Abstract
We evaluated whether the obesity-associated years of life lost (YLL) have decreased over calendar time. We implemented a meta-analysis including only studies with ≥2 serial BMI assessments at different calendar years. For each BMI category (normal weight: BMI 18.5 to <25 [reference], overweight: BMI 25 to <30, grade 1 obesity: BMI 30 to <35, and grade 2–3 obesity: BMI ≥35), we estimated the YLL change between 1970 and 1990. Due to low sample sizes for blacks, results are reported on whites. Among men aged≤60 years YLL for grade 1 obesity increased by 0.72 years (p<0.001) and by 1.02 years (p=0.01) for grade 2–3 obesity. For men aged>60, YLL for grade 1 obesity decreased by 1.02 years (p<0.001), and increased by 0.63 years for grade 2–3 obesity (p=0.63). Among women aged≤60, YLL for grade 1 obesity decreased by 4.21years (p<0.001) and by 4.97 years (p<0.001) for grade 2–3 obesity. In women aged>60, YLL for grade 1 obesity decreased by 3.98 years (p<0.001) and by 2.64 years (p=0.001) for grade 2–3 obesity. Grade 1 obesity’s association with decreased longevity has reduced for older white men. For white women, there is evidence of a decline in the obesity YLL association across all ages.
Keywords: Body mass index (BMI), years of life lost (YLL), parametric survival, meta-analysis, calendar time, maturation, recency, length of follow-up, study-level variation, recency
Introduction
Obese individuals (body mass index [BMI, kg/m2] ≥ 30) are more prone to many adverse medical conditions, such as cardiovascular disease, stroke, type 2 diabetes mellitus, and some cancers, and have increased mortality risk from multiple causes (e.g., heart disease and colon cancer)(1, 2). However, data from the National Health and Nutrition Examination Survey (NHANES) I (1971–1975), NHANES II (1976–1980), and NHANES III (1988–1994) have suggested that the BMI-mortality association may have decreased over calendar time (3). Mehta and Chang, using data from the Framingham Heart Study, the National Health Interview Survey , and NHANES, also concluded that the excess all-cause mortality associated with grade 1 obesity has declined over calendar time (4). Gregg et al. (5) found that, compared with obese persons in the 1960s, those in 1999–2000 had significantly lower levels of cholesterol, hypertension, and smoking (32% vs. 20%). A recent meta-analysis based on U.S. as well as non U.S. data present evidence that the excess mortality risk associated with grade 1 obesity is not statistically significant (6). However, other studies (7–12) have found that the obesity-mortality association has not decreased in recent years. A declining association would imply that years of life lost (YLL) associated with obesity have reduced (13, 14). This declining association, if present, would have substantial public health implications. A temporal decline in YLL estimates associated with obesity might suggest that advances in medicine, such as improved screening and interventions for obesity-associated diseases, have been at least somewhat successful in reducing obesity’s detrimental health effects. Advances in medicine would not be, however, the only possible explanation.
Following the Institute of Medicine’s suggestion (15), we conducted a thorough analysis of data from 17 large, well-characterized U.S. prospective studies to examine whether there is a decline in the obesity-YLL association
Materials and Methods
Study Selection and Data Extraction
Inclusion criteria were: (a) prospective U.S. based cohort studies with at least 1500 participants; (b) mortality follow-up ≥ 5 years; (c) an accumulation of at least 500 deaths; (d) BMI (measured or self-reported) available at ≥2 separate time points; and (e) subjects varied in age at baseline (this last criterion allowed us to construct two different waves matched for baseline age within each study).
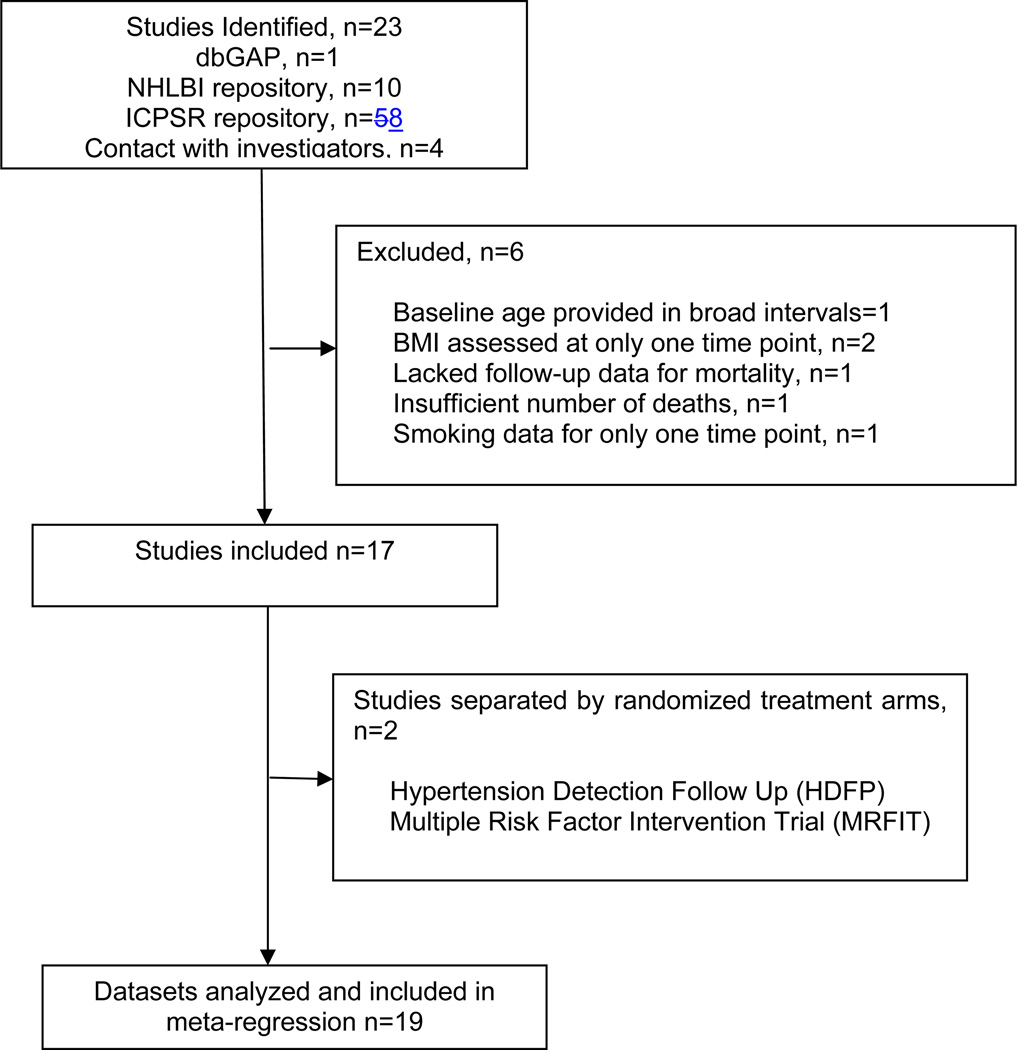
Studies (16–32) were identified from searches via Medline, PubMed, Scopus, Web of Science, Journal Citation Reports, and Biological Abstracts (Figure 1). Individual-level data for these studies were acquired from repositories of the National Heart, Lung and Blood Institute (NHLBI), Database for Phenotype and Genotype, Inter-University Consortium for Political and Social Research (ICPSR), and direct contact with investigators. We considered the 4 study sites of Established Populations for Epidemiologic Studies of the Elderly (EPESE) as different studies. Data for the Multiple Risk Factor Intervention Trial (MRFIT) and Hypertension Detection Follow Up (HDFP) consisted of a control group and an interventional group. Hence, we split these studies into 2 separate cohorts (see Glossary in the Supporting Information), leading to a total of 19 separate prospective studies for the meta-analysis (see Table 1).
Figure 1. Selection of Prospective Studies.
U.S. based prospective studies with serial BMI measurements were searched and individual-level data collected.
Table 1.
Characteristics of Prospective Studies
| Dataset | Study Dates |
Sample Size |
Total Death |
% Female |
Age (Years) |
Total Waves |
BMI | Source |
|---|---|---|---|---|---|---|---|---|
| Hypertension Detection Follow-Up (HDFP)–Referred Care (RC)(22) | 1973–1982 | 5,334 | 789 | 45.8 | 30–69 | 3 | M | NHLBI |
| Hypertension Detection Follow-Up (HDFP)–Stepped Care (SC)(22) | 1973–1982 | 5,512 | 709 | 46.0 | 30–69 | 3 | M | NHLBI |
| Multiple Risk Factor Intervention Trial (MRFIT)–Referred Group(21) | 1973–1985 | 6,416 | *496 | 0.0 | 35–57 | 7 | M | NHLBI |
| Multiple Risk Factor Intervention Trial (MRFIT)–Usual Group(21) | 1973–1985 | 6,429 | 537 | 0.0 | 35–57 | 7 | M | NHLBI |
| Alameda County [California] Health and Ways of Living Study (ACHWL)(23) | 1965–2000 | 5,956 | 3,095 | 53.8 | 18–85 | 2 | SR | ICPSR |
| Framingham Offspring Study(19) | 1971–2009 | 5,010 | 1,111 | 51.6 | 39–70 | 8 | M | dbGap |
| Atherosclerosis Risk in Communities (ARIC)(28) | 1987–2002 | 15,581 | 2,362 | 55.1 | 45–64 | 4 | M | NHLBI |
| Cardiovascular Health Study(17) | 1989–2002 | 5,770 | 2,634 | 57.4 | 65–90 | 6 | M | NHLBI |
| Tecumseh County Health Study (TCH)(26, 27) | 1959–1979 | 4,647 | 971 | 52.1 | 18–92 | 3 | M | ICPSR |
| Established Populations for Epidemiologic Studies of the Elderly (EPESE)–East Boston(25) | 1982–1993 | 3,431 | 1,633 | 60.4 | 65–100 | 4 | SR | ICPSR |
| Established Populations for Epidemiologic Studies of the Elderly (EPESE)–Duke(25) | 1986–1999 | 3,497 | 1,968 | 62.6 | 64–99 | 2 | SR | ICPSR |
| Established Populations for Epidemiologic Studies of the Elderly(EPESE)–New Haven(25) | 1982–1992 | 2,468 | 1,355 | 55.9 | 65–99 | 3 | SR | ICPSR |
| Established Populations for Epidemiologic Studies of the Elderly (EPESE)–Iowa(25) | 1982–1994 | 2,997 | 1,487 | 62.6 | 65–101 | 3 | SR | ICPSR |
| Framingham Heart Study(16) | 1948–2003 | 2,951 | 2,671 | 22.4 | 29–62 | 21 | M | NHLBI |
| Panel Study Income Dynamics (PSID)(29) | 1986–2007 | 7,936 | 2,077 | 57.5 | 18–98 | 4 | SR | PI |
| Charleston Heart Study(29) | 1960–2000 | 2,177 | 1,646 | 52.1 | 26–97 | 4 | M | ICPSR |
| Nurses’ Health Study II | 1989–2010 | 115066 | 2,468 | 100.0 | 25–42 | 10 | SR | PI |
| Nurses’ Health Study(18) | 1976–2008 | 109112 | 23,077 | 100.0 | 29–63 | 16 | SR | PI |
| Rancho Bernardo Heart Study (RBHS)(31, 32) | 1988–2008 | 1,692 | 887 | 59.3 | 44–98 | 4 | M | PI |
A total of 19 datasets were extracted from 17 prospective U.S. studies. Descriptive measures were computed after excluding subjects < 18 years old, excluding subjects with any missing mortality or covariate data, excluding pregnant women where known, and excluding subjects with BMI <14 and BMI >100. The Hypertension Detection Follow Up (HDFP) and Multiple Risk Factor Intervention Trial (MRFIT) cohorts were split into two different cohorts. In the BMI column, “M” stands for measured and “SR” stands for self-reported BMI. In the Source column, “PI” stands for principal investigators.
The inclusion criteria were applied at the study level. MRFIT-Referred group had <500 deaths however the total number of deaths in MRFIT study is >500.
Individual-level data for two waves from each study were extracted to account for stable study-level factors. From each study wave, we extracted information on BMI, sex, baseline age, race, smoking status, and all-cause mortality. BMI values within each study were divided into 5 categories: underweight (BMI <18.5), normal weight (BMI 18.5 to <25 [reference category]), overweight (BMI 25 to <30), grade 1 obesity (BMI 30 to < 35), and grade 2–3 obesity (BMI ≥35), consistent with Flegal et al. (3) and federally defined categories (33). Race was coded as white, black, and other. Smoking was coded as former smoker, never smoker, and current smoker.
Rationale for Setting Up Successive Waves Within Each Study
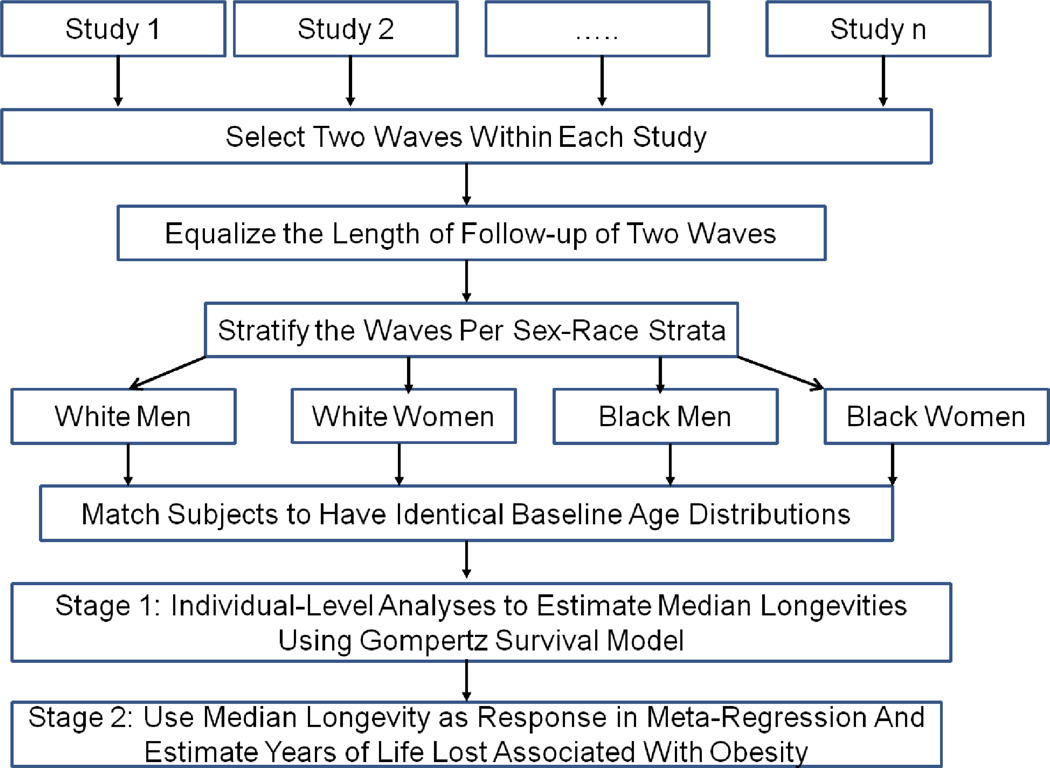
Any changes in the obesity-YLL association might be caused by 1) calendar time (history); 2) the modifying effects of an age-by-BMI interaction (maturation); 3) factors that varied across the different studies (study level variation); 4) the possibility that one’s BMI in the recent past has a different effect than one’s BMI in the distant past (recency); and 5) varying lengths of follow-up (see Glossary and Design Rationale in the Supporting Information) (34). We implemented a meta-analysis based on a multiple longitudinal design by including only datasets with serial BMI measurements and overlapping ages at two different calendar times within a study (Figure 2) (35). This design allows us to disentangle 4 of these 5 possible causes for an observed change in the obesity-YLL association: history, maturation, recency, and stable study-level factors (see Design Rationale in the Supporting Information) (36).
Figure 2. Analytic Pipeline.
Waves within a study were set up to be identical in terms of length of follow-up, baseline age distribution, race and sex.
To control for confounding due to length of follow-up, each wave within a study was restricted to have equal length of follow-up. Using the serial BMI assessments available within each study, we subdivided each study into two distinct “waves” at different points in calendar time (Figure 2), with each wave additionally stratified by race and sex (white men, white women, black men, and black women). To control for confounding due to maturation, subjects within each study specific race-sex strata were age-matched across the two waves so as to ensure an identical baseline age distribution (see Methods in the Supporting Information). Descriptive measures across all study waves following this matching procedure are provided in Table 2. Because the sample sizes and number of deaths among black men and women were insufficient after age matching, we report only the results for white respondents (see Table 2).
Table 2.
Post Age Matching Summary Measures Across All the US Prospective Cohort Studies
| White Men | White Women | |||
| Wave 1 | Wave 2 | Wave 1 | Wave 2 | |
| Sample Size | 12031 | 12031 | 62526 | 62526 |
| Deaths | 1955 | 1646 | 4679 | 3640 |
| Baseline Ages [25th, 50th, 75th percentile] | [46, 53.1, 59.6] | [45.5, 53.1, 59.6] | [40.2, 47.8, 52.4] | [40.1, 47.9, 52.5] |
| Calendar Time [25th, 50th, 75th percentile] | [1973, 1979, 1988] | [1976, 1989, 1992] | ||
| Black Men | Black Women | |||
| Wave 1 | Wave 2 | Wave 1 | Wave 2 | |
| Sample Size | 1748 | 1748 | 2308 | 2308 |
| Deaths | 265 | 256 | 258 | 258 |
| Calendar Time [25th, 50th, 75th percentile] | [1973, 1982, 1989] | [1974, 1983, 1989] | ||
Primary Analysis
We used a two-stage longitudinal meta-regression to test the hypothesis that calendar time was associated with changes in the obesity-YLL association (Figure 2 and Design Rationale in the Supporting Information). Separate analyses were conducted for white men and women. In stage 1, separately for every wave of a study, we estimated median longevities (i.e., median age at death) using a parametric Gompertz survival model. Gompertz distribution is widely used to model mortality data and is suitable for modeling data with monotone hazard rates that either increase or decrease exponentially with time (37, 38). The Gompertz distribution is based on the Gompertz law, which dictates that the force of mortality is exponential. Hence application of the Gompertz law on all-cause mortality implies that the probability of a person dying increases at a constant exponential rate as age increases (39, 40). Moreover, parametric survival models are able to provide more direct answers and consistent YLL estimates regardless of the particular distribution assumed (41). We computed median longevities for each subject in a study wave using age as time scale and including baseline age, BMI categories, and smoking status as predictors in the model (3, 42, 43). Delayed entry was accounted for by left truncating the survival observation period for each participant at the age they entered the study wave (43–45). Our survival model, which included time varying coefficients for BMI categories, allowed the hazard ratio (HR) of each BMI category (relative to normal weight individuals) to vary in three attained age intervals (≤60, >60 and ≤70, >70) similar to previous NHANES analyses (3, 38). All survival analyses were performed using Stata 12. Because the PSID, EPESE-Duke, and EPESE-New Haven datasets involved complex sampling designs, sample weights were utilized for these datasets.
In stage 2, a meta-regression was used to estimate the change in YLL over calendar time. The normal weight category was used as a reference when estimating YLL at a given calendar time. We fitted four separate longitudinal mixed models to estimate the changes in YLL across calendar time using linear contrasts: white men with baseline ages≤60, white men baseline with ages>60, white women baseline with ages≤60 and white women with baseline ages>60. YLL estimates associated with BMI category vary across baseline ages (12–14). Hence we summarized the changes in YLL for two baseline age strata within men and women: younger or middle-aged adults (baseline ages≤60) and older adults (baseline ages>60), similar to Greenberg (12). In each of these meta-regression models the estimated median longevities derived in stage 1 were regressed on BMI category, baseline age, calendar time, smoking status, and a BMI category by calendar time interaction. To account for the heterogeneity or systematic differences in the level of median longevities across studies at any calendar time, we also included "study" as a random effect (using the MIXED procedure in SAS 9.2). Moreover, to account for differences in the precision of the estimated median longevities, the inverse of the sampling variance of the estimated median longevities was used as weights in the mixed model. Finally, we excluded the underweight category in stage 2 because of the sparseness of data in this category and since this category is not the focus of our paper.
Sensitivity Analyses
We conducted two sensitivity analyses: (1) to assess the influence of individual studies on the estimates, we repeatedly fit the stage 2 model after removing each study one at a time, and (2) we modeled for nonlinear changes by fitting polynomial models that included quadratic terms for the calendar time by BMI category interactions. For additional methodological details, see Methods in the online only Supporting Information.
Results
Table 2 shows the descriptive measures of waves across all the studies. The waves within each study were identical in terms of baseline age distribution, sample-size, and length of follow-up. The differences in the sample size and number of deaths of white men and women were due to the inclusion of Nurses’ Health and Nurses’ Health II studies. While the majority of our white subjects were middle-aged adults, the age range included younger (age<40) as well as older adults (age>60).
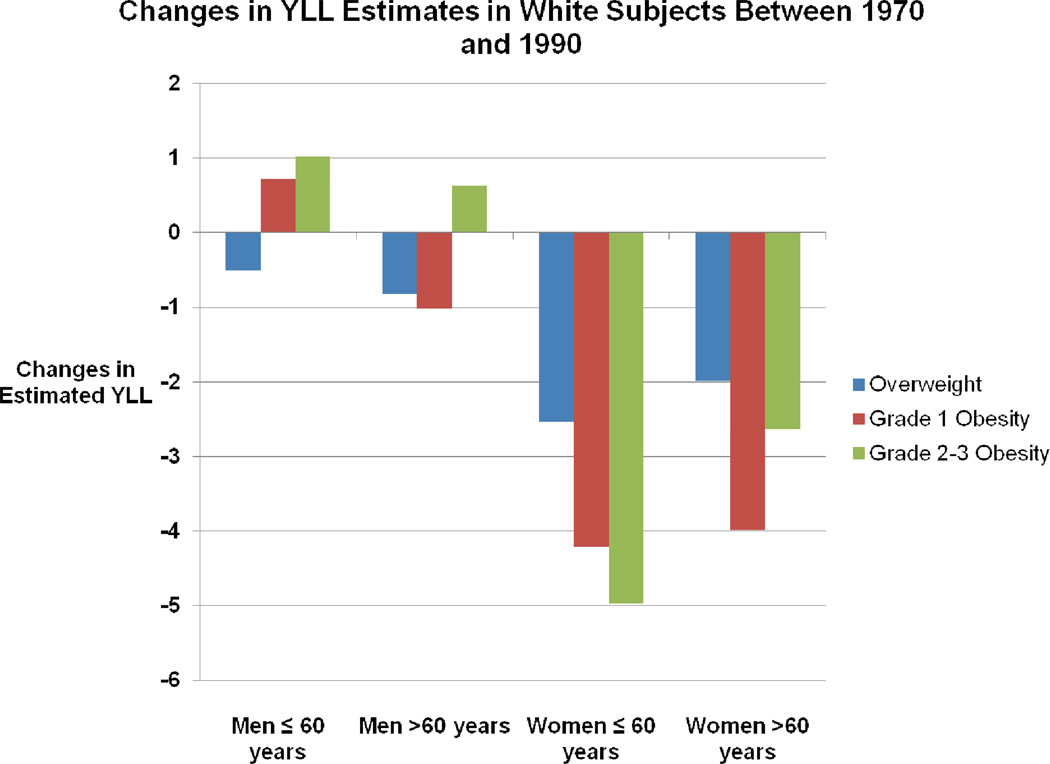
The results of our primary analysis are presented in Figure 3 and in detail in Table 3. YLL estimates for a given calendar year (e.g. 1970) were estimated relative to the normal weight as reference category. Briefly, in comparing YLL estimates between the years 1970 and 1990, we found that among white men ≤ 60, the estimates went down for overweight subjects, but up for obese subjects. Among white men >60, the YLL estimates decreased across overweight and grade 1 obesity categories but increased (though not statistically significantly) for those in the grade 2–3 category. Note that when a YLL estimate is said to decrease for a BMI category, this means a less deleterious association if being in that BMI category (relative to the reference category) with longevity. All changes, either positive or negative, among the white male subjects were fairly modest, with the largest being just over 1 YLL. Among women, the differences were more pronounced. Again comparing YLL estimates between 1970 and 1990, we found that among white women ≤ 60, YLL decreased quite markedly: overweight, 2.53 (p<0.001); grade 1 obesity, 4.21(p<0.001); and grade 2–3 obesity, 4.97 (p<0.001). Among white women >60, the data also show substantial YLL reductions: overweight, 1.99 (p<0.001); grade 1 obesity, 3.98 (p<0.001); and grade 2–3 obesity, 2.64 (p=0.001).
Figure 3. Stage 2 Meta-Regression Primary Analysis Results.
Changes in the years of life lost (YLL) between 1970 and 1990 estimated from primary analysis.
YLL were estimated relative to the normal weight. The negative value on the vertical axis indicates that the YLL estimate declined between 1970 and 1990. For example, the YLL associated with grade 2–3 obese white women aged > 60 years decreased by approximately 5 years between 1970 and 1990. All the changes were statistically significant at an alpha level of 0.05 except for the increase in the YLL of grade 2-3 obese men aged >60 years. See Table 3 for additional details.
Table 3.
Stage 2 Meta-Regression Results
| Group | BMI Category | Calendar Time |
Primary Analysis:
Only Linearity in Calendar Time |
Sensitivity Analysis: Model
with Non-Linearity in Calendar Time |
||
|---|---|---|---|---|---|---|
| YLL | Difference | YLL | Difference | |||
| White Men Aged ≤60 | Overweight | 1970 | 0.01 (−0.14, 0.16) |
−0.52 (p=0.001) |
0.00 (−0.144, 0.15) |
−0.82 (p<0.001) |
| 1990 | −0.51 (−0.75, −0.26) |
−0.82 (−1.16, −0.48) |
||||
| Grade 1 Obesity | 1970 | 0.48 (0.26, 0.71) |
0.72 (p<0.001) |
1.11 (0.86, 1.37) |
0.19 (p=0.42) |
|
| 1990 | 1.20 (0.89, 1.51) |
1.30 (0.98, 1.63) |
||||
| Grade 2–3 Obesity | 1970 | 3.00 (2.47, 3.52) |
1.02 (p=0.01) |
3.44 (2.73, 4.16) |
0.42 (p=0.43) |
|
| 1990 | 4.01 (3.51, 4.52) |
3.87 (3.34, 4.40) |
||||
| White Men Aged>60 | Overweight | 1970 | 0.61 (0.33, 0.89) |
−0.82 (p<0.001) |
0.29 (0.01, 0.56) |
−0.46 (p=0.002) |
| 1990 | −0.20 (−0.31, −0.10) |
−0.17 (−0.27, −0.07) |
||||
| Grade 1 Obesity | 1970 | 1.26 (0.73, 1.80) |
−1.02 (p<0.001) |
1.72 (1.15, 2.30) |
−1.43 (p<0.001) |
|
| 1990 | 0.24 (0.05, 0.43) |
0.29 (0.10, 0.47) |
||||
| Grade 2–3 Obesity | 1970 | −0.14 (−2.53, 2.25) |
0.63 (p=0.63) |
2.40 (−0.32, 5.12) |
−1.78 (p=0.22) |
|
| 1990 | 0.49 (0.08, 0.91) |
0.62 (0.21, 1.02) |
||||
| White Women Aged≤ 60 | Overweight | 1970 | 2.84 (2.78, 2.90) |
−2.53 (p<0.001) |
2.84 (2.76, 2.92) |
−2.57 (p<0.001) |
| 1990 | 0.31 (0.26, 0.36) |
0.27 (0.22, 0.32) |
||||
| Grade 1 Obesity | 1970 | 7.31 (7.20, 7.41) |
−4.21 (p<0.001) |
6.08 (5.92, 6.24) |
−2.77 (p<0.001) |
|
| 1990 | 3.10 (3.03, 3.17) |
3.31 (3.24, 3.39) |
||||
| Grade 2–3 Obesity | 1970 | 11.19 (10.99, 11.40) |
−4.97 (p<0.001) |
8.01 (7.73, 8.30) |
−1.04 (p<0.001) |
|
| 1990 | 6.22 (6.13, 6.32) |
6.97 (6.97, 7.07) |
||||
| White Women Aged>60 | Overweight | 1970 | 1.57 (1.23, 1.91) |
−1.99 (p<0.001) |
−0.44 (−0.79, −0.09) |
0.56 (p=0.007) |
| 1990 | −0.41 (−0.56, −0.26) |
0.12 (−0.03, 0.28) |
||||
| Grade 1 Obesity | 1970 | 3.87 (3.27, 4.47) |
−3.98 (p<0.001) |
1.99 (1.42, 2.57) |
−1.57 (p<0.001) |
|
| 1990 | −0.11 (−0.36, 0.13) |
0.42 (0.16, 0.67) |
||||
| Grade 2–3 Obesity | 1970 | 3.37 (2.16, 4.57) |
−2.64 (p=0.001) |
1.49 (0.36, 2.62) |
−0.20 (p=0.80) |
|
| 1990 | 0.72 (−0.13, 1.57) |
1.29 (0.43, 2.16) |
||||
YLL estimates based on the stage 2 meta-regression models are reported at calendar times 1970 and 1990. YLL were estimated relative to the normal weight. The change in YLL estimates across these calendar times and the corresponding p-values indicate whether within a group the deleterious association of an elevated BMI category to increased mortality has decreased over calendar time. A negative value in the difference estimated indicates that the YLL associated with the respective BMI category has declined between 1970 and 1990.
Presented in this way, the results are quite striking; however, our sensitivity analyses also raise some caveats. For example, among white men, our results were sensitive to the inclusion of the ARIC dataset. Specifically, in white men aged ≤60, the YLL increase of 0.72 years (p<0.001) for grade 1 obesity from 1970 to 1990 changed to a decrease of 2.97 years (p<0.001) when this dataset was excluded (1.43 [95% CI, 1.15 to 1.71] in 1970 and −1.55 [95% CI, −2.14 to −0.95] in 1990).
Similarly, in white women aged≤60, our results were particularly sensitive to the inclusion of Nurses’ Health Study (NHS). After excluding the NHS, from 1970 to 1990, the YLL associated with overweight and grade 2–3 obesity increased 2.74 (p<0.001) and 0.72 years (p=0.002), respectively. For grade 1 obesity the YLL declined by 0.02 years (p=0.92). In 1970, the YLL for overweight, grade 1 obesity and grade 2–3 obesity were −0.89 (95% CI, −1.02 to −0.74), 2.42 (95% CI, 2.2 to 2.66) and 2.61 (95% CI, 2.18 to 3.04), respectively. In 1990, the YLL for overweight, grade 1 obesity and grade 2–3 obesity had changed to 1.86 (95% CI, 1.75 to 1.97), 2.40 (95% CI, 2.25 to 2.56) and 3.33 (95% CI, 3.15 to 3.5), respectively.
We assessed the robustness of our primary analysis findings by allowing YLL changes to follow a non-linear pattern (Table 3). We found that our inferences about a decline in the YLL associations of grade 2–3 obesity and overweight categories in white women aged>60 were sensitive to the assumption of non-linearity. In white women aged>60, the decrease in YLL associated with grade 2–3 obesity, from 1970 to 1990, was only 0.20 (p=0.80) as against 2.64 (p=0.001) in the primary analysis. From 1970 to 1990, the decrease in YLL of 1.99 (p<0.001) associated with overweight category in the same group was reversed to an increase in YLL of 0.56 (p=0.007).
Discussion
We evaluated whether the detrimental association of obesity to YLL has declined over calendar time, between 1970 and 1990, after adjusting for confounding due to age-related effect modification, length of follow-up, recency and stable study level factors (Design Rationale in the Supporting Information). We found evidence of a consistent decline in the association of grade 1 obesity to YLL except in men aged≤60. This association remained deleterious in 1990 with YLL’s of < 1.5 in men and <3.5 in women. The decline in the association of grade 2–3 obesity to YLL across younger and middle-aged subjects was inconsistent. There was a statistically significant decline in women but not in men. Despite this decline, YLL estimates for grade 2–3 obesity in younger and middle-aged subjects remained elevated even in 1990. In 1990, compared to normal weight, grade 2–3 obese men could lose up to 4 years while women could lose up to 7 years. Any deleterious association of overweight to mortality existing in 1970 declined over time, and in some subjects, overweight was associated with decreased mortality relative to normal weight. In 1990, the most deleterious association of overweight we found was in women aged≤60 with a YLL of 0.31.
Sensitivity analyses revealed that our findings for women aged≤60 years were sensitive to the NHS. NHS, which has an especially large sample size and long follow-up, contributes to the majority of deaths in the meta-analysis and, thereby, greatly influences the findings for white women. Excluding NHS from the stage 2 meta-regression resulted in moderate but statistically significant increases in the YLL estimates of overweight and grade 2–3 obesity from 1970 to 1990 in contrast to the decrease in YLL observed in the primary analysis findings. NHS is a cohort of white registered nurses who seem to have lifestyles similar to the general population (46). Hence, it is plausible that inferences drawn without NHS are based on substantially lower number of person years and deaths. It is also plausible that nurses may have witnessed substantial improvements related to calendar time events such as better access to health care and lifestyle changes. Our grade 1 obesity findings among men aged≤60 were influenced by ARIC, a middle aged cohort with calendar times 1988 and 1994. The magnitude of increase in the median longevity of grade 1 obese men aged≤60 over calendar time was substantially greater when ARIC was excluded compared to the primary analysis where ARIC was included. The increase in median longevity of subjects in other BMI categories when ARIC was excluded was comparable to the findings in primary analysis. Hence, there was a statistically significant decline in the YLL associated with grade 1 obesity over calendar time when ARIC was excluded. Some of our findings were sensitive to the assumption of linear change in YLL over time. Interestingly, the NHANES analyses also seem to illustrate a non-linear pattern in the change in HRs especially in the ages 25–59 years as indicated in figure 1 of Flegal et al. (3).
Our estimates are generally consistent with those derived by Finkelstein and colleagues, who estimated YLL associated with overweight and obese categories, for white men and women, using the National Health Interview Survey respondent data from calendar times 1987 to 2000 (13). Finkelstein and colleagues reported ≤ 1 YLL for grade 1 obese white men while for white women their estimate was as high as three YLL. They also reported that YLL estimates for overweight were negative although not statistically significant. Our YLL estimates for 1990, except for white women aged ≤60 years, also suggest that overweight is no longer associated with elevated mortality or has a mildly protective association with all-cause mortality. Our findings were based on the standard normal weight category (BMI 18.5 to <25) while Finkelstein and colleagues used a narrower reference category (BMI 21 to <25). This suggests that our findings may be robust to the choice of reference category, a concern which has been recently cited in reporting results about broad BMI categories (6, 47). We used the broader category to allow comparability to the previous analysis of NHANES by Flegal et al. (3) which suggested that the deleterious association of obesity had reduced over calendar time. That said, we recognize and accept the value of the narrower BMI reference category. A subsequent study by Mehta and Chang(4) found that the grade 1 obesity-mortality association had declined and was not associated with increased mortality in the later time period. However, unlike Flegal et al. (3), they reported a lack of evidence for decline in the grade 2–3 obesity-mortality association. Both of these studies provide HR estimates for calendar times of BMI measured approximately in 1970s and 1990s. Our findings, which provide YLL estimates for whites only, are at least in partial agreement though not directly comparable. In contrast to Mehta and Chang’s findings (4), which were not specific to any race-sex strata, our estimates indicate that a deleterious association of grade 1 obesity to mortality for younger and middle-aged women remained in 1990. Again not directly comparable, the pattern of our YLL estimates seem to be in accord with the results recently reported by Greenberg for adults aged ≤ 55 years (12). Greenberg analyzed NHANES I, II and III data to estimate the extent to which overweight and obesity hastened mortality among young and middle-aged adults using a measure similar to YLL (12).
Our study has several strengths. This longitudinal meta-analysis used individual-level data and allowed for fitting consistent models to each dataset. To our knowledge this is the first study to estimate changes in obesity-mortality association over calendar time after accounting for recency, length of follow-up and other stable study level confounders (36). We used two waves per study which had equal length of follow-up, identical baseline ages of non-overlapping samples, and homogeneity in race and sex. This is also the first study to evaluate this question directly in terms of YLL and provide estimates specifically for whites. While HRs within an attained age category are useful measures, changes in YLL are directly interpretable and, from a public health perspective, potentially more useful (48). Interestingly, this was a metric Linus Pauling himself used to quantify the association of obesity with lifespan back in 1958 (49). We also followed upon Sir David Cox’s recommendation to use a parametric survival model when predicting outcomes specific to a subject (50). Our survival model in stage 1 allowed the HRs for BMI categories to change over three attained age intervals. The datasets possessed varying demographic characteristics and represented different geographical locations in the U.S. and covered a broad range of calendar time with varying and substantial lengths of follow-up, some over 15 years.
This study also had limitations, many of which involve logistical and data management issues. Due to the varying nature of questionnaires across waves within studies and to maintain analytic consistency, we did not include covariates such as physical activity, alcohol consumption, nutrition and other predictors that may improve predicting mortality in presence of BMI (47, 51). We used a two-stage analysis, pooling median longevity estimates from individual waves, instead of a single stage approach pooling all data and estimating all model parameters at once. The choice to follow a two-stage approach was driven by data sharing issues (e.g., not having permission to download mortality follow-up data on our server), which prevented us from pooling the mortality data across all the studies. However, there is reason to believe that our two-stage approach should yield similar results to a single-stage approach (52). Finally, the relative dearth of data on minority respondents prohibited us from deriving reliable estimates for non-whites.
Our findings concerning overweight and grade 1 obesity are potentially significant to our understanding of obesity and public health. One of the possibilities suggested, but not proven, through our findings is that advances in medicine, screening procedures and interventions may have reduced the excess mortality associated with overweight and grade 1 obesity. For example, Young et al. (53) estimated that half of the reduction in coronary heart disease (CHD) mortality in the US between 1980 and 2000 can be attributed to reductions in CHD risk factors. Our study findings also found differences in the YLL estimates and the reductions in YLL over calendar time between men and women. It has been reported that women with diabetes are at greater risk of cardiovascular disease (CVD) and CHD than men compared to their counterparts without diabetes mellitus (54, 55). The differences in YLL of obesity in men versus women could perhaps be explained by these reported differences in CVD and CHD risk. The decreases in YLL in women also suggest gender disparities and gaps in the quality of care may be reducing. Recent data suggests that women tend to use significantly more health care services and receive better preventive care especially in therapeutic areas such as CVD (56).
Despite the apparent medical advances in treating weight-related health risk factors, it is important to note that the disability burden of obesity may still be increasing (57, 58). Moreover, any potential reduction in the grade 1 obesity-mortality association is likely to increase healthcare costs (59, 60). The YLL associated with grade 2–3 obesity appear to remain elevated in younger and middle-aged subjects. The YLL associated with grade 2–3 obesity in men may have actually increased over time. One explanation could be due to the altered BMI distribution within this broad and open ended category over calendar time. Given that the U.S. population is getting heavier in general and the grade 2–3 obesity category includes a lower BMI limit but no upper limit, it is plausible that the mean BMI of this category in men has increased, perhaps markedly, over calendar time. However, grade 2–3 obesity is also associated with higher incidences of diabetes and other metabolic syndromes (61). Recently, Stokes and Mehta found that the deleterious dysglycemia-mortality association had not declined between the periods of 1988–2001 and 1999–2006 (62). Finally, the sensitivity of some of our findings suggests caution in interpreting results of purported changes in the association of BMI categories with functions of mortality over calendar time. These relationships may vary by study factors that are difficult to estimate stably such as the shifting of BMI distributions over time (across two waves within a study), changes in smoking prevalence over time and differences in survey instruments over time. Future studies may also focus on further identifying specific behavioral or medical practices or other environmental circumstances that may have contributed to reductions in the association of grade 1 obesity to mortality.
Supplementary Material
Acknowledgments
Funding/Support: This project was supported in part by NIH grants R01DK076771, P30DK056336, T32HL072757, and the UAB Doctoral Training Grant in Obesity and Nutrition Research funded by the Kraft Foods Corporation. The opinions expressed are those of the authors and not necessarily the NIH or any other organization.
Role of Sponsors: The sponsors had no role in design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
We are grateful to all the investigators who provided us the individual-level data for their respective studies, helped us in understanding their data, and responded to our queries. We would like to thank Dr. Kyle Grimes in helping us improve the presentation of our work in this manuscript. This manuscript also utilized limited access datasets obtained from the NHLBI and does not necessarily reflect the opinions or views of the NHLBI.
Footnotes
Author Contributions:
Mehta had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the analysis.
Study concept and design: Allison.
Acquisition of data: Mehta, Fontaine.
Curation and management of data: Mehta, Bangalore.
Analysis and interpretation of data: Mehta, Pajewski, Keith, de los Campos, Allison.
Drafting of the manuscript: Mehta, Fontaine.
Critical revision of the manuscript for important intellectual content: Fontaine, Allison, Pajewski, Mehta, Keith, de los Campos, Bartolucci.
Statistical analysis: Mehta.
Sensitivity analysis: Mehta.
Study supervision: Allison.
Conflict of Interest Disclosures: Mehta and Allison have consulted with GJORDING FOUSER PLLC. Mehta was supported by a UAB Doctoral Training Grant in Obesity and Nutrition funded by Kraft Foods.
Supporting Information
Supporting information is available at the journal’s website.
References
- 1.Flegal K, Graubard B, Williamson D, Gail M. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 2.Faeh D, Braun J, Tarnutzer S, Bopp M. Obesity but not overweight is associated with increased mortality risk. Eur J Epidemiol. 2011 doi: 10.1007/s10654-011-9593-2. [DOI] [PubMed] [Google Scholar]
- 3.Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NK, Chang VW. Secular Declines in the Association Between Obesity and Mortality in the United States. Popul Dev Rev. 2011;37(3):435–451. doi: 10.1111/j.1728-4457.2011.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calle E, Thun M, Petrelli J, Rodriguez C, Heath CJ. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg JA. Correcting biases in estimates of mortality attributable to obesity. Obesity (Silver Spring) 2006;14(11):2071–2079. doi: 10.1038/oby.2006.242. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JA, Fontaine K, Allison DB. Putative biases in estimating mortality attributable to obesity in the US population. Int J Obes (Lond) 2007;31(9):1449–1455. doi: 10.1038/sj.ijo.0803615. [DOI] [PubMed] [Google Scholar]
- 10.Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg JA. Obesity and early mortality in the united states. Obesity (Silver Spring) 2013;21(2):405–412. doi: 10.1002/oby.20023. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18(2):333–339. doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine K, Redden D, Wang C, Westfall A, Allison D. Years of life lost due to obesity. JAMA. 2003;289(2):187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 15.Estimating the Contributions of Lifestyle-Related Factors to Preventable Death: A Workshop Summary. The National Academies Press; 2005. [Google Scholar]
- 16.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. Am J Nurs. 1978;78(6):1039–1040. [PubMed] [Google Scholar]
- 19.Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol. 1980;46(4):649–654. doi: 10.1016/0002-9149(80)90516-0. [DOI] [PubMed] [Google Scholar]
- 20.Worth RM, Kagan A. Ascertainment of men of Japanese ancestry in Hawaii through World War II Selective Service registration. J Chronic Dis. 1970;23(5):389–397. doi: 10.1016/0021-9681(70)90022-6. [DOI] [PubMed] [Google Scholar]
- 21.The multiple risk factor intervention trial (MRFIT) A national study of primary prevention of coronary heart disease. JAMA. 1976;235(8):825–827. [PubMed] [Google Scholar]
- 22.Stamler J, Berkson DM, Gosch FC, et al. The new national cooperative Hypertension Detection and Follow-up Program. Chicago: Chicago Medicine; 1973. [Google Scholar]
- 23.Breslow L, Kaplan GA. Health and Ways of Living Study, 1965 Panel. Alameda County, California: Inter-university Consortium for Political and Social Research (ICPSR); 1996. [distributor] [Google Scholar]
- 24.Nietert PJ, Sutherland SE, Bachman DL, Keil JE, Gazes P, Boyle E. Charleston Heart Study, 1960–2000. Inter-university Consortium for Political and Social Research (ICPSR); 2010. [distributor] [Google Scholar]
- 25.Taylor JO, Wallace RB, Ostfeld AM, Blazer DG. Established Populations for Epidemiologic Studies of the Elderly, 1981–1993. East Boston, Massachusetts, Iowa and Washington Counties, Iowa, New Haven, Connecticut, and North Central North Carolina: Inter-university Consortium for Political and Social Research (ICPSR); 2006. [distributor] [Google Scholar]
- 26.Hawthorne V. Tecumseh Community Health Study, 1959–1969. Inter-university Consortium for Political and Social Research (ICPSR); 1989. [distributor] [Google Scholar]
- 27.Tecumseh Management C. Tecumseh Mortality Follow-up Study, 1978. Inter-university Consortium for Political and Social Research (ICPSR); 1993. [distributor] [Google Scholar]
- 28.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 29.Panel Study of Income Dynamics, public use and restricted use dataset. Ann Arbor, MI: Produced and distributed by the Survey Research Center, Institute for Social Research, University of Michigan; 2010. [Google Scholar]
- 30.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Metabolic precursors of hypertension. The San Antonio Heart Study. Arch Intern Med. 1996;156(17):1994–2001. [PubMed] [Google Scholar]
- 31.Austin MA, Berreyesa S, Elliott JL, Wallace RB, Barrett-Connor E, Criqui MH. Methods for determining long-term survival in a population based study. Am J Epidemiol. 1979;110(6):747–752. doi: 10.1093/oxfordjournals.aje.a112856. [DOI] [PubMed] [Google Scholar]
- 32.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108(5):367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 33.Panel NOEIE. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: NIH. 1998 Available from: http://www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm. [PubMed]
- 34.He J. Modeling the dynamic association of BMI and mortality in the Framingham Heart Study. Ann Epidemiol. 2011;21(7):517–525. doi: 10.1016/j.annepidem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. New York: Cambridge University Press; 2007. [Google Scholar]
- 36.Cook TD, Campbell DT. Quasi-Experimentation: Design & Analysis Issues for Field Settings. Boston: Houghton Mifflin Company; 1979. [Google Scholar]
- 37.Juckett DA, Rosenberg B. Comparison of the Gompertz and Weibull functions as descriptors for human mortality distributions and their intersections. Mech Ageing Dev. 1993;69(1–2):1–31. doi: 10.1016/0047-6374(93)90068-3. [DOI] [PubMed] [Google Scholar]
- 38.Cleves MA, Gould W, Gutierrez R, Marchenko Y. An Introduction to Survival Analysis Using Stata. Second ed. College Station, Texas: Stata Press; 2008. [Google Scholar]
- 39.Wetterstrand W. Parametric Models for Life Insurance Mortality Data. Transactions of Society of Actuaries. 1981;33:159–179. [Google Scholar]
- 40.Pollard JH, Valkovics EJ. The Gompertz Distribution and its Applications. Genus. 1992;48(3/4):15–28. [PubMed] [Google Scholar]
- 41.Robertson HT, de los Campos G, Allison DB. Turning the analysis of obesity-mortality associations upside down: Modeling years of life lost through conditional distributions. Obesity. 2013;21(2):398–404. doi: 10.1002/oby.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flegal K, Graubard B, Williamson D, Gail M. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol. 2007;166(8):975–982. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- 43.Gail M, Graubard B, Williamson D, Flegal K. Pencina Michael J, Larson Martin G, D'Agostino Ralph B., editors. Comments on 'Choice of time scale and its effect on significance of predictors in longitudinal studies'. Statistics in Medicine. 2007;26(8):1343–1359. doi: 10.1002/sim.2699. Stat Med. 2009;28:1315-7. [DOI] [PubMed] [Google Scholar]
- 44.Pencina M, Larson M, D’Agostino R. Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med. 2007;26(6):1343–1359. doi: 10.1002/sim.2699. [DOI] [PubMed] [Google Scholar]
- 45.Thiébaut A, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23(24):3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 46.Zapka JM, Lemon SC, Magner RP, Hale J. Lifestyle behaviours and weight among hospital-based nurses. J Nurs Manag. 2009;17(7):853–860. doi: 10.1111/j.1365-2834.2008.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient’s mortality risk? JAMA. 2013;309(1):87–88. doi: 10.1001/jama.2012.185445. [DOI] [PubMed] [Google Scholar]
- 48.Robertson HT, de los Campos G, Allison DB. Turning the analysis of obesity-mortality associations upside down: Modeling years of life lost through conditional distributions. Obesity. 2012 doi: 10.1002/oby.20019. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauling L. The Relation Between Longevity and Obesity in Human Beings. Proc Natl Acad Sci U S A. 1958;44(6):619–622. doi: 10.1073/pnas.44.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid N. A Conversation with Sir David Cox. Statistical Science. 1994;9(3) [Google Scholar]
- 51.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183(14):E1059–E1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson S, Kaptoge S, White I, et al. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39(5):1345–1359. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young F, Capewell S, Ford ES, Critchley JA. Coronary mortality declines in the U.S. between 1980 and 2000 quantifying the contributions from primary and secondary prevention. Am J Prev Med. 2010;39(3):228–234. doi: 10.1016/j.amepre.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18(12):598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Owens GM. Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm. 2008;14(3 Suppl):2–6. doi: 10.18553/jmcp.2008.14.S6-A.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alley D, Chang V. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 58.Jia H, Lubetkin EI. Obesity-related quality-adjusted life years lost in the U.S. from 1993 to 2008. Am J Prev Med. 2010;39(3):220–227. doi: 10.1016/j.amepre.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Beydoun M, Liang L, Caballero B, Kumanyika S. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 60.Allison DB, Zannolli R, Narayan KM. The direct health care costs of obesity in the United States. Am J Public Health. 1999;89(8):1194–1199. doi: 10.2105/ajph.89.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 62.Stokes A, Mehta NK. Mortality and excess risk in US adults with pre-diabetes and diabetes: a comparison of two nationally representative cohorts, 1988–2006. Popul Health Metr. 2013;11(1):3. doi: 10.1186/1478-7954-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.