Abstract
Background:
Endophytic bacteria do have several potential applications in medicine and in other various sectors of biotechnology including agriculture. Bacterial endophytes need to be explored for their potential applications in agricultural biotechnology. One of the potential applications of bacterial endophytes in agricultural is to enhance the growth of the agricultural crops. Hence, this study was undertaken to explore the plant growth promoting potential application of bacterial endophytes.
Objective:
The objective of this study was to examine the effect of endophytic bacteria from mangrove tree (Rhizophora apiculata Blume) for their efficacy in promoting seedling growth in rice.
Materials and Methods:
Eight endophytic bacterial isolates (EBIs) isolated from twig and petiole tissues of the mangrove were identified based on their 16S ribosomal ribonucleic acid (rRNA) gene sequence homology. Separately, surface sterilized paddy seeds were treated with cell-free broth and cell suspension of the EBIs. Rice seedlings were analyzed by various bioassays and data was recorded.
Results:
The gene sequences of the isolates were closely related to two genera namely, Bacillus and Pantoea. Inoculation of EBIs from R. apiculata with rice seeds resulted in accelerated root and shoot growth with significant increase in chlorophyll content. Among the isolates, Pantoea ananatis (1MSE1) and Bacillus amyloliquefaciens (3MPE1) had shown predominance of activity. Endophytic invasion was recognized by the non-host by rapid accumulation of reactive oxygen species (ROS) and was counteracted by the production of hydrogen peroxide (H2O2) and lipid peroxide. The results demonstrated that EBIs from mangrove tree can increase the fitness of the rice seedlings under controlled conditions.
Conclusion:
These research findings could be useful to enhance the seedling growth and could serve as foundation in further research on enhancing the growth of the rice crop using endophytic bacteria.
Keywords: 16S ribosomal deoxyribonucleic acid, bacteria, bioprospecting, endophytes, mangrove, Rhizophora apiculata, rice
INTRODUCTION
Endophytes are microorganisms that colonize the intercellular space by establishing either a symbiotic or a mutualistic or a commensalistic or trophobiotic association with host plants.[1,2,3] These microbes are often bacteria or fungi [found in various plant organs such as seeds, roots, stem, leaves, flowers, and fruits] that colonize host tissues similar to pathogen. Studies demonstrated that the endophytic association with the host contributes significantly in accelerated seedling emergence,[4] enhanced plant growth,[5,6,7,8,9,10] improved resistance against various phytopathogens,[11,12,13] and abiotic stresses.[14,15,16] Some endophytes synthesize novel metabolites[17] known for its antibiotic and antimicrobial activities. Owing to its potential benefits, a number of endophytes have been isolated from wide variety of plant species and from diverse environmental conditions ranging from permafrost sediments to agricultural field.
The rationale of host plant selection largely relies on promotion of growth and development of the plant under adverse conditions by endophytes. In the present study, the mangrove tree (Rhizophora apiculata Blume) served as a promising source for examining endophytes as its ecosystem characterized by broad range of salinity, temperature, and moisture[18] is similar to lowland rice ecosystem. Further, mangrove trees have remarkable adaptation and grow abundantly in saline coastal sediment. It has been proved that the endophytic colonization has played a major role in the ecological adaptation of the host and increased their survival under adverse conditions.[19,20]
Nearly 200 endophytic fungal species has been reported from mangrove biome[21] and the endophytic fungus Fusarium culmorum known to conferred salinity tolerance to several plant species including dune grass, panic grass, rice, and tomato.[22] Although fungal endophytes from mangrove are well-documented, there have been limited reports on bacterial endophytes of mangrove and its application. Further, there is no concrete evidence on mangrove endophytes ability in accelerating growth of rice. Besides, all endophytes display a degree of host specificity and exhibit stronger symbiotic association with host. Majority of these microbes were often appeared as pathogen because of their inappropriate response in non-host species.[23] This paper examines the compatible association of bacterial endophytes from R. apiculata in colonizing rice tissues and discuss the possibility of utilizing symbiotic association of EBIs to increase the fitness of rice seedlings.
MATERIALS AND METHODS
Isolation of endophytic bacteria from R. apiculata
Plant samples of R. apiculata (red mangrove) such as twigs and leaves were collected from the Merbok brackish river, Semeling, Kedah, Malaysia. The collected healthy samples were washed thoroughly under running tap water to remove surface adhering debris. The twigs, leaves, and petioles were cut into small pieces of about 1-2 cm length, disinfected with 70% ethanol for 30 s, and rinsed thoroughly with sterile distilled water, followed by surface sterilization with 3% sodium hypochlorite for 3 min. All the samples were plated on Luria Bertani (LB) agar medium in triplicate and incubated at 37 ± 2°C for 24 h. The colonies representing different morphologies were selected at random; and pure cultures were made by restreaking on the same medium. Pure cultures of the isolates were used to prepare glycerol stocks and stored at −80°C.
Identification of endophytes
Genomic DNA preparations were made from bacterial cells by heating a freshly isolated bacterial colony in 50 ml of distilled water to 100°C for 15 min; and centrifuged at 12,000 rpm for 5 min. The supernatant was transferred into a new eppendorf tube and used as DNA template. The conserved region of 16S ribosomal ribonucleic acid (rRNA) gene was amplified by using Bak11W-F (5’-AGTTTGATCMTGGCTCAG-3’) and Bak-R (5’-GACTACHAGGGTATCTAAT-3’) primers. Each polymerase chain reaction (PCR) was with 2.5 mM MgCl2, 0.16 mM dNTPs, 0.2 pM concentration of each primer, 5 μl supernatant (DNA template of respective isolate), and 0.75 U Taq DNA polymerase. The 16S rRNA encoding gene region amplification was carried out using an initial denaturation step of 3 min at 94°C, followed by 30 cycles of 30 s at 95°C, 30 s at 52°C, and 30 s at 72°C with a final extension step of 5 min at 72°C. PCR products were visualized in 0.8% agarose gels, and the products were excised and purified with Wizard® SV Gel and PCR clean up system by following the guidelines provided with kit. Alignment between the both strand's sequences was performed by using the Basic Local Alignment Search Tool (BLAST) [bl2seq] program available at National Center for Biotechnology Information (NCBI) to finalize the sequence of amplified 16S rRNA gene fragments. The annotated sequences of 16S rRNA encoding gene of isolates have been deposited in the GenBank/DNA Data Bank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL) nucleotide sequence database.
Rice seed treatments and germination
Paddy seeds (MR220) were surface-sterilized as described by Schmidt et al., (2004).[24] Bacterial suspensions in sterile distilled water (~108 cfu/ml) as well as cell-free broth were used for seed inoculation. The seeds treated only with sterile distilled water were used as control. The inoculated seeds (20–30 seeds) were incubated at room temperature overnight and transferred onto sterile filter papers (Whatman No. 1) in Petri dishes. The plates were incubated at room temperature (27 ± 2°C) with 12 h photoperiod. Ten days after inoculation, symbiotic association of the EBIs was analyzed by measuring the morphological parameters such as seed germination %, root and shoot length, shoot fresh and dry weight. The physiological indicators like hydrogen peroxide (H2O2) and malondialdehyde (MDA) were also estimated for the rice seedlings. Three independent experiments were carried out for all seedling assays.
Estimation of chlorophyll content
Total chlorophyll content was determined for the rice seedlings by following the method described by Harbone (1984).[25] Fresh leaves of about 250 mg were homogenized in 80% acetone at 4°C. The extract was centrifuged at 12,000 × g for 10 min. Absorbance of the supernatant was read at 646 and 663 nm using a spectrophotometer. The amount of chlorophyll in the leaf tissue was expressed as mg/g FW.
Determination of H2O2 content
H2O2 content was determined by following the method described by Velikova et al., (2000).[26] Leaf samples of about 500 mg were ground and homogenized in 5 ml of trichloroacetic acid (0.1%, w/v). After centrifuging the homogenate at 12,000 × g for 15 min, 0.5 ml of the aliquot was mixed with 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M potassium iodide (KI). The optical density (OD) was recorded at 390 nm.
Determination of lipid peroxidation
Lipid peroxidation was determined by using the method reported by Rao and Sresty (2000).[27] Leaf samples (~500 mg) were homogenized with 2.5 ml of trichloroacetic acid (0.1%); and the homogenate was centrifuged at 10,000 × g for 10 min. One milliliter aliquot from the supernatant was taken and mixed with 4 ml of 20% trichloroacetic acid and 0.5% of thiobarbituric acid (TBA). The mixture was heated at 95°C for 30 min and then cooled in an ice bath and then centrifuged at 10,000 × g for 15 min. The absorbance was measured at 532 nm. Measurements were corrected for unspecific turbidity by subtracting the absorbance at 600 nm.
Screening of endophytic bacteria for enzymatic activity
All the EBIs were individually screened for various enzymes such as amylase, lipase, protease, and cellulase by plate method.[28,29] All the isolates were spot inoculated on respective enzyme screening media and incubated at 28°C for 5-7 days.
Statistical analysis
The experiment was conducted in a completely randomized block design with three replications to assess the growth promoting role of the endophytes in rice. Analysis of variance (ANOVA) was performed using Statistical Package for Social Sciences (SPSS) version 13.0. The data was presented as the mean ± SE for each treatment. Means were compared using the least significant difference (LSD) test at the 5% probability level.
RESULTS
Isolation and identification of endophytes
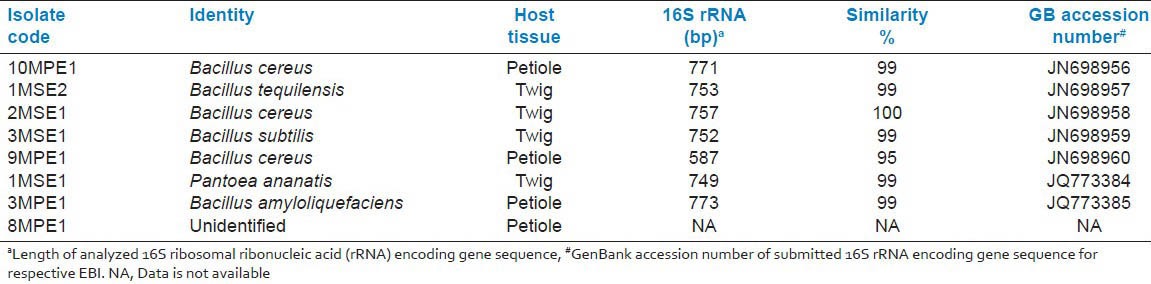
A total of eight endophytic strains were isolated from petiole and twigs tissues of the mangroves collected from Merbok river area, Kedah, Malaysia. All the isolates (except 8MPE1) were identified by analyzing respective amplified 16S rRNA encoding gene fragment sequence BLASTN output. All seven identified isolates were from two genera, namely Bacillus, and Pantoea. The homology between blasted 16S rRNA encoding gene sequence and hits from the database was either 99 or 100% with exception of isolate, 9MPE1. The accession numbers of the deposited 16S rRNA encoding gene sequences and identity of the isolates is depicted in the Table 1.
Table 1.
Isolated and identified seven endophytic bacterial isolates (EBIs) from Rhizophora apiculata Blume tissue samples collected from Merbok river area, Kedah, Malaysia
Effect of EBIs on seed germination and seedling growth
The study was laid to investigate the influence of mangrove EBIs on non-host rice seedlings. The growth promoting activity of EBIs was determined by analyzing the parameters such as seed germination (%), root and shoot length (cm) as well as fresh and dry weights of the seedlings. Germination was stared within 48 h and complete on the 8th day. The result indicated that the inoculation of mangrove EBIs has delayed the germination in both the pretreatments of rice seeds [Figure 1a]. The delay (4.76%) was more obvious where the seeds were treated with cell free broth of 3MSE1 isolate. However, no significant differences were noticed between the treatments (P < 0.05) for seed germination (%).
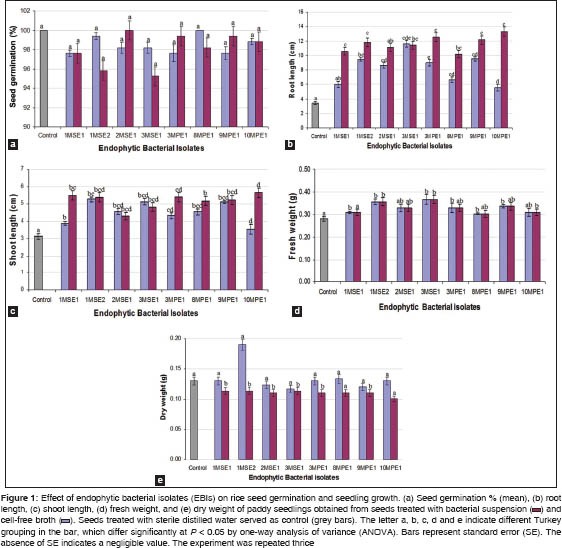
The result presented in Figures 1b and c showed that the EBIs had tremendously increased the seedling's root and shoot length as compared to control on the 10th day after seed germination. The increase in seedling root length ranged between 36.79 and 70.39% where seeds were treated (inoculated) with cell free broth, whereas it ranged from 66.17-74.11% where seeds were treated (inoculated) with bacterial suspension. Analysis of variation had registered a significant difference (P < 0.05) for the increase in root length between control and the seed treatments with EBIs. The increase in shoot length ranged from 11.79 to 40.53% where seeds were treated with cell free broth, while 26.98-44.52% where seeds were treated with bacterial suspension. A significant difference was also noticed for increased shoot length between the control and the seed treatments. The response of the non-host to mangrove endophytes can be explained clearly by examining the root to shoot length ratio. The ratios were comparatively higher in both the treatments than the control indicating that the endophytic invasion had greatly improved the root shoot balance in rice seedlings. Though, all the EBIs had improved the root and shoot length ratio, seeds treated with B. cereus (2 MSE1, 9MPE1, and 10 MPE1), B. amyloliquefaciens (3MPE1) and B. subtilis (3MSE1) had influenced the seedling growth to a greater extent.
The fresh and dry weight recorded for rice seedlings obtained is presented in Figure 1d and e. The result indicated that some strains of EBIs had increased the fresh weight as compared to control. Among the strains, B. tequilensis (1MSE2), B. amyloliquefaciens (3MPE1), and B. cereus (9MPE1) were more influential in enhancing the fresh weight of the seedlings [Figure 1d]. Result analyses of dry weight records suggest that there was no effect of treatments on the dry weight of the seedlings [Figure 1e]. However, a significant difference was noticed for both fresh and dry seedling weight (P < 0.05) between the control and the seed treatments.
Effect of EBIs on chlorophyll content of seedlings
The results presented in Figure 2a clearly indicate the influence of EBIs on the functional dynamics of rice seedling growth. The result revealed that the majority of stains had significantly increased the total chlorophyll content (P < 0.05). Among the treatments, seeds inoculated with bacterial suspension had recorded higher amount of chlorophyll pigments than the seeds inoculated with cell-free broth. Chlorophyll content was high in seedlings inoculated with Pantoea ananatis (1MSE1), B. cereus (2MSE1 and 10MPE1), B. subtilis (3MSE1), and an unidentified isolate (8MPE1).
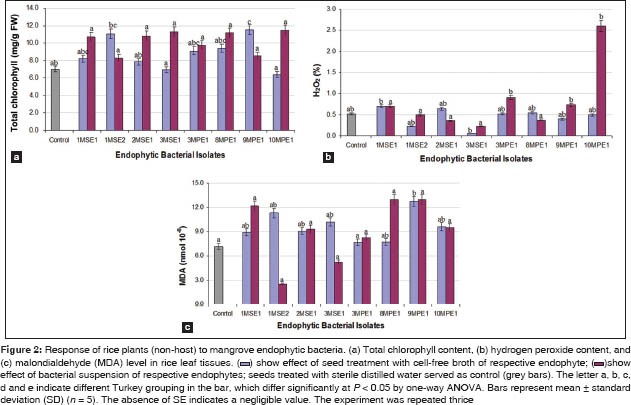
Oxidative changes in rice leaf tissue
Changes in H2O2 content are shown in Figure 2b. A significant oxidative change was observed in rice seedlings obtained from treated seeds. However, seeds inoculated with EBIs suspension had more pronounced effect on the H2O2 concentration [Figure 2b]. In general, effect was minimal in the seedlings obtained from seeds treated with cell-free broth of the EBIs. The H2O2 was relatively high in seedlings obtained from seeds inoculated with B. cereus (10MPE1). Lipid peroxidation levels in leaves of rice seedlings were determined, and the results are shown in Figure 2c. The concentration of MDA was very low in seedlings obtained from seeds inoculated with Bacillus tequilensis (1MSE2) and B. subtilis (3MSE1). The lower levels of H2O2 and lipid peroxidation in the leaf tissue are indicatives of an enhanced protection against oxidative damage.
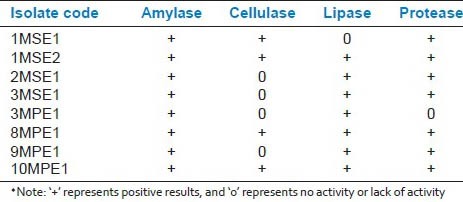
Enzymatic activities of isolated endophytes
Enzymatic activities of EBIs are presented in Table 2 has shown the presence of hydrolytic enzymes such as cellulose, amylase, lipase, and protease. All isolates of endophytic bacteria has produced positive result for amylase tests. Further, the test has shown that around 90% of the isolates were lipase and protease producers, only 4 out of 8 strain were cellulose producers.
Table 2.
Enzymatic activities of endophytic bacterial isolates (EBIs) isolated from mangrove tree Rhizophora apiculata Blume♦
DISCUSSION
Mangrove biome has unique ecological significance and harbors variety of endophytes which are known to enhance edaphic factor and ameliorate the detrimental effect of soil.[18,30] To exploit the potential benefit of mangrove endophytes, the present study was undertaken and eight EBIs were isolated from parts like petiole as well as twigs. The EBIs were identified using 16S rRNA encoding gene sequence homology based method, and the results revealed the predominance of Bacillus spp. [Table 1]. However, Gayathri et al., (2010) reported diverse colonies of S. aureus, B. subtilis, E. coli, Vibrio parahaemolyticus, V. anguillarum, and Fusarium spp. from Pitchavaram mangrove forest in Tamil Nadu.[31] On the other hand, bacterial strains such as B. licheniformis, M. chthonoplastes, Phyllobacterium myrsinacearum, V. aestuarianus, and V. proteolyticus were recovered from black mangrove.[32] Diverse array of heterotrophic bacteria associated with mangrove habitat also been reported by Dias et al., (2009) and most of them were in the order of Vibrionales, Actinomycetales, and Bacillales.[33] Further, the occurrence of Bacillus species as endophytes reported to promote growth of host plant species such as soybean,[34] pigeon pea,[35] and wheat.[36]
Rice itself harbors various entophytic bacteria, it has been reported that the bacteria, P. ananatis[37,38] and B. cereus[39] are localized in rice seeds; whereas B. subtilis[40] is prevalent in root, stem, and leaves of rice. These bacterial strains resides either in the seeds or may exist in the embryo or invade the plant through the wound of lateral roots or root tips.[41]
The present study was conducted to screen the efficacy of mangrove EBIs on the non-host rice (seedlings) by inoculating the EBIs with the rice seeds under controlled condition without supplementing nutrient. The results have shown a significant increase in root and shoot length where seeds were inoculated with B. cereus (10MPE1), B. amyloliquefaciens (3MPE1), and P. ananatis (1MSE1). The increased seedling growth observed in the present study might be due to the production of hydrolytic enzymes by the isolates [Table 2]. This is in accordance with the earlier reports where the authors explained that the increased hydrolytic enzyme activities of the endophytes improves synergistic effect of symbionts within root and substantially improve the plant growth by increasing root length, volume, and surface.[42,43,44,45] Further, endophytic inoculums had increased fresh weight, but not the dry weight of rice seedlings. It has been reported that endophytes promotes water conservation strategies in plants.[14] Difference in dry weight observed in 1MSE2 might be due to difference in the rate of development; however additional studies are needed to elucidate this mechanism on plant growth.
The increase in total chlorophyll content recorded in the study reflected the increased rate of chlorophyll synthesis which enhanced photosynthesis and resulted in better plant growth. The enhanced growth of seedling indicated that the rice had compatible interaction with EBIs used. The efficacy of endophytic bacteria in accelerating plant growth was reported by Hassen and Labuschagne (2010).[46] A significant increase in tomato root length, fresh, and dry weights was observed by them when inoculated with B. cereus. In addition, B. cereus is also known to activate cellular response and inhibit the blight caused by Phytopthora capsici Leon and root knot disease caused by Meloidogyne incognita.[47,48] Similarly, B. amyloliquefaciens is a rhizobacterium reported to stimulate plant growth and suppress plant pathogen,[49] whereas P. ananatis reported to tolerate high osmotic pressure.[38] Several mechanisms have been attributed to growth promotion activities of endophytes:
Facilitating nutrient uptake through nitrogen fixation or phosphate solubilization,[7]
Triggering induction of systemic resistance,[47]
Altering phytohormone homoeostasis either by producing indole-3-acetic acid (IAA) or by decreasing ethylene level,[4] and
Producing hydrolytic enzymes.[44]
The growth performance of the rice seedlings observed in the present study might be due to more than one of above said mechanism. However, active growth of the seedlings provides convincing evidence of the efficacy of recruitment of mangrove bacterial endophytes. Once it has been recognized by the non-host, the endophytes have to undergo specific adaptation. The compatibility of endophytes and host fitness was assessed by analyzing oxidative changes in rice leaves. H2O2 and MDA were appraised as promising criteria in determining the sensitivity of the host. Increased H2O2 and MDA level in rice leaves provided evidence for oxidative damage caused by reactive oxygen species (ROS). Previous studies indicated that the activity of antioxidant enzymes is correlated with plant tolerance.[50,51] The finding of Tanaka et al., (2006) suggested that generation of ROS negatively regulates the microbial development and inhibits excessive colonization in plant tissue.[51] However, defense related induction is prominent at early stage of infestation.[52] Since cell death is rarely observed in response to bacterial invasion, the results clearly demonstrated that rice plant remains metabolically balanced and confirm the fitness benefit from endophytic bacteria tested. These research findings could serve as foundation in further research to enhance the growth and development of the rice which may help in more rice production.
CONCLUSION
The EBIs from mangrove interacted positively with rice seedlings which resulted in the significant increase in root and shoot length, fresh weight, and chlorophyll content. A balance in production and scavenging of ROS maintained the defense responses between non-host rice and endophytic bacteria from mangrove. Further, it has shown that different endophytic bacteria had different degrees of competitiveness. Plant growth promoting features observed under normal condition demonstrated that rice seedlings had recognized mangrove endophytic bacteria as friendly intruder. However, further studies are necessary to assess the fitness of rice seedlings under adverse condition. In addition, the EBIs need to be explored for their other potential applications.
Footnotes
Source of Support: Authors are grateful to the Ministry of Agriculture and Agro-Based Industry (MoA), Malaysia for the financial support (Research Grant Code Number: 05-02-16-SF1001)
Conflict of Interest: None declared.
REFERENCES
- 1.Schulz B, Boyle C. What are endophytes? In: Schulz BJ, Boyle CJ, Sieber TN, editors. Microbial Root Endophytes. Berlin: Springer-Verlag; 2006. pp. 1–13. [Google Scholar]
- 2.Arnold AE. Understanding the diversity of foliar endophytic fungi: Progress, challenges and frontiers. Fungal Biol Rev. 2007;21:51–66. [Google Scholar]
- 3.Ryan RP, Germaine K, Franks A, Rayan DJ, Dowling DN. Bacterial endophytes: Recent developments and applications. FEMS Microbiol Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 4.Long HH, Schmidt DD, Baldwin IT. Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS One. 2008;3:e2702. doi: 10.1371/journal.pone.0002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kevin VJ. Plant growth promoting rhizobacteria as biofertilizer. Plant Soil. 2003;255:571–86. [Google Scholar]
- 6.Lee S, Flores-Encarnacion M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE, Kennedy C. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome C biogenesis genes. J Bacteriol. 2004;186:5384–91. doi: 10.1128/JB.186.16.5384-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakelin SS, Warren RA, Harvey PR, Ryder MH. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fertil Soils. 2004;40:36–43. [Google Scholar]
- 8.Woodward AW, Bartel B. Auxin: Regulation, action and interaction. Ann Bot. 2005;95:707–35. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glick BR, Todorovic B, Czarny J, Cheng ZY, Duan J, Mc Conkey. Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci. 2007;26:227–42. [Google Scholar]
- 10.Rijavec T, Lapanje A, Dermastia M, Rupnik M. Isolation of bacterial endophytes from maize kernels. Can J Microbiol. 2007;53:802–8. doi: 10.1139/W07-048. [DOI] [PubMed] [Google Scholar]
- 11.Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistics human pathogic bacteria. Environ Microbiol. 2005;7:1673–85. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 12.Gray EJ, Smith DL. Intracellular and extra-cellular PGPR commonalities and distinctions in the plant bacterium signalling processes. Soil Biol Biochem. 2005;37:395–412. [Google Scholar]
- 13.Kloepper JW, Ryu CM. Bacterila endophytesas elicitors of induced systemic resistance. In: Schulz BJ, Boyle CJ, Sieber TN, editors. Microbial Root Endophytes. Berlin: Springer-Verlag; 2006. pp. 33–52. [Google Scholar]
- 14.Malinowski DP, Belesky DP. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000;40:923–40. [Google Scholar]
- 15.Madhaiyan M, Poonguzhali S, Ryu J, Sa T. Regulation of ethylene levels in canola (Brassica compestris) by 1-aminocyclopropane-1-carboxylate deaminase containing Methylobacterium fujisawaense. Planta. 2006;224:268–78. doi: 10.1007/s00425-005-0211-y. [DOI] [PubMed] [Google Scholar]
- 16.Domenech J, Ramos SB, Probanza A, Lucas GJ, Gutierrez MF. Elicitation of systemic resistance and growth promotion of Arabidopsis thaliana by PGPRs from Nicotiana glauca: A study of the putative induction pathway. Plant Soil. 2007;290:43–50. [Google Scholar]
- 17.Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257–68. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Dey N, Bera A, Tiwari A, Sathyaniranjan K, Chakrabarti K, et al. Culture independent molecular analysis of bacterial communities in the mangrove sediments of Sundarban, India. Saline Systems. 2010;6:1. doi: 10.1186/1746-1448-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumaresan V, Suryanarayanan TS. Endophytic assemblages in young, mature and senescent leaves of Rhizophora apiculata: Evidence for the role of endophytes in mangrove litter degradation. Fungal Divers. 2002;9:81–91. [Google Scholar]
- 20.Sgroy V, Cassán F, Masciarelli O, Del Papa MF, Lagares A, Luna V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol. 2009;85:371–81. doi: 10.1007/s00253-009-2116-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu AR, Wu XP, Xu T. Research advances in endophytic fungi of mangrove. [Article in Chinese] Ying Yong Sheng Tai Xue Bao. 2007;18:912–8. [PubMed] [Google Scholar]
- 22.Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: Diversity and functional roles. New Phytol. 2009;182:314–30. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 23.Schulz B, Boyle C. The endophytic continuum. Mycol Res. 2005;109:661–86. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt DD, Kessler A, Kessler D, Schmidt S, Lim M, Gase K, et al. Solanum nigrum: A model ecological expression system and its tools. Mol Ecol. 2004;13:981–95. doi: 10.1111/j.1365-294X.2004.02111.x. [DOI] [PubMed] [Google Scholar]
- 25.Harborne JB. 2nd ed. London New York: 1984. Phytochemical Methods; A guide to modern techniques of plant analysis. [Google Scholar]
- 26.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant system in acid rain treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- 27.Madhava Rao KV, Sresty TV. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stress. Plant Sci. 2000;157:113–28. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 28.Maria GL, Sridhar KR, Raviraja NS. Antimicrobial enzyme activity of mangrove fungi of south west coast of India. J Agric Tech. 2005;1:67–80. [Google Scholar]
- 29.Cho KM, Hong SY, Lee SM, Kim YH, Khang GG, Lim YP, et al. Endophytic bacterial communities in Ginseng and their antifungal activity against pathogens. Microb Ecol. 2007;54:341–51. doi: 10.1007/s00248-007-9208-3. [DOI] [PubMed] [Google Scholar]
- 30.Bashan Y, Holguin G. Plant growth promoting bacteria: A potential tool for arid mangrove reforestration. Trees: Structure and Function. 2002;16:159–66. [Google Scholar]
- 31.Gayathri S, Saravanan D, Radhakrishnan M, Balagurunathan R, Kathiresan K. Bioprospecting potential of fast growing endophytic bacteria from leaves of mangrove and salt-marsh plant species. Indian J Biotechnol Technol. 2010;9:397–402. [Google Scholar]
- 32.Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves growing in a semiarid coastal lagoon. Biol Fertil Soils. 2001;30:460–8. [Google Scholar]
- 33.Dias ACF, Andreote FD, Dini-Andreote F, Lacava PT, Sá ALB, Melo IS, et al. Diversity and biotechnological potential of culturable bacteria from Brazilian mangrove sediment. World J Microb Biot. 2009;25:1305–11. [Google Scholar]
- 34.Oehrle NW, Karr DB, Kremer RJ, Emerich DW. Enhanced attachment of Bradyrhizobium japonicum to soybean through reduced root colonization of internally seed borne microorganism. Can J Mirobiol. 2000;44:600–6. doi: 10.1139/w00-030. [DOI] [PubMed] [Google Scholar]
- 35.Rajendran G, Sing F, Desai AJ, Archana G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour Technol. 2008;99:4544–50. doi: 10.1016/j.biortech.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 36.Selvakumar G, Kundu S, Gupta AD, Shouche YS, Gupta HS. Isolation and characterization of nonrhizobial plant growth promoting bacteria from nodules of kudzu (Pueraria thunbergiana) and their effect on wheat seedling growth. Curr Microbiol. 2008;56:134–9. doi: 10.1007/s00284-007-9062-z. [DOI] [PubMed] [Google Scholar]
- 37.Mano H, Tanaka F, Watanabe A, Kaga H, Okunishi S, Morisaki H. Cultural surface and endophytic bacterial flora of the maturing seeds of rice plants (Oryza sativa) cultivated in a paddy field. Microb Environ. 2006;21:86–100. [Google Scholar]
- 38.Mano H, Tanaka F, Nakamura C, Kaga H, Morisaki H. Culturable endophytic bacterial flora of the maturing leaves and roots of rice plants (Oryza sativa) cultivated in paddy field. Microb Environ. 2007;22:175–85. [Google Scholar]
- 39.Okunishi S, Sako K, Mano H, Imamura A, Morisaki H. Bacterial flora of endophytes in the maturing seeds of cultivated rice (Oryza sativa) Microb Environ. 2005;20:168–77. [Google Scholar]
- 40.Stolzfus JR, So R, Malarvithi PP, Ladha JK, de Bruiji FJ. Isolation of endophytic bacteria from rice and assessment of their potential for supplying rice with biologically fixed nitrogen. Plant Soil. 1997;194:25–36. [Google Scholar]
- 41.Mano H, Morisaki H. Endophytic bacteria in the rice plant. Microbes Environ. 2008;23:109–17. doi: 10.1264/jsme2.23.109. [DOI] [PubMed] [Google Scholar]
- 42.Verma SC, Ladha JK, Tripathi AK. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;91:127–41. doi: 10.1016/s0168-1656(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 43.Fry SC. Primary cell wall metabolism tracking the careers of wall polymers in living plants cells. New Phytol. 2004;161:641–75. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- 44.Adriano-Anaya ML, Salvador-Figueroa M, Ocampo JA, Garcia Romero I. Hydrolytic enzyme activities in maize (Zea mays) and sorghum (Sorghum bicolor) roots inoculated with Gluconacetobacter diazotrophicus and Glomus intraradices. Soil Biol Biochem. 2006;38:879–86. [Google Scholar]
- 45.Mostajeran A, Amooaghaie R, Emtiazi G. The participation of cell wall hydrolytic enzymes in the initial colonization of Azospirillum brasilense on wheat roots. Plant Soil. 2007;291:239–48. [Google Scholar]
- 46.Hassen AI, Labuschagne N. Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World J Microb Biot. 2010;26:1837–46. [Google Scholar]
- 47.Prime-A-Plant Group, Conrath U, Beckers GJ, Flors V, Garcia-Agustin P, Jakab G, Mauch F, et al. Priming: Getting ready for battle. Mol Plant Microbe Interact. 2006;19:1062–71. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 48.Guo JH, Wei LH, Li SM. The strain for biocontrol root-knot disease of vegetable. 2009; State Intellectual Property Office of the People's Republic of China. Patent number: ZL 200810088388X. [Google Scholar]
- 49.Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, et al. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant growth promoting effect. Microbiology. 2002;148:2097–109. doi: 10.1099/00221287-148-7-2097. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez R, Redman R. Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci U S A. 2005;102:3175–6. doi: 10.1073/pnas.0500367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka A, Christensen MJ, Takemoto D, Park P, Scotta B. Reactive oxygen play a role in regulating a fungus perennial rye grass mutualistic interaction. Plant Cell. 2006;18:1052–66. doi: 10.1105/tpc.105.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia Garrido JM, Ocampo JA. Regulation of the plant defense response in arbuscular mycorrhizal symbiosis. J Exp Bot. 2002;53:1377–86. [PubMed] [Google Scholar]


