Abstract
Aminoparthenolide derivatives have been prepared by reaction of parthenolide with various heterocyclic amines to afford corresponding Michael addition products. These novel compounds were evaluated for their modulatory effects on Xenopus oocyte maturation. Two compounds, 6e and 6f, were identified that promote G2-M cell cycle progression.
Keywords: Parthenolide, Oocyte maturation, Progesterone, Michael addition, Heterocyclic amines
Parthenolide (PTL) (1, Fig. 1), an abundant sesquiterpene lactone found in the medicinal herb Feverfew (Tanacetum parthenium), has undergone intense pharmacological research,1 and has been noted for its remarkable antileukemic properties.2 Initial efforts pertaining to the biomechanistic study of parthenolide and its analogs revealed that the compound appears to promote apoptosis by inhibiting the activity of the NF-κB transcription factor complex, thereby down-regulating anti-apoptotic genes under NF-κB control.3 Several other related sesquiterpenes have also been shown to possess similar biological activity, including melampomagnolide-B (MMB)4 (2, Fig. 1) and micheliolide (MCL)5 (3, Fig. 1). In this respect, our group has demonstrated that PTL induces robust apoptosis of primary acute myeloid leukemic (AML) cells in culture.6,7 In addition, our laboratory has been successful in overcoming the poor water-solubility of PTL without loss of its anti-leukemic activity, by derivatizing PTL into several alkylamino analogs via Michael addition chemistry (5, Fig. 1); such analogs can then be converted into water-soluble organic salts with improved druglike properts.8 In more recent work, David and coworkers have reported on some fluorinated amino-derivatives of PTL, which showed antiproliferative activity in HL-60 (human promyelocytic leukemia) cells.9
Figure 1.
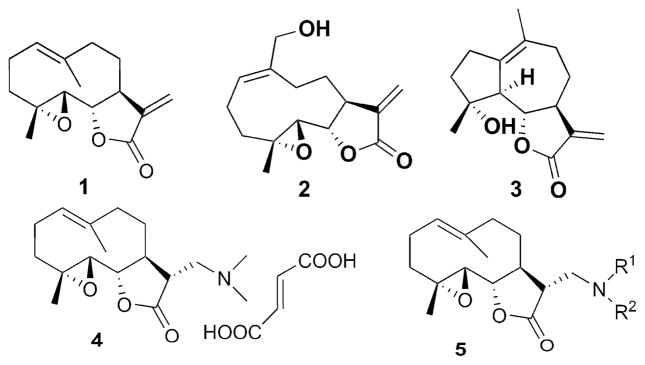
Structures of PTL, MMB, MCL, DMAPT, and PTL Michael adducts
In addition to its inhibitory effect on the NF-κB transcription factor complex, we have recently shown that PTL also selectively induces almost complete glutathione depletion and severe cell death in CD34+ acute myelogenous leukemia (AML) cells.10 Interestingly, PTL only induces limited and transient glutathione depletion as well as significantly less toxicity in normal CD34+ cells. PTL perturbs glutathione homeostasis by a multifactorial mechanism, which includes inhibiting key glutathione metabolic enzymes (GCLC and GPX1), as well as direct depletion of glutathione. These new findings demonstrate that primitive leukemia cells are uniquely sensitive to agents that target aberrant glutathione metabolism, an intrinsic property of primary human AML cells. The dimethylamino analog of PTL, DMAPT (4, Fig. 1), is currently in phase 1 clinical studies for evaluation as a treatment for AML.
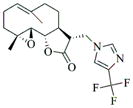
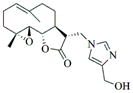
With regard to the continuing need to identify bioactive PTL analogs with increased water-solubility, and acceptable druglike properties, i.e. improved oral bioavailability and in vivo half-life, we have synthesized a series of Michael adducts of PTL (5, Fig. 1) by reacting PTL with various heterocyclic amines, i.e. imidazole, benzimidazole, morpholine, piperidine, triazole, pyrazole, and other related amines (Compounds 6a-6l; Table 1). These novel aminoparthenolides were evaluated for their modulatory effects in Xenopus oocyte maturation assays.
Table 1.
Michael addition reaction products, conditions and yields
| Amine | Product | Yield (%) | Time (hr) | Mp °C |
|---|---|---|---|---|
|
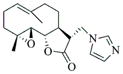 6a |
79 | 30 | 185 |
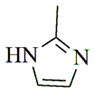
|
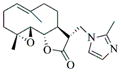 6b |
80 | 15 | 161 |
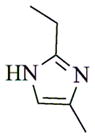
|
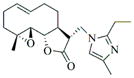 6c |
78 | 15 | 72 |
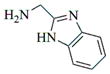
|
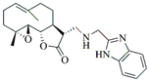 6d |
75 | 12 | 105 |
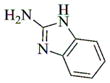
|
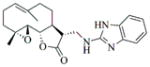 6e |
77 | 15 | 152 |
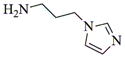
|
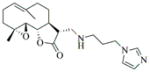 6f |
82 | 12 | Oil |
|
 6g |
68 | 72 | 165 |
|
 6h |
75 | 30 | 235 |
|
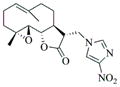 6i |
65 | 48 | 160 |
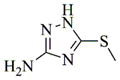
|
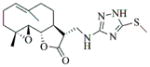 6j |
70 | 36 | 85 |
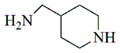
|
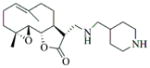 6k |
85 | 12 | 74 |
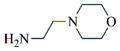
|
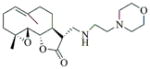 6l |
84 | 15 | Oil |
All Michael addition reactions were carried out in methanol at ambient temperature, and all products were obtained in good yields as the corresponding diastereoselective C-11 S-isomer. Structural characterization of all reaction products were determined by 1H and 13C NMR spectral analysis.11 The majority of the compounds could be isolated after simple aqueous work-up without any further purification.
As a drug discovery platform, Xenopus oocytes present a unique combination of evolutionary relatedness to humans and experimental malleability.12 Cell cycle control mechanisms are conserved between Xenopus and humans, since inhibitory drugs developed against mammalian cell growth also modulate cell cycle progression in Xenopus oocytes.13 Reciprocally, several studies have employed Xenopus embryo extracts to identify small-molecule inhibitors of conserved components of mammalian actin assembly, spindle assembly, and cell cycle progression.14 Moreover, Xenopus embryo phenotypic screening has identified compounds that are efficacious for inhibiting murine and human tumor cell growth.15 The newly synthesized heterocyclic aminoparthenolide analogs were thus screened for modulation of G2-M cell cycle progression during Xenopus oocyte maturation. In this assay, the steroid hormone progesterone triggers G2-arrested stage VI oocytes to re-enter the cell cycle and progress through meiosis to culminate in a fertilizable egg, arrested at metaphase of Meiosis II. In response to progesterone, maternal mRNAs are selectively translated in a strict temporal manner, resulting in the sequential activation of MAP kinase and cyclin-dependent kinase (CDK). MAP kinase activation triggers downstream activation of cyclin B/CDK1 (also known as maturation/M-phase promoting factor, MPF) which in turn leads to germinal vesicle (nuclear) breakdown (GVBD), characterized by the appearance of a white spot on the darkly pigmented animal hemisphere.16 We utilize the oocyte maturation assay here to assess the consequences of the heterocyclic aminoparthenolides’ effect on oocyte G2-M transition.
Xenopus oocytes were isolated and cultured as described previously.17 Oocytes were induced to mature with 2μg/ml progesterone.18 The rate of germinal vesicle breakdown (GVBD) was scored morphologically by observing the appearance of a white spot on the animal pole. Because oocytes from different frogs mature at different rates in response to progesterone, the culture times were standardized between experiments to the time taken for 50% of oocytes to undergo GVBD (designated GVBD50). As indicated, oocytes were pre-treated with test compounds or DMSO vehicle overnight, prior to progesterone addition. Animal protocols were approved by the UAMS Institutional Animal Care and Use committee, in accordance with Federal regulations.
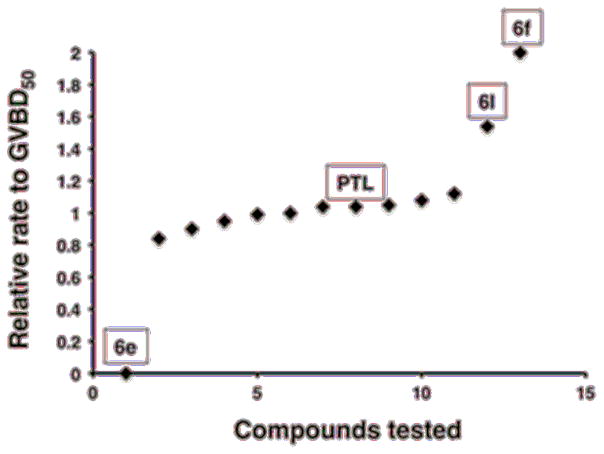
The PTL Michael addition products were dissolved in DMSO and added to oocyte media at 100 μM starting concentration in 1% DMSO (v/v), cultured overnight in 24-well plates (20 oocytes/well) and checked the next morning for the morphological appearance of a white spot, indicative of GVBD. Progesterone was added to oocytes which did not display white spots after overnight incubation, and progression through the cell cycle to GVBD was assessed relative to DMSO-treated control oocytes (Fig. 2).
Figure 2.
The Effect of compounds 6a-6l on the rate of progression of Xenopus oocytes to germinal vesicle breakdown (GVBD) was assessed for each test compound relative to the time taken for 50% of DMSO-treated control oocytes to complete GVBD (GVBD50) (Fig. 2). A value of 1.0 indicates that the compound did not differ from DMSO-treated control oocytes. A value greater than 1.0 indicates a delay to maturation, with a value of 2.0 indicating that no maturation occurred. A value less than 1.0 indicates acceleration of maturation, with a value of 0.0 indicating spontaneous progression to GVBD without added progesterone. Only compounds differing by more than 20% from DMSO-treated control oocytes were assessed further. Compound 6e induced spontaneous maturation in the absence of added progesterone. Compound 6f inhibited progesterone-stimulated maturation. Compound 6l appeared to delay time to GVBD, but this modest effect was not reproducible. None of the other compounds tested (including PTL and DMAPT, exerted any reproducibly significant phenotypic effects upon progression to GVBD during oocyte maturation.
Compound 6e was able to induce maturation in the absence of progesterone over several different doses (Fig. 3). To examine the possible point of action of 6e, we assessed its effect upon endogenous oocyte signaling pathways.
Figure 3.
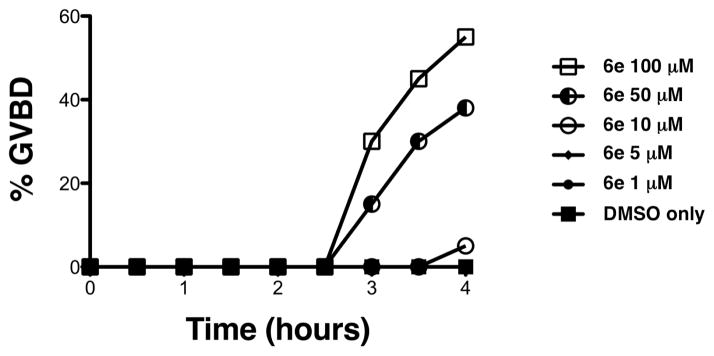
Compound 6e induces maturation in the absence of progesterone in a dose-dependent manner. The rate of oocyte progression to GVBD was assessed by appearance of a white spot on the animal pole over a range of 6e concentrations relative to DMSO-treated control oocytes. 6e induced GVBD in the absence of progesterone at 100 and 50 μM, and to a lesser extent at 10 μM.
Compound 6e triggers progesterone-independent activation of endogenous MAP kinase and cyclin B/CDK1 signaling pathways (Fig. 4). Drug and control-treated oocytes were lysed in NP40 lysis buffer containing sodium vanadate and a protease inhibitor cocktail (Sigma).19 Protein lysates were then spun, clarified and transferred immediately to 1x sample buffer (Nupage). The lysates were run on a 10% Nupage gel and transferred to a 0.2 μm-pore-size nitrocellulose filter (Protran; Midwest Scientific). The membrane was blocked with 1% bovine serum albumin (Sigma) in TBST for 60 min at room temperature. The phospho-specific Cdc2 antibody (Cell Signaling) was used at 1:1000 and detects the inhibitory Tyr15 phosphorylation.
Figure 4.
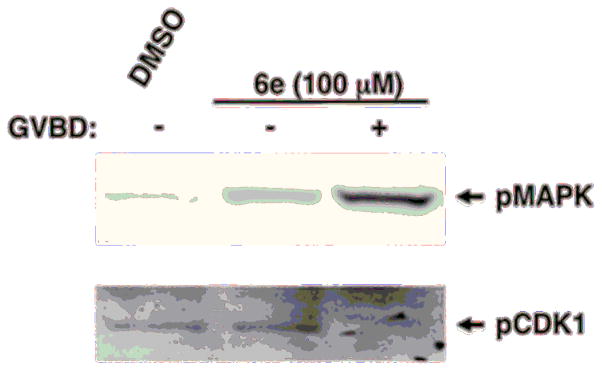
Compound 6e triggers progesterone-independent activation of endogenous signaling pathways. Oocytes treated with 6e were harvested when 50% of the oocytes completed GVBD; they were segregated into those which had not (−) or had (+) completed GVBD. 6e-treated oocytes which had not completed GVBD displayed activation of MAP kinase signaling above basal, but had not activated MPF (assessed by retention of the inhibitory phosphorylation of CDK1). After GVBD, 6e-treated oocytes showed augmented MAP kinase activation and de-phosphorylation and activation of MPF.
The phospho-specific MAP kinase antibody (Cell Signaling) was used at 1:1000 and detects the activating phosphorylations at Thr202/Tyr204. Following incubation with primary antibody, filters were incubated with horseradish peroxidase conjugated secondary antibody using enhanced chemiluminescence in a Fluorchem 8000 Advanced Imager (Alpha Innotech Corp).
Consistent with the sequence of signaling events in response to progesterone stimulation, oocytes treated with 6e displayed activation of MAP kinase signaling above basal levels in oocytes prior to GVBD, but these oocytes did not show activation of MPF (assessed by retention of the inhibitory phosphorylation of CDK1). After GVBD, 6e-treated oocytes showed augmented MAP kinase activation and de-phosphorylation and activation of MPF (Fig. 4).
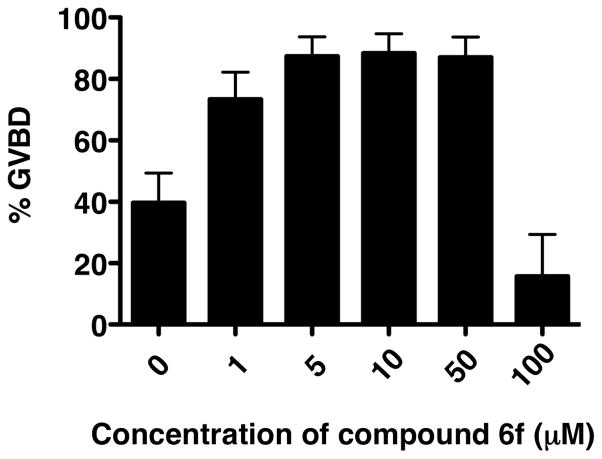
We next analyzed the inhibitory effect of 6f on progesterone-stimulated progression to GVBD (Fig. 5). We confirmed that 6f inhibits progesterone-stimulated maturation at 100 μM. However, at lower doses 6f was not inhibitory. In fact, 6f accelerated progesterone-stimulated maturation at lower doses, relative to DMSO-treated oocytes. 6f did not, however, induce maturation in the absence of progesterone.
Figure 5.
6f inhibits maturation at high concentration, but accelerates maturation at lower concentrations. The time-matched effects of varying 6f concentration on progesterone-stimulated maturation were assessed relative to DMSO-treated oocytes (0) and scored when oocytes treated with 50 μM 6f induced maximal GVBD. At 5, 10 and 50 μM, 6f doubled the rate of progression to GVBD. The mean values are plotted for three independent experiments.
In conclusion, heterocyclic aminoparthenolide derivatives have been synthesized by Michael addition chemistry and screened for modulatory effects on Xenopus oocyte maturation. Out of 12 compounds tested (6a-6l; Table 1), compound 6e was able to induce maturation in the absence of progesterone. This effect was observed over several doses and resulted in progesterone-independent activation of MAP kinase and MPF signaling. Compound 6f mediated inhibition of progesterone-stimulated maturation at 100 μM but mediated acceleration at lower doses with an EC50 of <5 μM. The other compounds did not exert any obvious phenotypic effects upon oocyte maturation.
Our results suggest that two PTL derivatives, 6e and 6f (at lower concentrations), can modulate cell cycle control at least in the Xenopus model system. It should be noted that while progesterone signaling triggers G2 arrested oocytes to re-enter the cell cycle, oocyte maturation culminates in cell cycle arrest at metaphase of meiosis II at which point it is competent to be fertilized.20 Key cell cycle regulatory proteins must be synthesized to mediate oocyte maturation, but the lack of transcriptional control21 suggests that 6e and 6f impinge on cellular signaling pathways that control maternal mRNA translation. Recent evidence suggests that regulated mRNA translation plays a key role in controlling stem cell growth and survival, and is thus an important, and underdeveloped therapeutic target for cancer control.22 Thus, while somewhat counter-intuitive, the ability of 6e and 6f to modulate Xenopus oocyte maturation may nonetheless indicate a potential to arrest or attenuate mammalian cell proliferation. Future studies will determine the ability of 6e and 6f attenuate mammalian cell proliferation and their cellular target(s) of action
Acknowledgments
We are grateful to the NIH for grant R01 CA158275 (P.A.C.); NIH grant RO1 HD35688, a Sturgis Diabetes Research Pilot Award, an Arkansas Breast Cancer Research Program award, the UAMS College of Medicine Research Council (to AMM); and a UAMS Translational Research Institute award supported by the NIH National Center for Research Resources grants UL1 TR0000039 and KL2TR000063 (to A.M.M. and P.A.C) for supporting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Knight DW. Nat Prod Rep. 1995;12:271. doi: 10.1039/np9951200271. [DOI] [PubMed] [Google Scholar]
- 2.Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. PLoS ONE. 2009;4:e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. FEBS Lett. 1997;402:85. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]; (b) Wen J, You KR, Lee SY, Song CH, Kim DG. J Biol Chem. 2002;277:38954. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]; (c) Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. J Biol Chem. 1998;273:1288. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]; (d) Sweeney CJ, Li L, Shanmugam R, Bhat-Nakshatri PB, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Clin Cancer Res. 2004;10:5501. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]; (e) Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Mol Cancer Ther. 2005;4:587. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]; (f) Nozaki S, Sledge GW, Nakshatri H. Oncogene. 2001;20:2178. doi: 10.1038/sj.onc.1204292. [DOI] [PubMed] [Google Scholar]
- 4.Nasim S, Pei S, Hagen FK, Jordan CT, Crooks PA. Bioorg Med Chem. 2011;19:1515. doi: 10.1016/j.bmc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Yaxin Lu, Ding Y, Zhai J, Ji Q, Ma W, Yang M, Fan H, Long J, Tong Z, Shi Y, Jia Y, Han B, Zhang W, Qiu C, Ma X, Li Q, Shi Q, Zhang H, Li D, Zhang J, Lin J, Li LY, Gao Y, Chen Y. J Med Chem. 2012;55:8757. doi: 10.1021/jm301064b. [DOI] [PubMed] [Google Scholar]
- 6.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. Blood. 2005;105:4163. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman ML, Jordan CT. Expert Opin Biol Ther. 2005;5:1147. doi: 10.1517/14712598.5.9.1147. [DOI] [PubMed] [Google Scholar]
- 8.Nasim S, Crooks PA. Bioorg Med Chem Lett. 2008;18:3870. doi: 10.1016/j.bmcl.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 9.James RW, Huaping M, Andrew AB, Tanja A, David AC. J Med Chem. 2011;54:7934. [Google Scholar]
- 10.Pei S, Minhajuddin M, Callahan KP, Balys M, Ashton JM, Neering SJ, Lagadinou ED, Corbett C, Ye H, Liesveld JL, O’Dwyer KM, Li Z, Shi L, Greninger P, Settleman J, Benes C, Hagen FK, Munger J, Crooks PA, Becker MW, Jordan CT. J Biol Chem. 2013;288:33542. doi: 10.1074/jbc.M113.511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
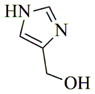
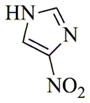
- 11.General synthetic procedure and characterization data for selected heterocyclic aminoparthenolide compounds. To a stirred solution of parthenolide (50 mg, 0.201 mmol) in methanol (2 mL), was added the appropriate heterocyclic amino compound (16.53 mg, 0.201 mmol). The reaction mixture was stirred at ambient temperature for 15h. After completion of the reaction (monitored by TLC), the reaction mixture was concentrated under reduced pressure to afford the crude compound, water (5 mL) was added to the crude compound and extracted with dichloromethane(2×5 mL). The organic layer was dried over Na2SO4 and concentrated to afford pure compound (6b) as white solid. 6a: 1H NMR (CDCl3, 400 MHz): δ 7.49 (s, 1H), 7.11 (s, 1H), 6.96 (s, 1H), 5.09 (d, J = 11.2 Hz, 1H), 4.55 (dd, J = 2.8, 14.4 Hz, 1H), 4.29 (dd, J = 4.8, 14.8 Hz, 1H), 3.87 (t, J = 8.4 Hz, 1H), 2.67-2.63 (m, 1H), 2.49 (d, J = 8.8 Hz, 1H), 2.37-2.25 (m, 2 H), 2.16-2.08 (m, 2H), 1.98-1.92 (m, 1H), 1.71-1.70 (m, 6H), 1.26 (s, 3H), 1.19-1.12 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 174.2, 137.6, 133.7, 130.5, 125.5, 119.5, 82.4, 65.9, 61.5, 49.1, 45.5, 43.4, 40.7, 36.3, 29.4, 23.9, 17.1, 16.8 ppm. 6b: 1H NMR (CDCl3, 400 MHz): δ 7.06 (s, 1H), 6.78 (s, 1H), 5.17 (d, J = 10 Hz, 1H), 4.36 (dd, J = 5.6, 15.2 Hz, 1H), 4.24 (dd, J = 6.8, 14.8 Hz, 1H), 4.04 (t, J = 9.2 Hz, 1H), 3.01-2.95 (m, 1H), 2.76 (d, J = 9.6 Hz, 1H), 2.40-2.29 (m, 4H), 2.15-1.97 (m, 4H), 1.89 (t, J = 12.4 Hz, 1H), 1.66-1.57 (m, 4H), 1.36 (dd, J = 6.4, 15.2 Hz, 1H), 1.20 (s, 3H), 1.15-1.07 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 175.7, 144.5, 134.6, 127.2, 124.9, 120.3, 82.0, 65.6, 61.6, 47.8, 46.7, 44.1, 40.7, 36.4, 28.9, 24.0, 17.2, 16.9, 13.2 ppm. 6c: 1H NMR (CDCl3, 400 MHz): δ 6.70 (s, 1H), 5.13 (d, J = 10.4 Hz, 1H), 4.24 (dd, J = 5.2, 14.8 Hz, 1H), 4.12 (dd, J = 6.4, 14.8 Hz, 1H), 4.00 (t, J = 8.8 Hz, 1H), 2.91-2.89 (m, 1H), 2.72 (d, J = 8.8 Hz, 1H), 2.63-2.58 (m, 2H), 2.32-2.29 (m, 1H), 2.08-1.94 (m, 7H), 1.84 (t, J = 12.4 Hz, 1H), 1.59-1.56 (m, 4H), 1.32 (dd, J = 5.6, 14.8 Hz, 1H), 1.21-1.08(m, 7H). 13C NMR (100 MHz, CDCl3): δ 175.3, 147.9, 135.0, 134.2, 124.5, 115.8, 81.6, 65.2, 61.2, 47.5, 46.3, 43.1, 40.2, 36.0, 28.6, 23.6, 19.2, 16.8, 16.5, 13.5, 12.2 ppm. 6d:1H NMR (CDCl3, 400 MHz): δ 7.58 (s, 2H), 7.24-7.22 (m, 2H), 5.05 (d, J = 11.3 Hz, 1H), 4.19 (s, 2H) 3.89 (t, J = 8.4 Hz, 1H), 3.11 (dd, J = 3.2, 12.8 Hz, 1H), 2.94 (dd, J = 6.0, 12.8 Hz, 1H), 2.69 (d, J = 8.8 Hz, 1H), 2.47-2.31 (m, 2H), 2.23-2.07 (m, 5H), 1.96 (t, J = 12.8 Hz, 1H), 1.84 (dd, J = 5.6, 15.2 Hz, 1H), 1.66-1.63 (m, 5H), 1.29 (s, 3H), 1.24-1.14 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 177.3, 153.9, 152.9, 134.4, 133.9, 125.3, 122.4, 82.8, 66.2, 61.8, 48.5, 47.6, 46.5, 45.9, 41.1, 36.6, 29.8, 24.2, 17.3, 16.9 ppm. 6e: 1H NMR (CDCl3, 400 MHz): δ 7.42 (d, J = 8 Hz, 1H), 7.137 (t, J = 8 Hz, 1H), 7.05 (t, J = 8 Hz, 1H), 6.99 (d, J = 8 Hz, 1H), 5,46 (brs, 1H), 5.02 (d, J = 12 Hz, 1H), 4.37-4.25 (m, 2H), 3.88 (t, J = 8 Hz, 1H), 2.79 (d, J = 12 Hz, 1H), 2.59 (d, J = 12 Hz, 1H), 2.36-2.27 (m, 2 H), 2.17-1.93 (m, 6H), 1.79-1.70 (m, 1H), 1.65 (s, 3H), 1,24 (s, 3H), 1.34-1.07 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 176.7, 154.6, 141.8, 134.7, 133.9, 125.9, 122.4, 120.1, 116.8, 107.2, 83.3, 65.6, 61.9, 48.8, 46.7, 40.7, 40.4, 36.4, 30.1, 24.1, 17.2, 16.9 ppm. 6f: 1H NMR (CDCl3, 400 MHz): δ 7.59 (s, 1H), 7.15 (s, 1H), 6.87 (s, 1H), 5.22 (d, J =10.4 Hz, 1H), 4.02 (m, 3H), 2.79 (d, J = 9.2 Hz, 3H), 2.45 (t, J = 6 Hz, 3H), 2.37-2.21 (m, 2H), 2.15-1.99 (m, 4H), 1.85-1.80 (m, 4H), 1.71-1.64 (m, 4H), 1.20 (s, 3H), 1.20-1.09 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 176.8, 137.2, 134.4, 128.3, 124.4, 119.3, 81.5, 65.4, 61.1, 47.5, 46.1, 46.0, 45.48, 43.8, 40.4, 36.1, 30.6, 28.9, 23.7, 16.8, 16.6 ppm. 6g: 1H NMR (CDCl3, 400 MHz): δ 7.56 (s, 1H), 7.35 (s, 1H), 5.13 (d, J = 12.8 Hz, 1H), 4.54 (dd, J = 3.2, 14.8 Hz, 1H), 4.32 (dd, J = 4.8, 15.2 Hz, 1H), 3.90 (t, J = 8 Hz, 1H), 2.71 (d, J = 10.8 Hz, 1H), 2.55 (d, J = 8.8 Hz, 1H), 2.38-2.30 (m, 2H), 2.18-2.10 (m, 2H), 1.96 (d, J = 10.8 Hz, 1H), 1.76-1.63 (m, 6H), 1.27 (s, 3H), 1.22-1.14 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 173.6, 138.4, 134.9, 133.4, 125.7, 122.4, 119.6 (q, JCF = 12 Hz, 1C) 82.4, 65.6, 61.5, 48.9, 45.8, 43.8, 40.6, 36.2, 29.2, 23.8, 17.0, 16.7 ppm. 6h: 1H NMR (DMSO-d6, 400 MHz): δ 7.71 (s, 1H), 6.85 (s, 1H), 5.21-5.13 (m, 2H), 4.48-4.39 (m, 3H), 4.27 (dd, J = 8, 14.8 Hz, 1H), 4.02 (t, J = 9.2 Hz, 1H), 3.17-3.08 (m, 1H), 2.77 (d, J = 8.8 Hz, 1H), 2.34-2.23 (m, 2H), 2.06 (t, J = 11.2 Hz, 2H), 1.85 (d, J = 6 Hz, 2H), 1.60-1.50 (m, 4H), 1.19-1.05 (m, 5H). 13C NMR (100 MHz, DMSO d6): δ 175.7, 138.9, 134.7, 132.1, 127.7, 124.6, 82.1, 65.6, 61.5, 52.7, 47.2, 47.0, 45.9, 43.8, 36.4, 29.0, 23.9, 17.0, 16.9 ppm. 6i: 1H NMR (CDCl3, 400 MHz): δ 7.84 (s, 1H), 7.49 (s, 1H), 5.14 (d, J = 11.2 Hz, 1H), 4.58 (dd, J = 2.8, 14.8 Hz, 1H), 4.35 (dd, J = 5.2, 14.8 Hz, 1H), 3.94 (t, J = 8.8 Hz, 1H), 2.77 (d, J = 6.4 Hz, 1H), 2.58 (d, J = 8.8 Hz, 1H), 2.38-2.32 (m, 2H), 2.18-2.09 (m, 2H), 2.02 (t, J = 9.6 Hz, 1H), 1.82-1.77 (m, 3H), 1.68 (s, 3H), 1.27 (s, 3H), 1.21-1.13 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 173.6, 148.5, 136.6, 133.4, 125.9, 119.7, 82.6, 65.6, 61.7, 48.8, 46.0, 44.8, 40.7, 36.2, 29.3, 23.9, 17.0, 16.7 ppm. 6j: 1H NMR (CDCl3, 400 MHz): δ 5.18 (d, J = 12.4 Hz, 1H), 4.93 (s, 1H), 4.33 (d, J = 14.8 Hz, 1H), 4.19 (d, J = 15.2 Hz, 1H), 3.94 (t, J = 9.2 Hz, 1H), 2.67 (d, J = 8.8 Hz, 2H), 2.51-2.08 (m, 9H), 1.71 (m, 3H), 1.58 (s, 3H), 1.29 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 176.6, 158.6, 156.3, 134.6, 125.0, 83.7, 65.9, 61.7, 48.7, 45.4, 42.9, 40.5, 36.5, 29.1, 24.0, 17.1, 16.9, 14.1 ppm.6l: 1H NMR (CDCl3, 400 MHz): δ 5.17 (d, J = 12 Hz, 1H), 3.85 (t, J = 8 Hz, 1H), 3.70 (t, J = 4 Hz, 4H), 3.00 (dd, J = 4, 12 Hz, 1H), 2.82-2.69 (m, 4 H), 2.49-2.02 (m, 15 H), 1.99-1.89 (m, 1H), 1.67 (s, 3 H), 1.26 (s, 3H), 1.19-1.18 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 176.6, 134.4, 125.1, 82.5, 66.9, 66.2, 61.4, 57.9, 53.6, 47.7, 47.5, 46.8, 46.4, 41.0, 36.5, 30.0, 24.0, 17.1, 16.8 ppm.
- 12.(a) Wheeler GN, Liu KJ. Genesis. 2012;50:207. doi: 10.1002/dvg.22009. [DOI] [PubMed] [Google Scholar]; (b) Cross MK, Powers MA. Dis Model Mech. 2009;2:541. doi: 10.1242/dmm.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tomlinson ML, Hendry AE, Wheeler GN. Methods Mol Biol. 2012;917:155. doi: 10.1007/978-1-61779-992-1_9. [DOI] [PubMed] [Google Scholar]; (d) Wheeler GN, Brandli AW. Dev Dyn. 2009;238:1287. doi: 10.1002/dvdy.21967. [DOI] [PubMed] [Google Scholar]
- 13.(a) Gaffre M, Martoriati A, Belhachemi N, Chambon JP, Houliston E, Jessus C, Karaiskou A. Development. 2011;138:3735. doi: 10.1242/dev.063974. [DOI] [PubMed] [Google Scholar]; (b) Schwab MS, Kim SH, Terada N, Edfjall C, Kozma SC, Thomas G, Maller JL. Mol Cell Biol. 1999;19:2485. doi: 10.1128/mcb.19.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Flament S, Bodart JF, Bertout M, Browaeys E, Rousseau A, Vilain JP. Zygote. 2000;8:3. doi: 10.1017/s0967199400000770. [DOI] [PubMed] [Google Scholar]; (d) Jessus C, Rime H, Haccard O, Van Lint J, Goris J, Merlevede W, Ozon R. Development. 1991;111:813. doi: 10.1242/dev.111.3.813. [DOI] [PubMed] [Google Scholar]; (e) Gard DL, Cha BJ, Roeder AD. Zygote. 1995;3:17. doi: 10.1017/s0967199400002331. [DOI] [PubMed] [Google Scholar]; (f) Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. Curr Biol. 2000;10:430. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]; (g) Bagowski CP, Myers JW, Ferrell JE., Jr J Biol Chem. 2001;276:37708. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]; (h) Huchon D, Ozon R. Reprod Nutr Dev. 1985;25:465. doi: 10.1051/rnd:19850311. [DOI] [PubMed] [Google Scholar]; (i) Keady BT, Kuo P, Martinez SE, Yuan L, Hake LE. J Cell Sci. 2007;120:1093. doi: 10.1242/jcs.03416. [DOI] [PubMed] [Google Scholar]
- 14.(a) Verma R, Peters NR, D’Onofrio M, Tochtrop GP, Sakamoto KM, Varadan R, Zhang M, Coffino P, Fushman D, Deshaies RJ, King RW. Science. 2004;306:117. doi: 10.1126/science.1100946. [DOI] [PubMed] [Google Scholar]; (b) Peterson JR, Lokey RS, Mitchison TJ, Kirschner MW. Proc Natl Acad Sci U S A. 2001;98:10624. doi: 10.1073/pnas.201393198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wignall SM, Gray NS, Chang YT, Juarez L, Jacob R, Burlingame A, Schultz PG, Heald R. Chem Biol. 2004;11:135. [PubMed] [Google Scholar]; (d) Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, Kopelovich L, Jasin M, Baer R, Paull TT, Gautier J. Nat Chem Biol. 2008;4:119. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, Langdon E, Tomlinson ML, Mosher J, Kaufman C, Chen F, Long HK, Kramer M, Datta S, Neuberg D, Granter S, Young RA, Morrison S, Wheeler GN, Zon LI. Nature. 2011;471:518. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dush MK, McIver AL, Parr MA, Young DD, Fisher J, Newman DR, Sannes PL, Hauck ML, Deiters A, Nascone-Yoder N. Chem Biol. 2011;18:252. doi: 10.1016/j.chembiol.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kalin RE, Banziger-Tobler NE, Detmar M, Brandli AW. Blood. 2009;114:1110. doi: 10.1182/blood-2009-03-211771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacNicol MC, MacNicol AM. 2010;77:662. doi: 10.1002/mrd.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machaca K, Haun S. J Cell Biol. 2002;156:75. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard EL, Charlesworth A, Welk J, MacNicol AM. Mol Cell Biol. 1999;19:1990. doi: 10.1128/mcb.19.3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNicol AM, Muslin AJ, Williams LT. Cell. 1993;73:571. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 20.Ferrell JJ. Bioassays. 1999;21:833. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.(a) Lasko P. Prog Mol Biol Transl Sci. 2009;90:211. doi: 10.1016/S1877-1173(09)90006-0. [DOI] [PubMed] [Google Scholar]; (b) Wickens M, Goodwin EB, Kimble J, Strictland S, Hentze MW. Translational Control of Developmental Decisions. In: Sonenberg N, Hershey J, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; New York, NY: 2000. pp. 295–370. [Google Scholar]
- 22.Grzmil M, Hemmings BA. Cancer Res. 2012;72:3891. doi: 10.1158/0008-5472.CAN-12-0026. [DOI] [PubMed] [Google Scholar]