Abstract
Purpose.
Delayed rod-mediated dark adaptation (DA) is characteristic of early age-related macular degeneration (AMD) and also can be observed in some older adults in normal macular health. We examine cross-sectional associations between rod-mediated DA and risk factors for AMD in older adults in normal macular health.
Methods.
The sample consisted of adults aged ≥60 years old in normal macular health per grading of fundus photos using an established disease classification system. Rod-mediated DA was measured psychophysically following a photobleach using a computer-automated dark adaptometer with targets centered at 5° on the inferior vertical meridian. The speed of DA was characterized by the rod-intercept value, with abnormal DA defined as rod-intercept ≥ 12.3 minutes. We assessed several health and functional characteristics that the literature has suggested increase AMD risk (e.g., smoking, alcohol use, inflammatory markers, apolipoproteins, low luminance visual acuity, chronic medical conditions, body mass, family history).
Results.
Among 381 participants (mean age, 68.5 years; SD, 5.5), 78% had normal and 22% had abnormal DA, with the prevalence of abnormal DA increasing with age. After age-adjustment, abnormal DA was associated with increased odds of elevated C-reactive protein (CRP), heavy use of or abstention from alcohol, high blood pressure, and drop in visual acuity under mesopic conditions.
Conclusions.
Despite having normal macular health according to accepted definitions of AMD presence, approximately one-quarter of older adults recruited from primary eye care clinics had abnormal DA, which was associated with known risk factors for AMD, including elevated CRP.
Keywords: aging, dark adaptation, rod function, age-related macular degeneration
Abnormal rod-mediated dark adaptation in older adults in normal macular health is associated with elevated plasma C-reactive protein levels.
Introduction
Scotopic dysfunction is characteristic of early age-related macular degeneration (AMD) even though visual acuity remains relatively unimpaired.1–7 Patients with early AMD report vision problems under dim lighting or at night,8,9 which are associated with their psychophysically measured scotopic thresholds.10–12 Persons with early AMD tend to exhibit deficits in rod-mediated light sensitivity that are more severe than cone-mediated deficits measured in the same retinal areas.2–4,13,14 Rod-mediated dark adaptation also is slowed in early AMD,1,5,15,16 which occurs even in cases where cone-mediated dark adaptation in the same retinal area is undisturbed6 and when steady state thresholds are relatively unimpaired.5 The mechanisms underlying slowed rod-mediated dark adaptation in early AMD are not completely understood, but previous research strongly suggests candidate mechanisms. Depositions rich in hydrophobic esterified cholesterol in aging Bruch's membrane (BrM) and in the sub-RPE space in early AMD17,18 are hypothesized to create a diffusion barrier to metabolic exchange between the choroid and photoreceptors, thus impairing transport of essential nutrients, such as vitamin A.1 This accumulation impedes translocation of multimolecular complexes, such as plasma lipoproteins, that deliver lipophilic essentials for rapid outer retinal uptake and distribution.19,20 It is well established that vitamin A deficiency preferentially causes rod dysfunction and eventual photoreceptor death.21–25 In terms of the impact on visual function, diminished amounts of 11-cis-retinal (a derivative of vitamin A) available to combine with the protein opsin to form the visual pigment rhodopsin can lead to slowing in the rate of rhodopsin regeneration and recovery of light sensitivity after light exposure.26 Cones have alternative sources of vitamin A through the retinal vasculature, that is, Müller cells, and cone-selective retinoid targeting mechanisms.27,28 Thus, they may be less impacted by a BrM/RPE nutritional barrier, unlike rods that derive vitamin A preferentially from the RPE. We previously have provided data consistent with this nutritional barrier/retinoid deficiency hypothesis in that the rate of rod-mediated dark adaptation in older adults with normal retinal health or AMD became more rapid after a 30-day course of high-dose retinol.12 Other potential contributing factors to slowed dark adaptation in early AMD include genetic alterations in vitamin A metabolism29 or age- and disease-related deficits in pathways within RPE that remain to be characterized.30 However, regardless of the precise mechanisms underlying slowed rod-mediated dark adaptation in early AMD, it has been established clearly as a functional deficit present in the earliest phases of AMD.
A critical question that remains unanswered is whether rod-mediated dark adaptation impairment precedes the development of early AMD as defined by the historical gold standard definition of AMD based on color fundus photography grading systems.31 Does rod-mediated dark adaptation impairment appear before AMD is clinically visible in images obtained by this imaging modality? Previous research has shown that some older adults with a normal macular appearance have rod-mediated dark adaptation delays that are more excessive than their age-mates.32 Could this rod-mediated dark adaptation delay be a signal that AMD is developing in these older adults even though it is invisible in the fundus? This hypothesis is attractive because the topography of rod loss in aging, that is, in central macula,33 matches well the topography of lipid-rich drusen, basal linear deposit, and prebasal linear deposit in aging and early AMD.34,35 This is a practical question for several reasons. If rod-mediated dark adaptation impairment does precede widely accepted clinical signs of the disease, then its assessment would have potential as a diagnostic biomarker for the earliest emergence of the disease, could be used to identify persons at high-risk for early AMD for targeted enrollment in clinical trials for new candidate treatments, and/or could serve as an outcome measure in clinical trials evaluating preventive treatments or treatments targeted at arresting the progression of early AMD. This question also is important, since it focuses on the factors (e.g., medical, biological, functional, lifestyle) that foster a transition from normal chorioretinal aging to disease.
The Alabama Study on Early Age-Related Macular Degeneration (ALSTAR) is a prospective cohort study funded by the National Institute on Aging designed to address the question of whether slowed rod-mediated dark adaptation in adults recruited with normal macular health at baseline is associated with the incident development of AMD three years later. In ALSTAR, normal macular health, and AMD presence and severity were determined by the grading of color fundus photographs using an AMD severity scale, the current gold-standard for identifying the disease in clinical research. In addition to measuring rod-mediated dark adaptation, we also assessed several health and functional characteristics that may increase the risk of AMD (i.e., smoking, heavy alcohol use, inflammatory markers, apolipoproteins, low-luminance visual acuity, chronic medical conditions of aging, body mass index, family history).18,36–43 We report key aspects of the baseline data that address whether previously reported risk factors for AMD are related to rod-mediated dark adaptation impairment in older adults in normal macular health. Associations among biomarkers or risk factors for AMD in older adults absent of the disease could inform biological and epidemiological models on the transition of normal retinal aging to early AMD.
Methods
Subjects
This study was approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB) and followed the tenets of the Declaration of Helsinki. Participants were recruited from two primary care ophthalmology practices in the Callahan Eye Hospital at UAB. Eligibility criteria were as follows:
Age ≥ 60 years;
Normal macular health in both eyes as determined by 3-field digital stereo-fundus photos (450 Plus camera; Carl Zeiss Meditec, Dublin, CA, USA) graded by an experienced grader who was masked to all other study variables and who used the 9-step Age-Related Eye Disease Study (AREDS) classification system.31 Photographers were certified by the AREDS2 coordinating center. For entrance to the study, the grade in each eye had to be grade 1 in the AREDS 9-step classification system,31 indicating normal macular health;
No previous diagnoses of glaucoma, other retinal conditions, optic nerve conditions, corneal disease, diabetes, Alzheimer's disease, Parkinson's disease, brain injury, or other neurological or psychiatric conditions as revealed by the medical record or by self-report;
Did not reside in a nursing home or was not bed-bound; and
Was willing to participate in a study that included a baseline visit to the Clinical Research Unit in the UAB Department of Ophthalmology and a follow-up visit three years later.
The baseline visit consisted of the following assessments after the informed consent process was completed. Through interviewer-administered questionnaires, demographic information (age, sex, race, education completed), smoking status,44 and alcohol use45 were collected. In addition, general health was assessed by asking about the presence or absence of 15 chronic medical conditions, which used the format of “Has a doctor ever told you that you have ….”.46 General cognitive status was estimated through the Mini-Mental State Examination (MMSE).47 Participants were asked about whether they were aware of any family history of AMD among first-degree relatives (defined as a parent, sibling, or child).
Best-corrected visual acuity for each eye was assessed via the Electronic Visual Acuity tester (EVA)48 under photopic conditions (100 cd/m2) and expressed as the logarithm of the minimum angle resolvable (logMAR). Low luminance visual acuity also was assessed using the EVA for each eye with participants viewing letters through a 1.5 log unit neutral density filter, a method described by Sunness et al.,39 which reduced background luminance to 3.16 cd/m2. To determine how much logMAR decreased by the lower light level compared to the photopic (100 cd/m2) assessment, we defined a drop in visual acuity by the increase in logMAR and by the number of lines on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart “lost” when acuity was measured under lower luminance.39 Contrast sensitivity for each eye was estimated by the Pelli-Robson chart49 with mean luminance of 100 cd/m2, the letter-by-letter scoring method,50 and expressed as logarithm of sensitivity. Height (cm) and weight (kg) were measured so that body mass index (BMI) could be computed.
Dark adaptation was measured in only one eye in each participant because of time constraints in the protocol and the high likelihood that both eyes have similar dark adaptation characteristics.16 The eye with better visual acuity was selected for testing. Dark adaptation was measured psychophysically using the AdaptDx (MacuLogix, Hummelstown, PA, USA), a computer-automated dark adaptometer described previously.7,15,16,51 Before testing, the eye was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride so that a pupil diameter of ≥6 mm was achieved. Trial lenses were added for the 30 cm viewing distance if needed to correct for optical blur. The fellow eye was occluded with an opaque patch. The participant placed his/her head in the forehead-chinrest of the adaptometer. An infrared camera positioned behind the fixation light displayed the eye on a monitor viewed by the examiner, who facilitated the positioning of the participant's test eye to the red fixation light using a reticule displayed on the eye's image. The procedure began with a photo-bleach exposure to a flash (0.25 ms duration, 58,000 scotopic cd/m2 s intensity; equivalent ∼83% bleach) while the participant was focused on the fixation light. This bleach has been shown to be sufficiently intense to generate impaired dark adaptation parameters in early AMD patients using a 20-minute duration test protocol.51 The photo-bleach flash, subtending 4°, was centered at 5° on the inferior vertical meridian (i.e., superior to the fovea on the retina) which also was the test target's position for measuring light sensitivity. Threshold measurement for a 2° diameter, 500-nm circular target began 15 seconds after bleach offset. During threshold measurement, the participant was instructed always to maintain fixation on the red fixation light and to press a response button when a flashing target first became visible within the bleached area. Threshold was estimated using a three-down/one-up modified staircase estimate procedure described previously,51 and continued at 30-second intervals for 20 minutes. Log thresholds were expressed as sensitivity in decibel (dB) units as a function of time from bleach offset. The speed of dark adaptation was characterized by the rod intercept value. The rod intercept is defined as the duration required for sensitivity to recover to a criterion sensitivity value of 5.0 × 10−3 scotopic cd/m2 (3.0 log units of attenuation of the stimulus).51 The criterion sensitivity level is located in the latter half of the second component of rod recovery.26 An increase in the rod intercept is caused by a slowing of the second component of rod-mediated dark adaptation and, thus, a rightward shift of the dark adaptation function. Impaired dark adaptation was defined as a rod-intercept of ≥12.3 minutes.51
Blood (4–8 mL) was collected by phlebotomy at the baseline visit and the resultant heparinized plasma collected for analysis. Plasma concentrations of apolipoprotein (apo) B and apo A-I, the major protein constituents of low (LDL) and high (HDL) density lipoprotein, respectively, were measured at Northwest Lipid Laboratory (Seattle, WA, USA) using an automated standardized immunoturbidimetric method calibrated against fresh pools of human plasma and commercial calibrators.52,53 Complement proteins (C3, C4, C5) and their respective activation fragments (C3a, C4a, C5a) were quantitated by protein-specific ELISAs using the manufacturers' protocols (C3 from Immunology Consultants Laboratory, Portland, OR, USA; C4 and C5 from Abcam, Cambridge, UK; C3a, C4a, and C5a from Quidel Corporation, San Diego, CA, USA). The concentration of C-reactive protein (CRP) was measured by ELISA as described previously.54
Statistical Analysis
To compare continuous and categorical variables for those with normal and abnormal dark adaptation, Student's t-tests and χ2 tests were used, respectively. Wilcoxon rank-sum tests were used to compare medians when continuous variables were not normally distributed. Logistic regression was used to calculate crude and age-adjusted odds ratios (OR) and 95% confidence intervals (CI). Statistical significance was defined as P < 0.05 (2-tailed).
Results
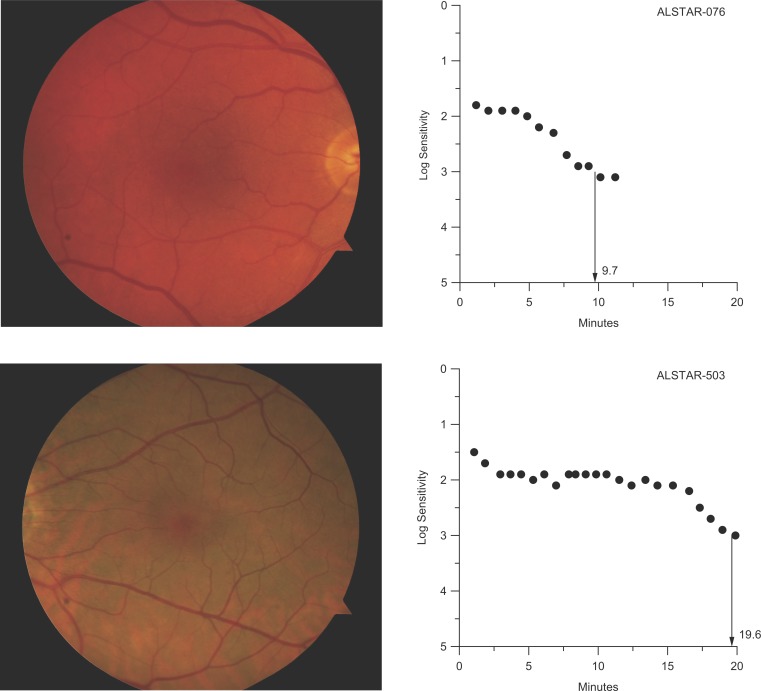
Table 1 provides baseline demographic information for participants. Approximately 96% were white, with most of the balance African American. Participants ranged in age from 60 to 88 years; two-thirds were in their 60s and almost one-third in their 70s, with the rest in their 80s. Approximately two-thirds of the sample was female. The mean rod-intercept for the entire sample (N = 381) was 11.1 minutes (SD 5.7). A total of 299 participants (78%) had normal dark adaptation (normal DA) and 82 (22%) had abnormal dark adaptation (abnormal DA). Mean rod-intercept for those with normal versus abnormal DA was 9.1 (SD 1.5) and 18.3 (SD 8.6) minutes, respectively. Figure 1 illustrates two participants whose digital color fundus photos indicate normal macular health, but one has normal DA and the other has abnormal DA as defined by the rod-intercept.
Table 1.
Demographic Characteristics of Study Participants
|
Characteristic |
N
(%) |
| Age, y | |
| 60–69 | 250 (65.6) |
| 70–79 | 120 (31.5) |
| 80–89 | 11 (2.9) |
| Sex | |
| Female | 252 (66.1) |
| Male | 129 (33.9) |
| Race/ethnicity | |
| White | 364 (95.54) |
| Black | 13 (3.42) |
| Asian or Pacific Islander | 2 (0.52) |
| Native American | 2 (0.52) |
Figure 1.
Top: Color fundus photograph (right eye) of a 75-year-old participant in normal macular health who has normal rod-mediated DA as defined by the rod-intercept. Bottom: Color fundus photograph (left eye) of 72-year-old participant also in normal macular health yet who has abnormal rod-mediated DA.
Tables 2 to 4 compare descriptive information on how the health and functioning variables collected in our protocol differ between those who have abnormal versus normal DA. Those with abnormal DA were more likely to be older, less educated, and female (Table 2). There was no difference between the groups in terms of smoking status. Those who had normal DA were more likely to report using alcohol in the past year compared to those with abnormal DA (P = 0.0033). This association was examined further in terms of number of reported alcohol drinks per week. Those who had normal DA were more likely to be moderate drinkers and those who had abnormal DA were more likely to report abstaining from alcohol (P = 0.047). With respect to general health, there was a marginally significant relationship between those with abnormal DA and a greater number of chronic medical conditions (P = 0.051). A history of high blood pressure and hearing problems were more likely to be present among those with abnormal DA. Having had cancer was more likely to be reported by those with normal DA. The MMSE scores were similarly distributed in both groups. No participant had an MMSE < 24, which is the accepted cut-point for cognitive impairment in older adults. Whether there was a family history of AMD did not differ for those with abnormal versus normal DA.
Table 2.
Demographic, Lifestyle and Chronic Medical Conditions of Sample by DA Status
|
N
(%) Unless Otherwise Noted |
P
Value |
||
|
DA Abnormal,
N
= 82 |
DA Normal,
N
= 299 |
||
| Demographics | |||
| Age, mean (SD) | 71.5 (5.6) | 67.7 (5.2) | <0.0001 |
| Sex | |||
| Male | 21 (25.6) | 108 (36.1) | 0.075 |
| Female | 61 (74.4) | 191 (63.9) | |
| Race | |||
| White | 79 (96.3) | 285 (95.3) | >0.99 |
| Nonwhite | 3 (3.7) | 14 (4.7) | |
| Education | |||
| <HS | 2 (2.4) | 5 (1.7) | 0.0001 |
| HS or equivalent | 28 (34.2) | 42 (14.1) | |
| Some college or more | 52 (63.4) | 252 (84.3) | |
| Lifestyle | |||
| Smoking status | |||
| Current | 5 (6.1) | 14 (4.7) | 0.87 |
| Former | 32 (39.0) | 119 (39.8) | |
| Never | 45 (54.9) | 166 (55.5) | |
| Alcohol use, past year | |||
| Yes | 45 (54.9) | 215 (71.9) | 0.0033 |
| No | 37 (45.1) | 84 (28.1) | |
| Alcohol use, drinks per wk | |||
| Abstainers* | 37 (45.1) | 84 (28.1) | 0.047 |
| <1 | 19 (23.2) | 85 (28.4) | |
| 1–7 | 16 (19.5) | 89 (29.8) | |
| 8–14 | 5 (6.1) | 26 (8.7) | |
| >14 | 5 (5.1) | 15 (5.0) | |
| Drinks per week, median (IQR) | 0 (0–2) | 0 (0–5) | 0.080 |
| Drinking consumption per wk* | |||
| Abstainers | 37 (45.1) | 84 (28.1) | 0.021 |
| Light | 19 (23.2) | 85 (28.4) | |
| Moderate | 18 (22.0) | 103 (34.5) | |
| Heavy | 8 (9.8) | 27 (9.0) | |
| BMI, kg/m2 | |||
| <18.50 | 2 (2.5) | 1 (0.3) | 0.23 |
| 18.50–24.99 | 24 (29.6) | 92 (31.4) | |
| 25.00–29.99 | 37 (45.7) | 121 (41.3) | |
| ≥ 30.00 | 18 (22.2) | 79 (27.0) | |
| BMI kg/m2, mean (SD)† | 27.4 (5.2) | 27.6 (5.1) | 0.69 |
| Chronic medical conditions | |||
| Number of medical conditions, median (IQR) | 3.0 (2.0–5.0) | 3.0 (1.0–4.0) | 0.051 |
| Heart problems | 28 (34.6) | 85 (28.6) | 0.30 |
| Circulation problems | 5 (6.2) | 26 (8.8) | 0.45 |
| High blood pressure | 47 (58.0) | 134 (45.1) | 0.039 |
| Low blood pressure | 10 (12.4) | 24 (8.1) | 0.23 |
| Neurological problems | 6 (7.4) | 20 (6.7) | 0.83 |
| Arthritis | 42 (51.9) | 153 (51.5) | 0.96 |
| Osteoporosis | 19 (23.5) | 47 (15.8) | 0.11 |
| Cancer | 13 (16.1) | 84 (28.3) | 0.026 |
| Chronic pulmonary problems | 11 (13.6) | 31 (10.4) | 0.43 |
| Digestive problems | 29 (35.8) | 95 (32.0) | 0.52 |
| Urinary problems | 21 (25.9) | 51 (17.2) | 0.075 |
| Kidney problems | 7 (8.6) | 16 (5.4) | 0.30 |
| Hearing problems | 28 (34.6) | 59 (19.9) | 0.0053 |
| MMSE, mean (SD) | 28.2 (1.8) | 28.5 (1.9) | 0.14 |
Abnormal dark adaptation was defined as a rod intercept ≥ 12.3.
Abstainers were defined as those who reported drinking no alcohol in the past year. Among those who reported drinking in the past year, light drinking was defined as less than one drink per week, moderate drinking was defined as one to seven drinks per week for women and 1 to 14 drinks per week for men, and heavy drinking was defined as eight or more drinks per week for women and 15 or more drinks per week for men.
Data are missing for seven participants, since they declined having their weight measured.
Table 4.
Visual Function, Macular Pigment Optical Density, and Family History of AMD by DA Status
|
Abnormal DA,
N
= 82 |
Normal DA,
N
= 299 |
P
Value |
|
| Visual function* | |||
| Visual acuity, logMAR, mean (SD) | −0.023 (0.08) | −0.013 (0.10) | 0.40 |
| Low luminance visual acuity, logMAR, mean (SD)* | 0.32 (0.10) | 0.30 (0.11) | 0.11 |
| Difference between low luminance visual acuity and visual acuity | |||
| 0.1–3.0 (%) | 25 (30.5) | 152 (51.4) | 0.0022 |
| 3.1–4.0 (%) | 41 (50.0) | 94 (31.8) | |
| 4.1–8.0 (%) | 16 (19.5) | 50 (16.9) | |
| Mean (SD) | 0.35 (0.08) | 0.31 (0.10) | 0.0053 |
| Contrast sensitivity, mean (SD) | 1.59 (0.10) | 1.63 (0.09) | 0.0027 |
| Family history of AMD (%) | |||
| Yes | 8 (9.8) | 21 (7.0) | 0.41 |
| No | 74 (90.2) | 278 (93.0) | |
Data from the eye tested for dark adaptation is presented.
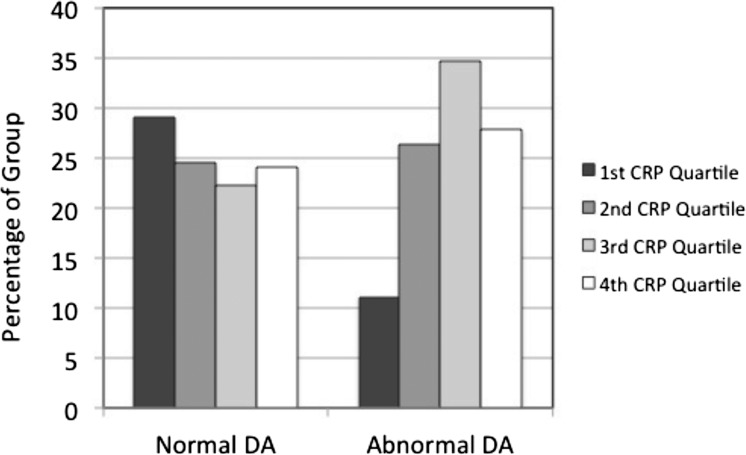
Table 3 presents blood chemistry variables for the abnormal and normal DA groups. The CRP values for those with abnormal DA were significantly higher than those with normal DA (P = 0.0074), mainly due to a decreased percentage of persons with abnormal DA in the lowest CRP quartile (Fig. 2). Complement components were all in the normal to high normal range, and there was no difference in the levels of any complement components between normal DA and abnormal DA groups. Apo-AI and Apo-B values were not significantly different between the groups.
Table 3.
Blood Chemistry Variables of Sample by DA Status
|
N
(%) Unless Otherwise Noted |
P
Value |
||
|
DA Abnormal,
N
= 82 |
DA Normal,
N
= 299 |
||
| CRP μg/mL quartiles* | |||
| ≤1.13 | 8 (11.1) | 82 (29.1) | 0.011 |
| 1.14–2.35 | 19 (26.4) | 69 (24.5) | |
| 2.36–4.92 | 25 (34.7) | 63 (22.3) | |
| >4.92 | 20 (27.8) | 68 (24.1) | |
| CRP μg/mL, median (IQR) | 3.2 (1.8–5.5) | 2.2 (1.0–4.8) | 0.0074 |
| C3 μg/mL quartiles | |||
| ≤818.95 | 13 (17.8) | 76 (27.1) | 0.31 |
| 818.96–957.21 | 17 (23.3) | 71 (25.3) | |
| 957.22–1245.44 | 21 (28.8) | 68 (24.2) | |
| >1245.44 | 22 (30.1) | 66 (23.5) | |
| C3 μg/mL, median (IQR) | 1016.5 (843.9–1252.4) | 941.7 (802.9–1213.5) | 0.20 |
| C4 μg/mL quartiles | |||
| ≤413.56 | 20 (27.4) | 69 (24.4) | 0.58 |
| 413.56–521.09 | 21 (28.8) | 68 (24.0) | |
| 521.10–672.13 | 14 (19.2) | 75 (26.5) | |
| >672.13 | 18 (24.7) | 71 (25.1) | |
| C4 μg/mL, median (IQR) | 490.0 (409.1–664.1) | 527.0 (415.0–673.3) | 0.54 |
| C5 μg/mL quartiles | |||
| ≤55.13 | 22 (30.1) | 67 (23.7) | 0.40 |
| 55.14–85.09 | 19 (26.0) | 70 (24.7) | |
| 85.10–115.79 | 13 (17.8) | 76 (26.9) | |
| >115.79 | 19 (26.0) | 70 (24.7) | |
| C5 μg/mL, median (IQR) | 78.22 (49.83–117.24) | 86.99 (57.73–115.61) | 0.42 |
| C3a μg/mL quartiles | |||
| ≤43.18 | 14 (19.2) | 75 (26.6) | 0.46 |
| 43.19–85.12 | 17 (23.3) | 72 (25.5) | |
| 85.13–128.68 | 22 (30.1) | 67 (23.8) | |
| >128.68 | 20 (27.4) | 68 (24.1) | |
| C3a μg/mL, median (IQR) | 94.61 (54.63–1329.71) | 82.69 (40.20–127.41) | 0.23 |
| C4a μg/mL quartiles | |||
| ≤12.74 | 18 (24.7) | 71 (25.2) | 0.085 |
| 12.75–26.66 | 19 (26.0) | 70 (24.8) | |
| 26.67–50.97 | 25 (34.3) | 64 (22.7) | |
| >50.98 | 11 (15.1) | 77 (27.3) | |
| C4a μg/mL, median (IQR) | 26.146 (13.591–38.648) | 2706.5 (12.740–58.360) | 0.44 |
| C5a ng/mL quartiles | |||
| ≤62.68 | 19 (26.4) | 70 (24.7) | 0.27 |
| 62.68–152.49 | 19 (26.4) | 70 (24.7) | |
| 152.50–427.83 | 12 (16.7) | 77 (27.2) | |
| >427.83 | 22 (31.0) | 66 (23.3) | |
| C5a ng /mL, median (IQR) | 147.0 (58.6–437.4) | 156.7 (66.3–423.7) | 0.65 |
| Apo–AI mg/dL quartiles† | |||
| ≤142.5 | 19 (25.0) | 72 (25.0) | 0.88 |
| 142.6–162.5 | 18 (23.7) | 73 (25.4) | |
| 162.6–182.0 | 22 (29.0) | 71 (24.7) | |
| >182.0 | 17 (22.4) | 72 (25.0) | |
| Apo–AI mg/dL, mean (SD) | 163.1 (29.2) | 163.7 (30.6) | 0.87 |
| Apo–B mg/dL quartiles | |||
| ≤75.5 | 19 (23.7) | 73 (25.4) | 0.58 |
| 75.6–90.0 | 25 (32.9) | 72 (25.0) | |
| 90.1–106.0 | 16 (21.1) | 70 (24.3) | |
| >106.0 | 17 (22.4) | 73 (25.4) | |
| Apo–B mg/dL, mean (SD) | 92.4 (25.0) | 93.0 (23.6) | 0.85 |
Abnormal DA was defined as a rod intercept ≥ 12.3.
Data for the inflammatory marker analyses were unavailable for 27 participants because the participant declined blood specimen collection or because of laboratory analysis failure.
Data for the apolipoprotein analyses were unavailable for 17 participants because the participant declined blood specimen collection or because of laboratory analysis failure.
Figure 2.
The distribution of CRP values by quartiles for those with normal rod-mediated DA and those with abnormal DA. Higher quartiles correspond to higher CRP values. Quartile values are as follows: first quartile, ≤1.13 μg/mL; second quartile, 1.14–2.35 μg/mL; third quartile, 2.36–4.92 μg/mL; fourth quartile, >4.92 μg/mL. Those with abnormal DA were less likely to have CRP values in the lowest quartile (see also Table 3).
Visual acuity was on average excellent in both groups, averaging better than 20/20, and did not differ between groups (Table 4). Low luminance visual acuity was worse on average in those with abnormal DA, but this difference was not statistically significant (P = 0.11). Persons with abnormal DA had on average a 0.35 logMAR drop in visual acuity under low luminance conditions (compared to high luminance conditions) and those with normal DA had a slightly smaller logMAR drop of 0.32 (P = 0.0053). Contrast sensitivity tended to be slightly worse in the abnormal DA group (P = 0.0027).
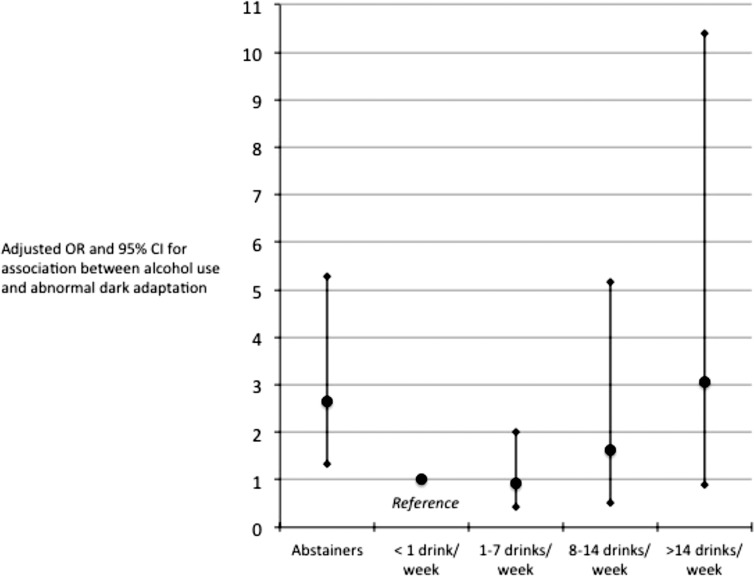
Those participants in their 70s and 80s were more likely to have abnormal DA compared to those in their 60s (for the 70s, OR = 4.39; 95% CI, 2.60–7.43; P < 0.0001 and for the 80s, OR = 5.89; 95% CI, 1.70–20.45; P = 0.0053). Table 5 displays the crude and age-adjusted ORs between abnormal DA and demographic, lifestyle, and chronic medical characteristics. Focusing on the age-adjusted associations, we found that females and those with less education were more likely to have abnormal DA. Smoking status was unrelated to abnormal DA. Those with abnormal DA were 59% less likely to report using alcohol in the past year, with further analysis indicating that this association was mediated by their being less likely to be moderate drinkers and more likely to be abstainers or heavy drinkers (Fig. 3). With respect to chronic medical conditions, after age-adjustment, those with abnormal DA had a 68% increased odds of having high blood pressure, and 64% decreased odds of having a history of cancer. Those with abnormal DA were 1.6 times more likely to have hearing impairments although this finding did not reach statistical significance (P = 0.086).
Table 5.
Crude and Adjusted Associations (OR) Between Abnormal DA and Demographics, Lifestyle, and Chronic Medical Conditions
| Crude |
Adjusted* |
|||
|
OR |
OR |
95% CI |
P
Value |
|
| Demographics | ||||
| Sex | ||||
| Female | 1.64 | 1.92 | 1.08–3.42 | 0.027 |
| Male | Ref | Ref | Ref | – |
| Education | ||||
| High school or less | 3.09 | 2.82 | 1.59–5.00 | 0.0004 |
| Some college or more | Ref | Ref | Ref | – |
| Lifestyle | ||||
| Smoking status | ||||
| Current | 1.32 | 1.40 | 0.46–4.23 | 0.55 |
| Former | 0.99 | 0.81 | 0.47–1.39 | 0.45 |
| Never | Ref | Ref | Ref | – |
| Alcohol use, past y | ||||
| Yes | 0.48 | 0.41 | 0.24–0.70 | 0.0011 |
| No | Ref | Ref | Ref | – |
| Alcohol use, drinks per wk† | ||||
| Abstainers | 1.97 | 2.64 | 1.32–5.29 | 0.0061 |
| <1 | Ref | Ref | Ref | – |
| 1–7 | 0.80 | 0.93 | 0.43–2.02 | 0.85 |
| 8–14 | 0.86 | 1.63 | 0.52–5.17 | 0.41 |
| >14 | 1.49 | 3.07 | 0.91–10.41 | 0.072 |
| Drinking consumption, drinks per wk† | ||||
| Abstainers | 1.97 | 2.65 | 1.33–5.30 | 0.0058 |
| Light | Ref | Ref | Ref | – |
| Moderate | 0.78 | 0.98 | 0.46–2.08 | 0.96 |
| Heavy | 1.33 | 2.38 | 0.86–6.57 | 0.096 |
| BMI kg/m2 | ||||
| ≤24.99 | Ref | Ref | Ref | – |
| 25.00–29.99 | 1.09 | 1.36 | 0.74–2.49 | 0.32 |
| ≥30.00 | 0.82 | 1.04 | 0.51–2.11 | 0.92 |
| Chronic medical conditions | ||||
| N of medical conditions | ||||
| 0 | Ref | Ref | Ref | – |
| 1–2 | 0.74 | 0.77 | 0.26–2.32 | 0.64 |
| 3–4 | 1.19 | 1.01 | 0.34–3.01 | 0.99 |
| 5 or more | 1.51 | 1.16 | 0.37–3.65 | 0.80 |
| Specific medical conditions‡ | ||||
| Heart problems | 1.32 | 1.18 | 0.68–2.04 | 0.56 |
| Circulation problems | 0.69 | 0.46 | 0.16–1.31 | 0.14 |
| High blood pressure | 1.68 | 1.68 | 1.00–2.83 | 0.049 |
| Low blood pressure | 1.60 | 1.30 | 0.57–2.97 | 0.54 |
| Neurological problems | 1.11 | 1.10 | 0.41–2.97 | 0.84 |
| Diabetes | 0.73 | 1.00 | 0.10–10.01 | 0.99 |
| Arthritis | 1.01 | 0.97 | 0.58–1.63 | 0.92 |
| Osteoporosis | 1.63 | 1.42 | 0.75–2.68 | 0.28 |
| Cancer | 0.49 | 0.36 | 0.18–0.71 | 0.003 |
| Chronic pulmonary problems | 1.35 | 1.52 | 0.71–3.27 | 0.28 |
| Digestive problems | 1.19 | 1.07 | 0.62–1.83 | 0.81 |
| Urinary problems | 1.69 | 1.45 | 0.79–2.66 | 0.23 |
| Kidney problems | 1.66 | 1.26 | 0.47–3.36 | 0.65 |
| Hearing problems, vs. no | 2.13 | 1.65 | 0.93–2.93 | 0.086 |
| Impaired MMSE§ | 1.22 | 0.88 | 0.16–4.69 | 0.88 |
Adjusted for age.
Abstainers were defined as those who reported drinking no alcohol in the past year. Among those who reported drinking in the past year, light drinking was defined as less than 1 drink per week, moderate drinking was defined as 1 to 7 drinks per week for women and 1 to 14 drinks per week for men, and heavy drinking was defined as 8 or more drinks per week for women and 15 or more drinks per week for men.
The referent (Ref) for each of the listed chronic medical conditions consists of those who do not have the medical condition.
Impaired MMSE is defined as a total score ≤ 23.
Figure 3.
Adjusted ORs and 95% CIs describing the association between various levels of alcohol use and abnormal DA. The reference consists of those who reported that they drink but did not drink in the previous week. Abstainers and those who drink more than 14 drinks per week were more likely to display abnormal DA (see also Table 5).
As summarized in Table 6, compared to persons with normal DA, those having abnormal DA were 3 to 4 times more likely to have a higher CRP value (i.e., in the second, third, and fourth quartiles) and were approximately 2.5 times more likely to have a C3 value in the highest quartile. With respect to visual function (Table 7), those with a drop in visual acuity under low luminance of >3 lines to 4 lines and >4 lines were 2.9 and 1.8 times, respectively, more likely to have abnormal DA compared to those with normal DA, though a drop of >4 lines was not statistically significant. Impaired contrast sensitivity (Pelli-Robson score worse than 1.6) was associated with a 70% increased odds of having abnormal DA, although this finding did not reach statistical significance (P = 0.064).
Table 6.
Crude and Adjusted Associations (OR) Between Abnormal DA and Blood Chemistry Variables
| Crude |
Adjusted* |
|||
|
OR |
OR |
95% CI |
P
Value |
|
| Clinical | ||||
| CRP μg/mL quartiles | ||||
| ≤1.13 | Ref | Ref | Ref | – |
| 1.14–2.35 | 2.82 | 2.82 | 1.14–7.03 | 0.026 |
| 2.36–4.92 | 4.07 | 4.25 | 1.75–10.32 | 0.0014 |
| >4.92 | 3.01 | 3.04 | 1.23–7.54 | 0.016 |
| C3 μg/mL quartiles | ||||
| ≤818.95 | Ref | Ref | Ref | – |
| 818.96–957.21 | 1.40 | 1.40 | 0.61–3.19 | 0.43 |
| 957.22–1245.44 | 1.81 | 2.01 | 0.90–4.47 | 0.087 |
| >1245.44 | 1.95 | 2.48 | 1.11–5.54 | 0.026 |
| C4 μg/mL quartiles | ||||
| ≤413.56 | Ref | Ref | Ref | – |
| 413.56–521.09 | 1.07 | 0.93 | 0.45–1.94 | 0.84 |
| 521.10–672.13 | 0.64 | 0.51 | 0.23–1.14 | 0.099 |
| >672.13 | 0.88 | 0.86 | 0.41–1.82 | 0.69 |
| C5 μg/mL quartiles | ||||
| ≤55.13 | Ref | Ref | Ref | – |
| 55.14–85.09 | 0.83 | 1.02 | 0.49–2.12 | 0.96 |
| 85.10–115.79 | 0.52 | 0.61 | 0.28–1.33 | 0.21 |
| >115.79 | 0.83 | 0.80 | 0.38–1.66 | 0.55 |
| C3a μg/mL quartiles | ||||
| ≤43.18 | Ref | Ref | Ref | – |
| 43.19–85.12 | 1.26 | 1.15 | 0.51–2.56 | 0.74 |
| 85.13–128.68 | 1.76 | 1.63 | 0.75–3.54 | 0.21 |
| >128.68 | 1.58 | 1.37 | 0.63–3.00 | 0.43 |
| C4a μg/mL quartiles | ||||
| ≤12.74 | Ref | Ref | Ref | – |
| 12.75–26.66 | 1.07 | 0.95 | 0.44–2.02 | 0.88 |
| 26.67–50.97 | 1.54 | 1.71 | 0.83–3.55 | 0.15 |
| >50.98 | 0.56 | 0.55 | 0.23–1.27 | 0.16 |
| C5a ng/mL quartiles | ||||
| ≤62.68 | Ref | Ref | Ref | – |
| 62.68–152.49 | 1.00 | 0.95 | 0.45–2.01 | 0.90 |
| 152.50–427.83 | 0.57 | 0.51 | 0.22–1.15 | 0.11 |
| >427.83 | 1.23 | 1.23 | 0.59–2.55 | 0.58 |
| APO–AI quartiles | ||||
| ≤142.5 | Ref | Ref | Ref | – |
| 142.6–162.5 | 0.93 | 1.19 | 0.56–2.53 | 0.66 |
| 162.6–182.0 | 1.17 | 1.39 | 0.67–2.99 | 0.38 |
| >182.0 | 0.90 | 1.19 | 0.55–2.58 | 0.65 |
| APO–B quartiles | ||||
| ≤75.5 | Ref | Ref | Ref | – |
| 75.6–90.0 | 1.41 | 1.68 | 0.82–3.45 | 0.16 |
| 90.1–106.0 | 0.93 | 0.98 | 0.45–2.14 | 0.96 |
| >106.0 | 0.94 | 1.18 | 0.54–2.56 | 0.68 |
Adjusted for age.
Table 7.
Crude and Adjusted Associations (OR) Between Abnormal DA and Visual Function, Macular Pigment Optical Density and Family History of AMD
| Crude |
Adjusted* |
|||
|
OR |
OR |
95% CI |
P
Value |
|
| Visual function† | ||||
| Visual acuity, logMAR | ||||
| ≤0.0, 20/20 or better | Ref | Ref | Ref | – |
| >0.0, worse than 20/20 | 0.93 | 0.72 | 0.42–1.26 | 0.25 |
| Low luminance, logMAR | ||||
| ≤0.30 | Ref | Ref | Ref | – |
| >0.30 | 1.32 | 1.05 | 0.63–1.77 | 0.84 |
| Number of lines of visual acuity “lost” under low luminance | ||||
| >0 and ≤3 | Ref | Ref | Ref | – |
| >3 and ≤4 | 2.65 | 2.92 | 1.62–5.26 | 0.0004 |
| >4 and ≤8 | 1.95 | 1.83 | 0.88–3.84 | 0.11 |
| Contrast sensitivity | ||||
| <1.6 | 2.08 | 1.70 | 0.97–2.97 | 0.064 |
| ≥1.6 | Ref | Ref | Ref | – |
| Family history of AMD | 1.43 | 1.54 | 0.63–3.77 | 0.35 |
Adjusted for age.
Data presented for eye tested for dark adaptation.
Discussion
In this study, approximately one-quarter of older adults with normal macular health as defined by a widely accepted AMD grading scale nevertheless had abnormal rod-mediated DA. The prevalence of abnormal DA increased with increasing age. Since our sample was a clinic-based sample, this prevalence estimate is not population-based, yet it does illustrate that abnormalities in rod-mediated DA are not uncommon among older adults with normal fundus appearance seeking primary eye care.
As discussed earlier, it is well established that scotopic dysfunction, including slowed rod-mediated DA, is observed commonly in laboratory studies on early and intermediate AMD,1–7,15,16 and patients' subjective reports on questionnaires vision problems under low luminance8–12 are consonant with the laboratory findings. The goal of this investigation was to examine to what extent previously identified risk factors and biomarkers for AMD are associated with delayed rod-mediated dark adaptation in older adults free of this disease. One of our most prominent findings is that those with abnormal DA are 3 to 4 times more likely to have elevated plasma CRP levels compared to those with normal DA. Previous epidemiologic research has indicated that elevated CRP is an independent risk factor for AMD and its progression,37,55,56 and it may be a marker of early oxidative stress.57 Furthermore, those with abnormal DA were 2.5 times more likely to have elevations in the complement protein C3, which is linked genetically to risk for AMD.58 Interestingly, although C3 and CRP were elevated in participants with abnormal DA, and CRP can activate complement,59 the activation fragment C3a was not elevated, consistent with the concept of aging as a state of para-inflammation60 (inflammation-preparedness). Both CRP and C3 are acute phase proteins synthesized by hepatocytes.61,62 C3 is also expressed by chorioretinal cells.63,64 C-reactive protein appears in choroidal vascular endothelium of normal aged eyes.64–66 Of importance to outer retinal barrier function, which is probed by dark adaptometry, CRP immunoreactivity in BrM of aged eyes is low,66 even though cholesterol, a prominent CRP binding partner67,68 is abundant in this tissue.69 It is possible that higher plasma CRP levels may drive its deposition in the choroid, or that low-level inflammation in the choroid may raise CRP levels specifically there, with consequences for outer retinal function. Independent assessments of choroidal health, perhaps through optical coherence tomography imaging70 and RPE health, through assessment of fundus autofluorescence, may be informative in answering this question.
Our analyses indicated that persons who reported abstaining from alcohol use or were heavy users were more likely to have abnormal DA, whereas those who were moderate drinkers were more likely to have normal DA. The literature on alcohol and dark adaptation is scarce. Previous research has addressed the acute effects of alcohol on dark adaptation71 and dark adaptation in persons with alcoholism,72 but there has been little to no attention to how different levels of alcohol use over extended periods of time (e.g., light, moderate, heavy drinkers, or abstainers) impact dark adaptation. Thus, there is little empirical framework for interpreting our findings. With respect to the abstainers' elevated risk for DA impairment, one might argue that these persons are abstainers because they are more likely to have significant medical comorbidities, and, thus, their frailer health is mediating the association between alcohol abstention and impaired DA. However, we further adjusted the analysis for the potentially confounding effects of high blood pressure, cancer, urinary problems, and hearing impairment, and the association between abstainers (adjusted OR = 2.82; CI, 1.37–5.80) and those who consumed >14 drinks per week (adjusted OR = 3.15; CI, 0.90–11.06) with abnormal DA persisted. Chronic, heavy alcohol use has been associated with increased risk for early and late AMD.43,73 The biological mechanisms underlying this risk remain unclear, although the neurotoxic properties of alcohol have led to speculation that oxidative stress or damage to the mechanisms protecting against oxidative stress contribute to AMD pathogenesis.73 To what extent slowed rod-mediated DA can be attributed to these mechanisms in chronic, heavy drinkers also is unknown.
The J-shaped relationship in Figure 2 between alcohol use and abnormal DA is reminiscent of findings in the cardiovascular literature suggesting a reduction in coronary heart disease risk with moderate consumption of alcohol.74,75 Alcohol use also is associated with CRP values in a similar fashion,76,77 which raises the question as to whether alcohol use and CRP are independently associated with abnormal dark adaptation, or whether alcohol use exerts its impact on DA through CRP. We computed a separate model to evaluate the association of drinking status on abnormal DA after further adjusting for CRP. The adjusted point estimates for drinking status (abstainers, OR = 2.56; 1–7 drinks/wk, OR = 0.90; 8–14 drinks/wk, OR = 1.69; >14 drinks/wk, OR = 2.63) and CRP (quartile 2, OR = 2.48; quartile 3, OR = 3.66; quartile 4, OR = 2.58) remained relatively unchanged after adjustment for each other, suggesting alcohol use and CRP have independent associations with abnormal DA.
Those with abnormal DA were more likely to have a greater drop in visual acuity under low luminance (mesopic) conditions compared to those with normal DA. This association persisted after further adjusting for intraocular lens status. At first glance, one could surmise that this association may represent the two tests' common reliance on rod photoreceptor function. However, the low luminance visual acuity test, although performed under mesopic conditions, was a foveal test and, thus, was presumably heavily reliant on cones; however, the mechanisms underlying this low luminance acuity measure have not been elucidated. Although our data suggested that the decrease in visual acuity under mesopic conditions is statistically associated with the rod-intercept parameter, the relationship between the low-luminance drop in visual acuity and the rod-intercept actually is quite weak (Spearman ρ = 0.14), indicating that one test is not an acceptable surrogate for the other.
Participants with high blood pressure were more likely to have abnormal rod-mediated DA. To our knowledge, there have been no previous reports on this relationship. If replicable, further research should explore potential retinal and vascular mechanisms underlying this relationship, including the role of antihypertensive medications in any observed association. Previous research suggests that high blood pressure moderately elevates the risk for AMD.78 We also observed an association between hearing impairment and abnormal DA, with hearing impairment increasing the odds of abnormal DA by 65% (although this finding was of borderline statistical significance). Many previous studies, including those assessing hearing and vision status psychophysically and by self-report,79,80 have noted the common co-occurrence of hearing and vision impairment in older adults; such findings are attributed usually to shared risk factors or a generalized biological aging of the central nervous system.79,81 In the vast majority of these studies, vision was measured by assessing visual acuity; there have been no previous reports on hearing's relationship to DA. Hearing impairment also has been reported to be a risk factor for AMD.82,83
Those persons with a self-reported history of cancer were more likely to have normal DA, a finding that is puzzling. Compared to those without cancer, participants with a history of cancer were older, more likely to have a history of arthritis and chronic pulmonary disease, and had lower C3a values and higher C4a values. However, after adjusting for these potential confounders, the association between cancer (OR = 0.29; CI, 0.13–0.63) and abnormal DA remained statistically significant. Future research may resolve whether this is a spurious or replicable finding.
Those with normal versus abnormal DA had similar levels of apo B and apo A-I. This finding parallels previous studies that have shown similar distributions of plasma lipids in early AMD and normal controls,84 and are consistent with the concept that lipoproteins involved in extracellular lipid deposition in BrM and drusen are produced within the eye.64,85–87 It is interesting that we have established that those older adults in normal macular health, regardless of their DA status, have similar levels of these serum-based apolipoproteins.
Strength and limitations of this study must be considered. To our knowledge, this is the first study to examine cross-sectional relationships between rod-mediated DA, an established functional risk factor for AMD, and a comprehensive assortment of risk factors for AMD. The sample size of older adults in normal macular health according to the traditional gold standard definition is very large compared to most previous studies on functional characteristics of older adults in normal macular health. A weakness is that our analyses in this study are focused on cross-sectional analyses of risk factors; however, it is important to recognize that ALSTAR is a prospective study with a repeat assessment at a three-year follow-up visit of most of the variables, including AMD presence and severity. In addition, the present study did not include analysis of other factors believed to be relevant in AMD pathogenesis (e.g., dietary intake, macular pigment density, subretinal pathology that might be revealed through optical coherence tomography imaging, genetic factors). However, ALSTAR included the collection of these data with analyses still ongoing with reports forthcoming. We did collect data on smoking status, an established risk factor for AMD. However, we did not find it related to abnormal DA in persons with normal macular health. Prospective data from ALSTAR may clarify the relationship between smoking, DA, and AMD incidence. However, the low representation of current smokers in our sample (approximately 6%) may hamper our ability to address this issue properly. The presence of chronic medical conditions in older adults was assessed by self-report, not medical evaluation. However, self-report has been determined to be a valid and reliable source of information on most chronic medical conditions.88,89 Another, perhaps more important, consideration is whether any lack of agreement is likely to be systematically different between those with and without impaired DA. This is highly unlikely given that the study participants were unaware of their quantitative DA findings at the time they enrolled in the study. Also, it is unlikely they were aware of any associations between these conditions and any DA problems they may have been experiencing. The vast majority of the sample was white so generalizability of our findings to other ethnic/racial groups remains to be determined.
This study has several implications for future research on AMD. It has established that a common functional accompaniment of early AMD, slowed rod-mediated DA, is not unusual among older adults who are in presumptive normal macular health, at least according to a widely accepted definition of disease presence. Clinical trials focused on evaluating AMD prevention strategies might consider rod-mediated DA as a practically efficient screening strategy for identifying candidates for clinical trial enrollment. Our study has demonstrated further that slowing in rod-mediated DA in older adults in normal macular health is associated with a panoply of known risk factors for AMD and its progression, including advanced age, plasma CRP levels, heavy alcohol use or abstention, hypertension, and perhaps hearing problems. These associations can potentially inform the development of epidemiological models of disease causation, provide guidance for basic biological investigations on early AMD pathogenesis, and ultimately may contribute toward an improved understanding about how normal retinal aging transitions into early AMD.
Acknowledgments
Supported by the National Institute on Aging (R01AG04212 and R01AG04212 Supplement), Research to Prevent Blindness, the EyeSight Foundation of Alabama, and the Alfreda J. Schueler Trust.
Disclosure: C. Owsley, Genentech (C), P; C. Huisingh, None; G.R. Jackson, Maculogix (E, S), P; C.A. Curcio, None; A.J. Szalai, None; N. Dashti, None; M. Clark, None; K. Rookard, None; M.A. McCrory, None; T.T. Wright, None; M.A. Callahan, None; L.B. Kline, None; C.D. Witherspoon, None; G. McGwin Jr, None
References
- 1. Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993; 77: 549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000; 41: 267–273 [PubMed] [Google Scholar]
- 3. Scholl HPN, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004; 45: 574–583 [DOI] [PubMed] [Google Scholar]
- 4. Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992; 33: 334–340 [PubMed] [Google Scholar]
- 5. Owsley C, Jackson GR, White MF, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001; 108: 1196–1202 [DOI] [PubMed] [Google Scholar]
- 6. Owsley C, McGwin G, Jackson G, Kallies K, Cone- Clark M. and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007; 114: 1728–1735 [DOI] [PubMed] [Google Scholar]
- 7. Clark M, McGwin G, Neely D, et al. Association between retinal thickness measured by spectral-domain OCT and dark adaptation in non-exudative age-related maculopathy. Br J Ophthalmol. 2011; 95: 1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999; 128: 45–53 [DOI] [PubMed] [Google Scholar]
- 9. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119: 1050–1058 [DOI] [PubMed] [Google Scholar]
- 10. Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002; 109: 1235–1242 [DOI] [PubMed] [Google Scholar]
- 11. Owsley C, McGwin G Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 528–535 [DOI] [PubMed] [Google Scholar]
- 12. Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 1310–1318 [DOI] [PubMed] [Google Scholar]
- 13. Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002; 1: 381–386 [DOI] [PubMed] [Google Scholar]
- 14. Curcio CA, Jackson GR, Owsley C. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000; 41: 2015–2018 [PubMed] [Google Scholar]
- 15. Jackson GR, Clark ME, Scott IU, Walter LE, Quillen DA, Brigell MG. Twelve-month natural history of dark adaptation in patients with AMD [published online ahead of print April 3, 2014]. Optom Vis Sci. [DOI] [PubMed] [Google Scholar]
- 16. Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic test sensitivity and specificity of the AdaptDx for the detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014; 55: 1427–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curcio CA, Johnson M, Huang JD, Aging Rudolf M. age-related macular degeneration, and the response-to-retention of apolipoprotein B-contacting lipoproteins. Prog Retin Eye Res. 2009; 28: 393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curcio CA, Johnson M, Rudolf M, Huang J-D. The oil spill in ageing Bruch's membrane. Br J Ophthalmol. 2011; 95: 1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cankova Z, Huang J-D, Kruth H, Johnson M. Passage of low-density lipoproteins through Bruch's membrane and choroid. Exp Eye Res. 2011; 93: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tserentsoodol N, Sztein J, Campos M, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006; 12: 1306–1318 [PubMed] [Google Scholar]
- 21. Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci U S A. 1958; 44: 648–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kemp CM, Jacobson SG, Faulkner DJ. The effects of vitamin A deficiency on human visual function. Exp Eye Res. 1988; 46: 185–197 [DOI] [PubMed] [Google Scholar]
- 23. Kemp CM, Jacobson SG, Borruat F-X, Chaitlin MH. Rhodopsin levels and retinal function in cats during recovery from vitamin A deficiency. Exp Eye Res. 1989; 49: 49–65 [DOI] [PubMed] [Google Scholar]
- 24. Katz ML, Chen D-E, Stientjes HJ, Stark WS. Photoreceptor recovery in retinoid-deprived rats after vitamin A replenishment. Exp Eye Res. 1993; 56: 671–682 [DOI] [PubMed] [Google Scholar]
- 25. Kemp CM, Jacobson SG, Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988; 46: 185–196 [DOI] [PubMed] [Google Scholar]
- 26. Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004; 23: 307–380 [DOI] [PubMed] [Google Scholar]
- 27. Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002; 36: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garlipp MA, Gonzalez-Fernandez F. Cone outer segment and Muller microvilli pericellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res. 2013; 113: 192–202 [DOI] [PubMed] [Google Scholar]
- 29. Thompson DA, Gal A. Vitamin A megatherapy for retinal abnormalities. Prog Retin Eye Res. 2003; 22: 683–703 [DOI] [PubMed] [Google Scholar]
- 30. Kim JY, Zhao H, Martinez J, et al. Noncanonical autophagy promotes the visual cycle. Cell. 2013; 154: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration. AREDS Report No. 17. Arch Ophthalmol. 2005; 123: 1484–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson GR, Owsley C, McGwin G Jr. Aging and dark adaptation. Vision Res. 1999; 39: 3975–3982 [DOI] [PubMed] [Google Scholar]
- 33. Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993; 34: 3278–3296 [PubMed] [Google Scholar]
- 34. Curcio CA, Messinger JD, Sloan KR, McGwin G Jr, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013; 33: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feurer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical cohorence tomography. Ophthalmology. 2011; 118: 2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005; 19: 935–944 [DOI] [PubMed] [Google Scholar]
- 37. Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between c-reactive protein and age-related macular degeneration. JAMA. 2004; 291: 704–710 [DOI] [PubMed] [Google Scholar]
- 38. Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999; 6: 125–143 [DOI] [PubMed] [Google Scholar]
- 39. Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997; 104: 1677–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hogg RE, Woodside JV, Gilchrist SECM, et al. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008; 115: 1046–1052 [DOI] [PubMed] [Google Scholar]
- 41. Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003; 121: 785–792 [DOI] [PubMed] [Google Scholar]
- 42. Shahid H, Khan JC, Cipriani V, et al. Age-related macular degeneration: the importance of family history as a risk factor. Br J Ophthalmol. 2012; 96: 427–431 [DOI] [PubMed] [Google Scholar]
- 43. Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2008; 145: 707–715 [DOI] [PubMed] [Google Scholar]
- 44. National Health Interview Survey. Questionnaires, datasets, and related documentation 1997 to the present. Centers for Disease Control and Prevention; 1997. Available at: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm. Accessed March 19, 2014 [Google Scholar]
- 45. West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults: the SEE Project. Invest Ophthalmol Vis Sci. 1997; 38: 72–82 [PubMed] [Google Scholar]
- 46. Owsley C, McGwin G Jr, Sloane ME, Wells J, Stalvey BT, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. JAMA. 2002; 288: 841–849 [DOI] [PubMed] [Google Scholar]
- 47. Folstein MF, Folstein SW, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 48. Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003; 135: 194–205 [DOI] [PubMed] [Google Scholar]
- 49. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1988; 2: 187–199 [Google Scholar]
- 50. Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vision Sci. 1991; 6: 471–475 [Google Scholar]
- 51. Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008; 1: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Albers JJ, Marcovina SM, Kennedy H. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. II. Evaluation and selection of candidate reference materials. Clin Chem. 1992; 38: 658–662 [PubMed] [Google Scholar]
- 53. Marcovina SM, Albers JJ, Henderson LO, Hannon WH. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. III. Comparability of apolipoproteins A-I values by use of international reference material. Clin Chem. 1993; 39: 773–781 [PubMed] [Google Scholar]
- 54. Szalai AJ, van Ginkel FW, Wang Y, McGhee JR, Volanakis JE. Complement-dependent acute-phase expression of C-reactive protein and serum amyloid P-component. J Immunol. 2000; 165: 1030–1035 [DOI] [PubMed] [Google Scholar]
- 55. Seddon J, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of c-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005; 123: 774–782 [DOI] [PubMed] [Google Scholar]
- 56. Klein R, Myers CE, Cruickshanks KJ, et al. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: The Beaver Dam Eye Study. JAMA Ophthalmol. 2014; 132: 446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abramson JL, Hooper WC, Jones DP, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005; 178: 155–121 [DOI] [PubMed] [Google Scholar]
- 58. Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk for age-related macular degeneration. New Engl J Med. 2007; 357: 553–561 [DOI] [PubMed] [Google Scholar]
- 59. Du Clos TW. Pentraxins: structure, function, and role in inflammation. ISRN Inflam. 2013; 2013: 379040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu H, Chen M, Forrester JV. Para-inflammatin in the aging retina. Prog Retin Eye Res. 2009; 28: 348–368 [DOI] [PubMed] [Google Scholar]
- 61. Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013; 190: 3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013; 190: 3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li M, Jia C, Kazmierkiewicz KL, et al. Comprehensive analysis of gene expression in human retina and supporting tissues [published online ahead of print April 1, 2014] Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozyous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006; 103: 17456–17461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431 [DOI] [PubMed] [Google Scholar]
- 66. Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011; 95: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taskinen S, Hyvonen M, Kovanen PT, Meri S, Pentikainen MO. C-reactive protein binds to the 3beta-OH group of cholesterol in LDL particles. Biochem Biophys Res Commun. 2005; 329: 1208–1216 [DOI] [PubMed] [Google Scholar]
- 68. Laine M, Jarva H, Seitsonen S, et al. Y402H polmorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007; 178: 3831–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Curcio CA, Millican CL, Bailey T, Kruth H. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001; 42: 265–274 [PubMed] [Google Scholar]
- 70. Spaide R, Koizumi H, Pozonni M. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500 [DOI] [PubMed] [Google Scholar]
- 71. Khan S, Timney B. Alcohol does not affect dark adaptation or luminance increment thresholds. J Stud Alcohol Drugs. 2007; 68: 493–502 [DOI] [PubMed] [Google Scholar]
- 72. Campbell TD, Sampliner RE, Russell RM, Garrett MS. Night driving (mesopic) visual acuity in sober male alcoholics with and without liver disease. Alcohol Clin Exp Res. 1981; 5: 34–37 [DOI] [PubMed] [Google Scholar]
- 73. Adams MKM, Chong EW, Williamson E, et al. 20/20—alcohol and age-related macular degeneration. Am J Epidemiol. 2012; 176: 289–298 [DOI] [PubMed] [Google Scholar]
- 74. Pearson TA. Nutrition Committee of the American Heart Association. Alcohol and heart disease. Circulation. 1996; 94: 3023–3025 [DOI] [PubMed] [Google Scholar]
- 75. Ronksley PE, Brien S, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011; 342:d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Imhof A, Froelich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001; 357: 763–737 [DOI] [PubMed] [Google Scholar]
- 77. Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003; 107: 443–447 [DOI] [PubMed] [Google Scholar]
- 78. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010; 10: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chia E-M, Mitchell P, Rochtchina E, Foran S, Golding M, Wang JJ. Association between vision and hearing impairments and their combined effects on quality of life. Arch Ophthalmol. 2006; 124: 1465–1470 [DOI] [PubMed] [Google Scholar]
- 80. Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults: United States, 1993–1997. MMWR CDC Surveill Summ. 1999; 48: 1331–1356 [PubMed] [Google Scholar]
- 81. Anstey KJ, Luszez MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. J Gerontol B Psychol Sci Soc Sci. 2001; 56B: P3–P11 [DOI] [PubMed] [Google Scholar]
- 82. Klein R, Cruickshanks KJ, Klein BEK, Nondahl DM, Wiley T. Is age-related maculopathy related to hearing loss? Arch Ophthalmol. 1998; 116: 360–365 [DOI] [PubMed] [Google Scholar]
- 83. Klein R, Cruickshanks KJ, Nash SD, et al. The prevelance of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010; 128: 750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dashti N, McGwin G, Owsley C, Curcio CA. Plasma apolipoproteins and risk for age related maculopathy. Br J Ophthalmol. 2006; 90: 1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li C-M, Presley JB, Zhang X, et al. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J Lipid Res. 2005; 46: 628–640 [DOI] [PubMed] [Google Scholar]
- 86. Tserentsoodol N, Gordiyenko NV, Pacual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006; 12: 1319–1333 [PubMed] [Google Scholar]
- 87. Zheng W, Reem R, Omarova S, et al. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One. 2012; 7: e37926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med. 2000; 18: 215–218 [DOI] [PubMed] [Google Scholar]
- 89. Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989; 79: 1554–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]