Abstract
Tendon-to-bone integration is a great challenge for tendon or ligament reconstruction regardless of use of autograft or allograft tendons. We mineralized the tendon, thus transforming the tendon-to-bone into a “bone-to-bone” interface for healing. Sixty dog flexor digitorum profundus (FDP) tendons were divided randomly into 5 groups: 1) normal FDP tendon, 2) CaP (Non-extraction and mineralization without fetuin), 3) CaPEXT (Extraction by Na2HPO4 and mineralization without fetuin), 4) CaPFetuin (Non-extraction and mineralization with fetuin), and 5) CaPEXTFetuin (Extraction and mineralization with fetuin). The calcium and phosphate content significantly increased in tendons treated with combination of extraction and fetuin compared to the other treatments. Histology also revealed a dense mineral deposition throughout the tendon outer layers and penetrated into the tendon to a depth of 200 μm in a graded manner. Compressive moduli were significantly lower in the four mineralized groups compared with normal control group. No significant differences in maximum failure strength or stiffness were found in the suture pull-out test among all groups. Mineralization of tendon alters the interface from tendon to bone into mineralized tendon to bone, which may facilitate tendon-to-bone junction healing following tendon or ligament reconstruction.
Keywords: Tendon Mineralization, Graded Mineral, Tendon-to-Bone Healing, Tendon Allografts
INTRODUCTION
Tendon-to-bone integration is generally recognized as the most difficult hurdle in tendon or ligament reconstructions. The tendon-to-bone insertion is physiologically characterized with a unique transitional fibrocartilage zone, with calcified collagen fibers connecting to bone and non-calcified collagen fibers connecting to tendon, which allows for more efficient force transmission without stress concentrations.1, 2 Direct reattachment of tendon to bone is indicated when a reconstructive tendon or ligament needs to be integrated into bone for healing to restore function. Unfortunately, because healing occurs between two types of tissues, tendon-to-bone insertion repair and the regeneration of the graded transitional zone is slow and difficult to achieve in both experimental3 and clinical studies.4 Previous attempts to improve tendon-to-bone healing include bone substitutes, periosteum autografts, growth factors and gene therapy, physical stimulation, and stem cells transplantation.5 However, these efforts have not been completely successful, and the naturally graded transitional zone has not been regenerated.
Tendon mineralization has long been recognized as a physiological adaptation found in some organisms such as birds and dinosaurs.6 However, it usually does not occur in humans, unless subjected to a pathological change resulting from injury,7 degenerative disorders,8 or tendinitis,9 which alter the normal tendon function. To prevent or treat this problem, many studies were conducted to explore the mechanism of mineralization, some of which indicated that a still unidentified macromolecular inhibitor plays an important role to preclude tendon or other soft tissue calcification.10-12 This theory is supported by data showing that tendon extraction with 3% Na2HPO4 leads to a quick mineralization and re-adding the extract significantly inhibits the rates of calcium and phosphate uptake from the soluble phase.11-13 However, a recent study showed that when the Na2HPO4-extracted tendon was incubated in a solution containing both calcium and phosphate, the mineralization process only lasted for several hours.12 A decline in free calcium occurred quickly because of the spontaneous formation of apatite crystals in solution containing increased levels of calcium and phosphate.14 Studies by Price et al.15, 16 indicated that fetuin, which is synthesized in the liver and is found at high concentrations in mammalian serum and bone, can sustain elevated calcium and phosphate levels in solution and favor mineralization within the collagen fibrils by selectively preventing apatite crystal growth in the solution outside the fibril, thereby enhancing intrafibrillar mineralization.
While the phenomenon of tendon mineralization has been investigated in the context of tendinopathy12, 13 or engineering material mineralization for bone formation,17 benefits of this abnormal mineralization to tendon-to-bone healing could be realized by converting a tendon partially into a bone-like tissue, thereby, adapting a tendon-to-bone interface into a “bone-to-bone” interface for better healing. We studied the conditions that would optimize tendon mineralization as a prelude to studying the ability of mineralized tendon to heal to bone.
MATERIALS AND METHODS
Preparation of Tendon Tissue
15 hind paws were obtained from 8 adult male mixed-breed dogs (weight range, 24 to 27 kg) that were euthanized for other IACUC approved studies. Immediately after death, the paws were harvested and frozen in a -80 °C freezer. Following thawing at room temperature, 60 flexor digitorum profundus (FDP) tendons resected 5-mm proximal to the tendon-to-bone insertion were dissected from the 2nd to 5th digits and divided randomly into 5 groups (Table 1). Before any treatment, the distal 1-cm of these FDP tendons were dissected and prepared for histology, scanning electron microscopy (SEM) and measurement of calcium and phosphate content (Table 1), while the middle 2-cm tendon segments were harvested centering on Okuda’s zone D18 and prepared for biomechanical testing. 24 segments served as a normal control group. The other 96 segments were immediately immersed in liquid nitrogen for 1 min, then thawed for 5 mins in normal saline solution at 37°C. This procedure was repeated 5 times to induce tenocyte necrosis.
Table 1.
Assignment of tendon specimens. The 12 distal 1-cm tendons in each group were used for histology, SEM and measurement of calcium and phosphate content. An additional 12 2-cm tendons in each group were used for indentation testing followed by suture pull-out testing.
| Groups | Interventions | Evaluations (Sample Size) | ||||
|---|---|---|---|---|---|---|
| Histology | Scanning Electron Microscopy | Calcium and Phosphate | Indentation Test | Suture Pull-out Test | ||
| Normal | Normal | 3 | 1 | 8 | 12 | 12 |
| CaP | Non-extraction and Mineralization without fetuin | 3 | 1 | 8 | 12 | 12 |
| CaPEXT | Extraction and Mineralization without fetuin | 3 | 1 | 8 | 12 | 12 |
| CaPFetuin | Non-extraction and Mineralization with fetuin | 3 | 1 | 8 | 12 | 12 |
| CaPEXTFetuin | Extraction and Mineralization with fetuin | 3 | 1 | 8 | 12 | 12 |
Extraction
The tendons in the CaPEXT and CaPEXTFetuin groups were suspended in 3% Na2HPO4 solution, PH 9.2, and shook slowly and continuously for 3 days at 4°C.10, 11 These tendons were washed by soaking in 4°C distilled water with water changes every 20 mins, then lyophilized and stored in the refrigerator until use. For groups CaP and CaPFetuin, the tendons were soaked in normal saline solution - instead of 3% Na2HPO4 solution - under the same conditions described for the CaPEXT and CaPEXTFetuin treatment groups.
Mineralization
The 24 non-extracted tendons from the CaP group and 24 extracted tendons from the CaPEXT group were incubated and mixed end-over-end at 37°C continuously for 72 hrs in the calcification solution consisting of 50 mM Tris buffer, 150 mM NaCl, 5 mM CaCl2, 5 mM KH2PO4 at a pH of 7.4. For the CaPFetuin and CaPEXTFetuin groups, in addition to the aforementioned chemicals, the calcification solutions also contained fetuin (1 mg/ml, Sigma, St. Louis, MO). To prevent bacterial growth, all the solutions also contained 100 units/ml of penicillin and 100 μg/ml streptomycin.
Histological Evaluation
After mineralization, 3 randomly selected 1-cm specimens from each group were mounted and frozen in Tissue-Tek (Sakura Finetek, California), and subsequently sectioned transversely or longitudinally at 10 μm using a cryostat (CM1850; Leica). The sections were collected on charged glass slides (Fisher Scientific) and air-dried overnight. The sections were stained with the use of the Hematoxylin-Eosin or von Kossa kit (MasterTech) according to the standard procedure. These slides were observed with a microscope (Olympus BH-2).
SEM
One randomly selected 1-cm specimen from each group was used for SEM. The samples were dehydrated in a graded ethanol series and critical-point dried. The samples were then sputter-coated with an ultra-thin layer of gold-palladium alloy. They were examined at 3.0 kV with an SEM (Hitachi S-4700 FE-SEM).
Calcium and Phosphate Content Measurement
8 randomly selected 1-cm tendons in each group were incubated for 48 hrs at room temperature with 5 ml of 0.2 M HCl per tendon. Calcium and phosphate levels in the acid extracts were determined with the Calcium and Phosphate Colorimetric Assay Kit (Biovision). The absorbance was measured using a microplate reader (FLUOstar Omega), and the concentrations were calculated from the standard curve.
Biomechanical testing
Indentation testing was carried out according to a previously established method.19 The 12 2-cm tendon specimens from each group were placed on an aluminum platform. Indentation testing was performed using a Bose ElectroForce 3200 test system (Bose Corp). A 3-mm diam indenter with a flat surface was brought into contact with the center of specimens, and a preload force of 0.1 N was applied. Indentation testing was performed at a loading rate of 10 N/min to a maximum force of 5.0 N. The stress was calculated by dividing the force by the area of indenter, and the strain was calculated based on the indentation depth relative to the specimen height under a force of 0.03 N. The slope of the linear region of the stress–strain curve was considered the compressive modulus. Following testing, a single loop suture of 3-0 Ethibond (Ethicon Inc.) was placed through the tendon 5 mm from the distal end. The suture was knotted with a single closed loop with a 6 cm perimeter. Suture pullout testing was carried out to evaluate the tendon holding strength, which is also related to tendon tissue structure and mechanical properties.20 The sutured tendon was mounted onto an MTS machine (MTS 858 Mini Bionix II). The suture construct was distracted at 20 mm/min until failure. The maximum pull-out load and linear stiffness were calculated.
Statistical Analysis
The quantitative data were represented as the mean with standard deviation, and were analyzed using a one-way analysis of variance, followed by the Tukey HSD post hoc test for each pairwise comparison. The significance level was set at P < 0.05.
RESULTS
Histology
Only a small amount of mineral was attached to the tendon surface in the CaP group (Fig, 1, B & G). There was some mineral penetration into the tendon in the CaPEXT group, but the depth of mineral was minimal (Fig. 1, C & H), Although mineralization seemingly penetrated deeper into the CaPFetuin group than either the CaP or CaPEXT groups, mineralization was still limited (Fig. 1, D & I). However, the specimens in CaPEXTFetuin group revealed more mineral deposits inside the tendon. Mineral deposition in the CaPEXTFetuin group was visible inside the tendon to a depth of 200 μm (Fig. 1, E & J). Mineral deposition could be seen in a graded manner in this group. Although a quantitative assessment of the number of cells was not performed, by gross observation the number of cells inside tendons of the 2 extracted groups appeared to be less compared to the 2 non-extracted groups and the normal control group. The structure of tendon fibers became looser, and fibers were less aligned in all 4 mineralized groups (Fig. 1, L-O) compared with the structure of normal tendon (Fig. 1, K).
Figure 1.
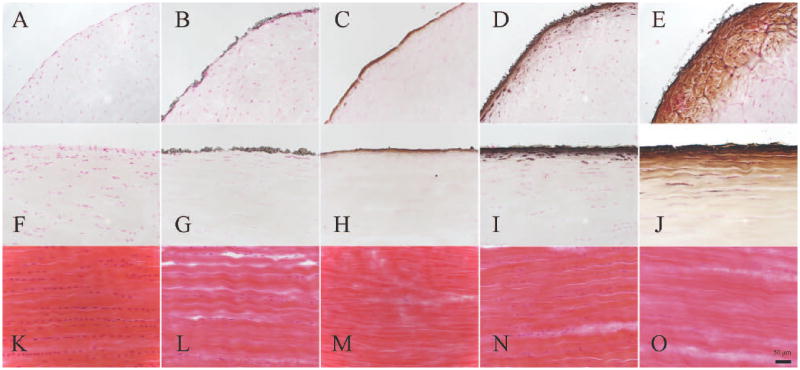
Normal tendon (A, F and K); CaP (B, G and L); CaPEXT (C, H and M); CaPFetuin (D, I and N); and CaPEXTFetuin (E, J and O). Figure A-E von Kossa staining of transverse section; Figure F-G: von Kossa staining of longitudinal section; Figure K-O: HE staining of longitudinal section. Magnification: ×200. The black bar = 50 μm.
SEM
In the CaP and CaPEXT groups, a large amount of calcium phosphate crystals precipitated on the tendon surfaces (Fig. 2, B & C). However, in the CaPFetuin and CaPEXTFetuin groups, the mineral was mainly deposited within the tendons’ collagen fibers. The deposition of mineral into collagen fibers appeared to have little influence on the size of the fibers compared with the size of normal collagen fibers (Fig. 2A). No calcium phosphate precipitation was apparent on the surfaces of collagen fibers in the 2 fetuin-treated groups (Fig. 2, D & E).
Figure 2.

SEM of tendon surfaces. Normal tendon (A); CaP (B); CaPEXT (C); CaPFetuin (D); and CaPEXTFetuin (E). A large amount of calcium phosphate crystal precipitated on the tendon surfaces (B and C). No calcium phosphate crystal was precipitated on the collagen fiber surfaces (D and E). Magnification: ×50000.
Calcium and Phosphate Content
Significantly higher calcium and phosphate contents were present in the CaPEXTFetuin group (4.82±0.59 and 2.83±0.37μmol per tendon) as compared with normal tendons (0.18±0.1 and 0.02±0.01 μmol), CaP (1.08±0.3 and 0.65±0.21 μmol), CaPEXT (1.6±0.45 and 0.96±0.27 μmol), and CaPFetuin (3.27±0.35 and 1.9±0.2 μmol) groups (p < 0.05) (Fig. 3). Calcium and phosphate contents in the CaPFetuin group were also significantly higher compared to the CaP and CaPEXT groups (p < 0.05). However, no significant difference was found in calcium (P=0.14) and phosphate (P=0.1) content between the CaP group and CaPEXT group (Fig. 3). The molar Ca:P ratios ranged from 1.62 to 1.74.
Figure 3.
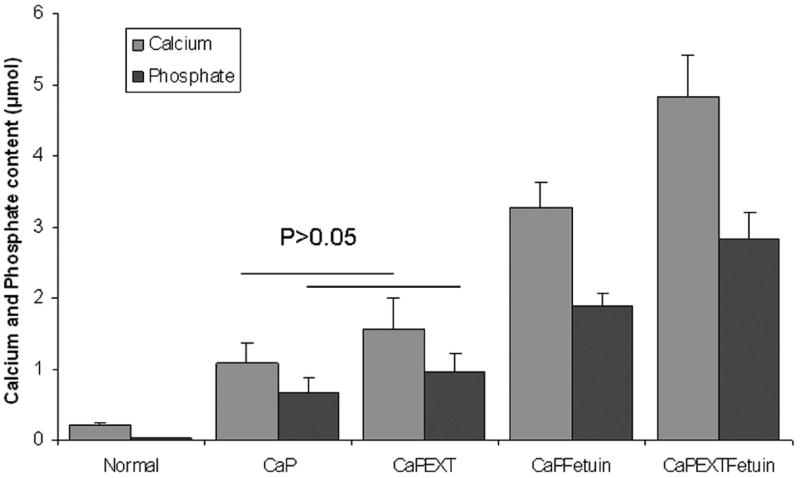
Significant differences on calcium and phosphate contents were found between all pairs, except between CaP group and CaPEXT group (P=0.14 for calcium, P=0.1 for phosphate).
Biomechanical Testing
Compared with the normal group (4.34±0.59 MPa), the compressive moduli in the CaP (3.43±0.2), CaPEXT (3.12±0.2), CaPFetuin (3.56±0.31), and CaPEXTFetuin (3.33±0.18) groups were significantly lower (p < 0.05). The mean ventro-dorsal thickness of normal fresh tendon (1.32±0.12 mm) was significantly less compared with the 4 mineralized groups (1.57±0.18, 1.63±0.18, 1.64±0.13 and 1.65±0.17 mm for CaP, CaPEXT, CaPFetuin, and CaPEXTFetuin, respectively, p < 0.05). No significant differences in maximum failure strength for suture pull-out testing were found among normal (14.11±4.07 N), CaP (13.98±4.27), CaPEXT (14.3±4.32), CaPFetuin (15.7±4.05), and CaPEXTFetuin (16.57±4.56) groups (p > 0.05) (Fig. 5). Furthermore, no significant differences in linear stiffness were found between normal (3.06±0.89 N/m), CaP (3.11±0.55), CaPEXT (3.31±0.77), CaPFetuin (3.09±0.61), and CaPEXTFetuin (3.04±0.84) groups (p > 0.05) (Fig. 5).
Figure 5.
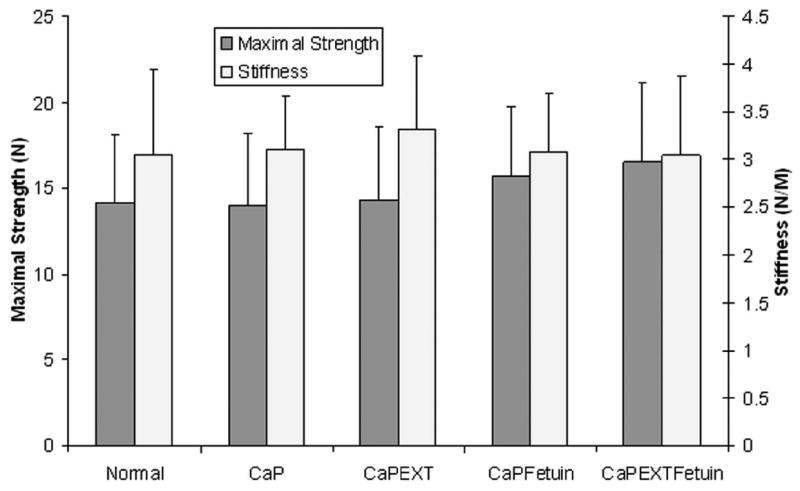
Suture pull-out test. Left Y axis and left column: Maximum failure strength and there were no significant differences among the groups. Right Y axis and right column: Stiffness in linear region. No significant differences could be found among the groups.
DISCUSSION
Tendon mineralization has long been studied to explore the mechanism of natural calcification of the Achilles tendon in turkeys and other large birds6 and to determine why tendon calcification only occurs in pathological conditions in human, such as calcific tendinopathy21 and ossification of the ligamentum flavum.13 Although the mechanism of calcification is still not fully understood, two factors seem to play an important role: fetuin22 and the inhibitor extracted by Na2HPO4.13 However, to our knowledge, these factors have not been previously studied in combination, and their effect has not been investigated on tendon calcification for the purpose of accelerating tendon-to-bone interface healing. Our findings showed that tendon was mineralized in vitro in both the extracted and fetuin-treated groups. However, the maximum mineralization was noticed when combining these two treatment methods together. Quantitative analysis of calcium and phosphate content confirmed this finding. Additionally, mineralization resulting from extraction and fetuin treatment was formed in a graded manner that may help to generate a native transitional zone within tendon.
Interestingly, even when we combined the Na2HPO4 extraction with fetuin enhancement, the whole tendon could not be mineralized thoroughly, forming a graded mineral concentration zone in only the outer layer. Mineral deposition was visible to a depth of only 200 μm. This may be because the penetration of the fetuin-mineral complex could be blocked by a mineral coating. A recent study also showed precursor droplets were blocked by the mineral coating during turkey tendon mineralization via the polymer-induced liquid-precursor process.17 However, a 200-μm-deep graded mineral concentration zone may be enough to enhance the regeneration of a graded interface zone in vivo. In addition, a previous in vivo study showed that when transplanting the extracted - but non-mineralized - ligament into dorsal muscles of rats, numerous calcareous deposits were distributed throughout the entire extracted graft, while less calcareous deposits were located in only a part of the non-extracted grafts.13 This indicates that the extracted tendon may be calcified thoroughly in vivo because Na2HPO4 extraction has made calcification possible.
Na2HPO4 extraction clearly caused the tendons to exhibit calcification. However, until now, the exact calcification inhibitor extracted by Na2HPO4 has not been identified, though many studies have suggested its existence.11, 12, 23 Adding the tendon extracts (obtained during preparation of extracted tendons) in the calcium and phosphate homogenous system can also inhibit precipitation of calcium and phosphate ions as a mineral phase.12 Based on previous studies, two hypotheses could explain calcification produced by Na2HPO4 extraction: (1) Na2HPO4 specifically promotes dissociation from the tissue of a substance that inhibits calcification in situ; and (2) phosphate residuals alone, or in combination with endogenous Ca2+, bind at appropriate matrix sites, thus providing centers for nucleation and growth of calcium phosphate crystals.10, 11, 13
The indentation test was used to evaluate mechanical properties of tendon in previous studies.19 Our original hypothesis was that the compressive moduli of tendons would increase after mineralization. However, the compressive modulus of tendons in the 4 mineralized groups decreased after the procedures of extraction, lyophilization, and mineralization compared to the normal tendon. The tendons treated with these procedures became thicker and softer in comparison with normal tendon. Although the thin outer layer of tendon had been calcified, it could not restore the compressive modulus to normal because the compression was mainly supported by the large portion of non-calcified tendon tissue. The fiber structure became looser and less aligned in the 4 mineralized groups. Usually, lyophilized tendons are rehydrated in normal saline for 30 mins to 24hrs before use.24, 25 In our study, the lyophilized tendons were mineralized in calcification solution at 37°C for 72 hrs. Long-time rehydration of lyophilized tendons might account for the decreased compressive moduli in the 4 mineralized groups.
Qualitative analysis revealed that only a small number of cells remained in the 2 Na2HPO4 extracted groups. The CaPEXTFetuin-treated tendon scaffolds may not only elicit less immunogenic response, but may also provide good porosity for cell migration into the tendon, which may further improve tendon-to-bone healing.26 Although the tendon segments became a bit thicker after treatment, the maximal suture pullout strength and linear stiffness were not significantly affected. This might indicate that these morphological changes do not negatively affect mechanical properties of the repair. Previous studies also showed that Na2HPO4 extraction affects the removal of soluble proteins while not significantly affecting the integrity of collagen fibers.10, 27, 28
The calcification process is far more complicated in vivo than in vitro, in which calcium and phosphate deposit only into tendon tissue, although the term of “mineralization” or calcification” has been used in vitro studies 16, 17. The SEM results showed the mineral was mainly deposited inside the collagen fibers that had been treated with combined extraction and fetuin. However, we did not evaluate where mineralization mainly occurred in levels of fibrils. Previous studies found fetuin-regulated collagen mineralization mainly deposited the mineral inside collagen fibrils (i.e., intrafibrillar mineralization).16, 29 Martin et al. found in canine whole bones that 58% of the mineral was intrafibrillar, 14% extrafibrillar, and 28% within the gaps between the ends of collagen fibrils.30 Tendon is mainly composed of type I collagen with almost the same amino acid composition as bone collagen. By combining Na2HPO4 extraction and fetuin enhancement, we successfully generated a 200-μm-layer of “bone” inside the tendon. Most importantly, the fiber continuity was not disrupted based on our histological and mechanical results. With this technique, we believe that we can adapt tendon-to-bone healing into “bone-to-bone” healing, with a graded interface zone regenerated inside the tendon rather than at the tendon-to-bone interface, which is the weak point for healing. Of course, in vivo animal studies are required for proving the effectiveness of this novel natural tendon-derived scaffold.
This study has several limitations. First, this was only an in vitro investigation. The biological effect of this natural tendon scaffold with gradations in mineral content on tendon-to-bone healing in vivo is unknown. Second, we only used SEM to assess the nature of the calcium phosphate mineral deposited on the tendons by these procedures. Transmission electron microscopy and synchrotron X-ray diffraction should be performed to examine the characteristics of the mineral inside the tendons. However, the molar Ca:P ratios in the mineralized tendons ranged from 1.62 to 1.74, which is not different from the ratios of bovine non-demineralized bone (1.64 to 1.66).16 Third, the depth of mineral deposition and residual cells inside the tendons were not quantitatively evaluated in histology due to the small sample size.
In summary, we combined Na2HPO4 extraction and fetuin treatment to explore a potential method for tendon calcification, though phosphate extraction for tendon calcification and fetuin treatment for bone regeneration were independently studied previously. Our findings may have important implications for improving tendon-to-bone healing and regenerating the graded transitional interface zone for tendon or ligament reconstruction. Further in vivo animal study is necessary to verify this concept.
Figure 4.
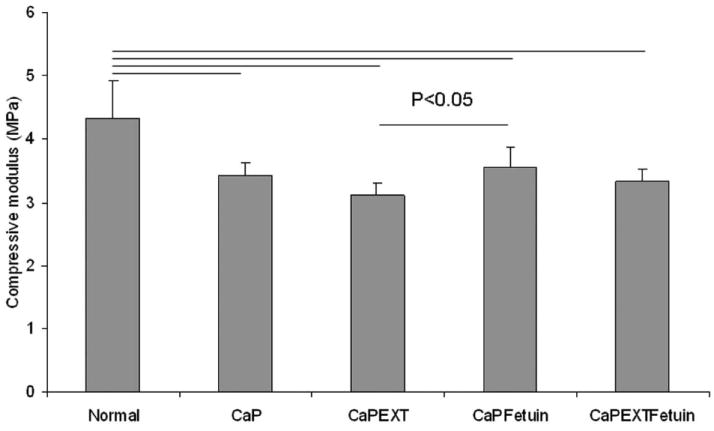
The compressive modulus of each group. There was significantly lower modulus in the four intervention groups compared with normal group. The modulus in CaPEXT group was significant lower compared to the CaPFetuin group.
Acknowledgments
This study was supported by grants from Mayo Foundation and NIH/NIAMS (AR057745).
References
- 1.Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 2007;17:303–15. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 2.Genin GM, Kent A, Birman V, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Nebelung W, Becker R, Urbach D, et al. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158–63. doi: 10.1007/s00402-002-0463-y. [DOI] [PubMed] [Google Scholar]
- 5.Kuang GM, Yau WP, Lu WW, et al. Osteointegration of soft tissue grafts within the bone tunnels in anterior cruciate ligament reconstruction can be enhanced. Knee Surg Sports Traumatol Arthrosc. 2010;18:1038–51. doi: 10.1007/s00167-009-0910-1. [DOI] [PubMed] [Google Scholar]
- 6.Vandenberge JC, Storer RW. Intratendinous Ossification in Birds - a Review. J Morphol. 1995;226:47–77. doi: 10.1002/jmor.1052260105. [DOI] [PubMed] [Google Scholar]
- 7.Valencia H, Gavin C. Infrapatellar heterotopic ossification after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:39–42. doi: 10.1007/s00167-006-0131-9. [DOI] [PubMed] [Google Scholar]
- 8.Riley GP, Harrall RL, Constant CR, et al. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis. 1996;55:109–15. doi: 10.1136/ard.55.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia TC, Chandler JT. Ossific tendonitis of the achilles with tendon fracture. Orthopedics. 2006;29:453–5. doi: 10.3928/01477447-20060501-11. [DOI] [PubMed] [Google Scholar]
- 10.Thomas WC, Jr, Tomita A. Mineralization of human and bovine tissue in vitro. Am J Pathol. 1967;51:621–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Quittner C, Wadkins CL. A macromolecular inhibitor of in vitro calcification of tendon matrix. Calcif Tissue Res. 1978;25:161–8. doi: 10.1007/BF02010764. [DOI] [PubMed] [Google Scholar]
- 12.Tandon CD, Forouzandeh M, Aggarwal S, et al. Inhibitors of in vitro mineralization from flexor tendons of rabbits and their role in biological mineralization. Mol Cell Biochem. 1997;171:29–35. doi: 10.1023/a:1006894400172. [DOI] [PubMed] [Google Scholar]
- 13.Maruta K, Ichimura K, Matsui H, et al. Calcification inhibitors in human ligamentum flavum. J Orthop Res. 1993;11:92–103. doi: 10.1002/jor.1100110111. [DOI] [PubMed] [Google Scholar]
- 14.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–52. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 15.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–47. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 16.Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem. 2009;284:17092–101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jee SS, Kasinath RK, DiMasi E, et al. Oriented hydroxyapatite in turkey tendon mineralized via the polymer-induced liquid-precursor (PILP) process. Crystengcomm. 2011;13:2077–2083. [Google Scholar]
- 18.Okuda Y, Gorski JP, An KN, et al. Biochemical, histological, and biomechanical analyses of canine tendon. J Orthop Res. 1987;5:60–8. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda J, Zhao C, Chen Q, et al. Compressive properties of cd-HA-gelatin modified intrasynovial tendon allograft in canine model in vivo. J Biomech. 2011;44:1793–6. doi: 10.1016/j.jbiomech.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C, Sun YL, Zobitz ME, et al. Enhancing the strength of the tendon-suture interface using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and cyanoacrylate. J Hand Surg Am. 2007;32:606–11. doi: 10.1016/j.jhsa.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien EJ, Frank CB, Shrive NG, et al. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–31. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brylka L, Jahnen-Dechent W. Epub ahead of print. The Role of Fetuin-A in Physiological and Pathological Mineralization. Calcif Tissue Int. 2013 doi: 10.1007/s00223-012-9690-6. [DOI] [PubMed] [Google Scholar]
- 23.Tandon CD, Aggarwal S, Forouzandeh M, et al. Inhibitors of in vitro mineralization from rabbit aorta and their role in biomineralization. J Cell Biochem. 1998;68:287–97. [PubMed] [Google Scholar]
- 24.Mahirogullari M, Ferguson CM, Whitlock PW, et al. Freeze-dried allografts for anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:625–37. doi: 10.1016/j.csm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Sun YL, Ikeda J, et al. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am. 2010;92:2817–28. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustmann M, Schmidt T, Gangey I, et al. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16:360–9. doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 27.Einbinder J, Schubert M. Binding of mucopolysaccharides and dyes by collagen. J Biol Chem. 1951;188:335–41. [PubMed] [Google Scholar]
- 28.Gohr CM, Fahey M, Rosenthal AK. Calcific tendonitis : a model. Connect Tissue Res. 2007;48:286–91. doi: 10.1080/03008200701692362. [DOI] [PubMed] [Google Scholar]
- 29.Nudelman F, Pieterse K, George A, et al. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 2010;9:1004–9. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin RB, Burr D, Sharkey N. Skeletal Tissue Mechanics. Springer-Verlag New York Incorporated; New York: 1998. p. 406. [Google Scholar]


