in our study included 54 out of 72 adenocarcinoma variants and all the mucinous variants. Most of these cases presented with a higher TNM Stage. Thus, we found that the cases with a higher TNM stage had higher serum CEA levels.
By radioimmunoassay the concentration of CA-125 antigen was determined in 26 patients with CRCs and it was shown that CA-125 is without clinical value in large bowel cancer.[14] Similarly, in our study, we found only 18 cases out of 90 (20%) with raised preoperative serum levels of CA-125 (>35 U/ml). Most of the cases had normal preoperative serum CA-125 levels, thus highlighting the fact that serum CA-125 is not a good screening tumor marker for CRC.
Among the 18 cases with CA-125 >35 U/ml, 12 cases presented with TNM stage III, four cases were TNM stage IV, and two were TNM stage II tumors.
Among those 18 cases, 10 cases had mucinous histology accounting for 55.55%. The others were adenocarcinoma (six cases) and signet ring variant (two cases) accounting for 33.33 and 11.11%, respectively.
Hsu et al., demonstrated three factors that influence the survival, including more than two organs involved, higher CEA level, and different salvage treatment.[15] In our study, there were only four cases presenting with distant metastasis. In other studies like those by Lin et al., early postoperative CEA concentration is an independent prognostic factor for CRC and patients with high postoperative CEA values should receive aggressive follow-up examinations for early relapse of CRC, with special attention paid to recurrence at the liver.[16]
CONCLUSION
Our study established the relation of the mucin stains and tumor markers with the clinicopathological characteristics of CRC. There was increased secretion of mostly acidic mucins and also neutral mucins in CRC. Also, the majority of mucins present in CRC, both acidic as well as neutral, stained intensely with both PAS and Alcian blue. The mucinous variant showed predilection for patients younger than 40 years of age. Most of the cases occurred in males, presented in the right colon with higher stage, corresponding to Dukes’ stage C and TNM stage III. All the mucinous variants were strongly Alcian blue positive indicating the presence of acidic mucins in abundance. There were two cases of signet ring cell carcinoma in the study, which presented in young male patients in the rectum with TNM stage III, these being the most common site and stage of presentation as shown by the previous studies. Mucin histochemistry showed the intracellular mucin of the signet rings to stain intensely with PAS indicating their neutral nature. Conclusively, it can be said that the mucin histochemistry still remains one of the valuable methods of mucin evaluation in CRCs. Routine use of mucin histochemistry is required, especially in the early malignant lesions where histochemical changes precede morphological changes.
Tumour marker study with preoperative serum CEA level assessment is an indispensible adjunct to the diagnosis and prognosis of CRC, raised levels providing an indirect evidence of advanced disease and the need for proper and immediate therapeutic intervention to decrease the mortality. However, preoperative serum CA-125 level measurement is not an efficient tool for prognostication in CRC and should not be recommended for routine use.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9:288. doi: 10.1186/1471-2407-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: A population-based study from an Asian country. Dis Colon Rectum. 2004;47:78–85. doi: 10.1007/s10350-003-0014-9. [DOI] [PubMed] [Google Scholar]
- 4.Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161–8. doi: 10.1007/s10350-004-0932-1. [DOI] [PubMed] [Google Scholar]
- 5.Song W, Wu SJ, He YL, Cai SR, Zhang CH, Zhang XH, et al. Clinicopathologic features and survival of patients with colorectal mucinous, signet-ring cell or non-mucinous adenocarcinoma: Experience at an institution in southern China. Chin Med J (Engl) 2009;12:1486–91. [PubMed] [Google Scholar]
- 6.Perea J, Alvaro E, Rodríguez Y, Gravalos C, Sánchez-Tomé E, Rivera B, et al. Approach to early-onset colorectal cancer: Clinicopathological, familial, molecular and immunohistochemical characteristics. World J Gastroenterol. 2010;16:3697–703. doi: 10.3748/wjg.v16.i29.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew MH, Yeo SA, Ng ZP, Lim KH, Koh PK, Ng KH, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25:1221–9. doi: 10.1007/s00384-010-1033-3. [DOI] [PubMed] [Google Scholar]
- 8.Chao A, Thun MJ, Jacobs EJ, Henley SJ, Rodriguez C, Calle EE. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J Natl Cancer Inst. 2000;92:1888–96. doi: 10.1093/jnci/92.23.1888. [DOI] [PubMed] [Google Scholar]
- 9.Nabi U, Nagi AH, Riaz S, Sami W. Morphological evaluation of colorectal carcinoma with grading staging and histological types. J Pak Med Assoc. 2010;60:998–1001. [PubMed] [Google Scholar]
- 10.Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, et al. Mucinous differentiation in colorectal cancer — indicator of poor prognosis? Histopathology. 2012;60:1060–72. doi: 10.1111/j.1365-2559.2011.04155.x. [DOI] [PubMed] [Google Scholar]
- 11.Ionilă M, Mărgăritescu C, Pirici D, Mogoantă SS. Mucinous adenocarcinoma of the colon - a histochemical study. Rom J Morphol Embryol. 2011;52:783–90. [PubMed] [Google Scholar]
- 12.Zakout YM, Ezeldeen YA. Assessment of qualitative changes of acid mucins among sudanese colorectal carcinoma patients. J Gastrointest Cancer. 2011;43:205–8. doi: 10.1007/s12029-010-9246-9. [DOI] [PubMed] [Google Scholar]
- 13.Gara S, Meziou S, Mtar A, Ghanem A, Harzallah L, Rahal K, et al. Prognostic value of preoperative carcinoembryonic antigen level in colorectal cancer in Tunisia. Tunis Med. 2012;90:41–4. [PubMed] [Google Scholar]
- 14.Bukowski J, Góźdź S, Słuszniak J, Korejba W, Zieliński A. CA 19-9 and CA 125 antigens in the sera of patients with cancer of the large intestine in relation to its clinical progress. Wiad Lek. 1989;42:30–4. [PubMed] [Google Scholar]
- 15.Hsu CW, King TM, Wang HT, Wang JH. Factors that influence survival in unresectable metastatic or locally advanced colorectal cancer. Int J Colorectal Dis. 2011;26:1559–66. doi: 10.1007/s00384-011-1231-7. [DOI] [PubMed] [Google Scholar]
- 16.Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26:1135–41. doi: 10.1007/s00384-011-1209-5. [DOI] [PubMed] [Google Scholar]


