Abstract
Background: The present study was aimed to determine the effect of Germinated Barley Foodstuff (GBF) administration on serum C-reactive protein (CRP) levels and clinical signs in patients with Ulcerative Colitis (UC).
Methods: Forty-six patients were randomly allocated into GBF group and control group. Subjects in control group received only conventional drug therapy, while the GBF group received 30g GBF per day (3 times a day) by oral administration during 2 month along with routine medications.
Results: The mean serum CRP in the GBF group decreased significantly (P=0.017) compared with the baseline. Although the frequency of clinical signs including the number of episodes diarrhea, degree of visible blood in stool, degree of abdominal pain or cramping, nausea, vomiting, and anorexia decreased in the GBF group but it was statistically significant only in the case of abdominal pain and cramping. However, this reduction was only significant in the case of abdominal pain and cramping (P=0.016)
Conclusions: The consumption of GBF along with the current medication may be efficient in attenuating the inflammation and clinical signs of UC patients.
Keywords: Germinated barely foodstuff, Ulcerative colitis, C - reactive protein, Clinical signs
Introduction
Inflammatory bowel disease (IBD) which includes two main forms, Crohn's disease and ulcerative colitis (UC), is a common disorder characterized by recurrent and serious inflammation of the gastrointestinal tract immunologically mediated.1 The worldwide incidence and prevalence of IBD is constantly rising. Between 20,000 and 100,000 people are diagnosed with these diseases annually in North America.2 Although the highest incidence and prevalence rates have been reported from northern Europe, the UK, and North America, but these rates are continuing to rise in low-incidence areas such as southern Europe, Asia, and most developing countries.3 Patients suffer periodic attacks of cramps, abdominal pain, diarrhea, fever, loss of appetite, rectal bleeding, mucous in the stool, and weight loss.4,5 IBD has a significant impact on an individual’s health-related quality of life.6 UC is an idiopathic, chronic inflammatory disorder of the colon.7
The knowledge of the etiology and pathogenesis of the inflammation in ulcerative colitis is still insufficient.8 The pathogenesis of UC includes an abnormal immunological response to disturbed intestinal microflora.9 Under normal situations, the intestinal mucosa is in a state of controlled inflammation regulated by a delicate balance of pro-inflammatoryand anti-inflammatory cytokine and stimulation of the mucosal immune system through gut microbiota determining a state of low-grade physiological inflammation. Once this balance is disturbed, it induces abnormal immune responses to luminal bacteria, disturbs regulatory balance, increases production and release of strong destructive immunological and inflammatory molecules.10-12
Prebiotics are selectively fermented ingredients which escape from digestion in the upper intestinal tract and allow specific changes both in the composition and/or activity of gastrointestinal microflora.12,13 Germinated barley foodstuff (GBF) is a prebiotic product derived from the aleurone and scutellum fractions of germinated barley and consists of insoluble glutamine-rich protein and dietary fiber.13-15
Since C-reactive protein (CRP) is an objective marker of inflammation in gastrointestinal diseases16 and the clinical signs are common in UC.17 Therefore, this study was aimed to investigate the effects of orally administered germinated barely foodstuff on serum CRP levels and clinical signs in ulcerative colitis patients in remission course.
Material and Methods
GBF preparation
GBF mainly consists of water-insoluble dietary fibers and glutamine-rich proteins. The detailed production process of GBF was described previously. Briefly, after germination, barley is mashed and filtered to extract the endosperm, and then residue is milled and sieved to obtain germinated barley foodstuff.13,15
Subjects and treatments
This study was an open-labeled trial as a pilot study. The participants (28 males and 18 females) were selected from UC patients in remission course. In all patients, the disease activity was mild or moderate according to the Truelove & Witts criteria based on the number of f bloody stools per day, hemoglobin, Erythrocyte sedimentation rate (ESR), pulse rate and body temperature.18 Exclusion criteria were having UC disease in recurrence course or requiring hospitalization in the last 3 months, using pro or probiotic, antioxidant and omega-3 supplements within the past 3 months, having any other disease or inflammatory condition and using corticosteroid drugs. UC diagnosis was confirmed by a gastroenterologist based on clinical and endoscopic features. From a total of 46 participants, 29 had left-side colitis and 17 had pancolitis.
Approval of this trial was granted by the Ethical Committee of the Nutrition and Food Sciences Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Besides, all subjects were given an informed written consent to be completed and signed.
The patients were randomly allocated into two groups: GBF group and control group (23 patients in each group). Subjects in the control group received only conventional medications while the GBF group received orally administered of 30g germinated barley foodstuff per day (3 times in a day) during 2 month along with conventional medications over the study. Pre- and post-treatment values of serum hs-CRP were measured using a commercial cytokine-specific ELISA kit according to the manufacturer’s instructions (Diagnostic Biochem Canada hs-CRP Elisa kit). Clinical signs including the number of episodes diarrhea, degree of visible blood in stool, degree of abdominal pain or cramping, nausea and vomiting, and anorexia were asked before and after the GBF treatment.
Statistical Analysis
Results were expressed as Mean ± SD. Thevariables were normally distributed using Kolmogorov–Smirnov test. Differences in mean values were analyzed with paired t-test. McNemar test was used to analyze the effect of GBF on clinical signs. Statistical significance value was set at P <0.05.
Results
Three patients from the GBF group and two patients from the control group were excluded from the study because of using corticosteroid drugs (2 patients) and gastrointestinal discomfort (3 patients). Consequently, the data were analyzed for 26 males and 15 females. The demographic characteristics and serum CRP levels of patients are presented in Table 1.
Table 1. The baseline characteristics of participants in GBF and control groups .
| Characteristic | GBF# n = 20 Mean ± SD | Control n = 21 Mean ± SD | P |
| Age (yr) | 33.90±11.76 | 33.04±12.41 | 0.77 |
| Weight(kg) | 67.85±14.84 | 71.0±13.13 | 0.30 |
| Height(cm) | 168.75±9.99 | 172.88±9.91 | 0.19 |
| BMI (kg/m2) | 23.66±4.05 | 23.64±3.10 | 0.98 |
| Disease duration(yr) | 5.25±3.09 | 5.47±1.91 | 0.77 |
| Started age of disease(year) | 27.10±11.43 | 29.04±12.61 | 0.43 |
| Serum CRP (mg/L) | 4.91±1.06* | 3.20±0.64* | 0.17 |
# GBF, germinated barley foodstuff/ * Standard error of the mean (SEM)/ P values based on Independent t tests.
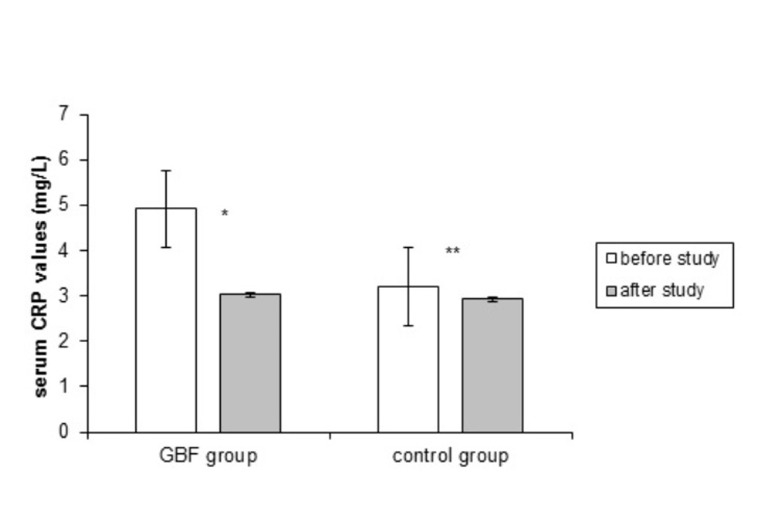
No significant difference was found at baseline characteristics and pre-treatment serum CRP values between the two groups (P=0.17). Figure 1 presents the effect of GBF consumption on serum CRP values. The mean serum CRP in the GBF group decreased significantly (P= 0.017) whereas there was a non-significant decrease in the control group (P= 0.46).
Fig. 1 .
The effect of GBF consumption on serum CRP level (mg/L). Error bars show standard error of the mean
*P-value= 0.017/** P-value=0.46
The effects of GBF consumption on clinical signs of UC were shown in table 2. The frequency of clinical signs reduced in GBF group; however, this reduction was only significant in the case of abdominal pain and cramping (P=0.016). No significant differences were found in other variables in the control group over the study.
Table 2. Comparison of frequency of patients with clinical signs before and after study .
| GBF group(n=20) | P value | Control group(n=21) | P value | |||||||
| Before study N (%) | After study N (%) | Before study N (%) | After study N (%) | |||||||
| Clinical sign | Increased clinical sign | Reduced clinical sign (%) | Not changed (%) | Increased clinical sign | Reduced clinical sign (%) | Not changed (%) | ||||
| chronic diarrhea | 7(35.00) | ----- | 5(25.00) | ----- | 0.50 | 5(23.80) | 1(4.76) | 1(4.76) | 2(9.52) | 1.00 |
| visible blood in stool | 10(50.00) | ----- | 7(35.00) | 1(5.00) | 0.50 | 5(23.80) | 1(4.76) | ----- | 4(19.04) | 1.00 |
| abdominal pain or cramping | 10(50.00) | ----- | 2(10.00) | 1(10/00) | 0.01 | 9(42.9) | 10(47.61) | 2(9.52) | 3(14.28) | 0.07 |
| nausea and vomiting | 4(16.00) | ----- | ----- | 1(5.00) | 0.25 | 3(14.3) | 2(9.52) | ----- | 1(4.76) | 1.00 |
| Anorexia | 6(30.00) | ----- | 1(5.00) | ----- | 0.06 | 4(19) | 1(4.76) | ----- | 3(14.28) | 1.00 |
P values based on Mc Nemar tests
Discussion
In the present study, no side effects were observed on the consumption of germinated barley foodstuff. Although serum CRP levels decreased in both groups, only the reduction in the GBF group was statistically significant. Since the common conventional medications were used in both groups over the study, the cause of this reduction were justifiable in the control group. To the best of our knowledge, there were limited studies assessing the effect of GBF on serum levels of CRP in UC patients. The consumption of GBF in UC patients resulted in the reduction of some inflammatory markers such as serum CRP level.19
We observed a remarkable trend in the reduction of clinical signs in patients with UC after GBF consumption. Mitsuyama, et al. showed a significant improvement in clinical activity index scores in UC patients after 4 weeks of GBF consumption.19 The efficacy of GBF in UC patients in remission state showed that clinical activity index values improved significantly in the GBF compared with the control group.20 Besides, in Kanauchi et al. study, the GBF-treated group showed a significant decrease in the clinical activity index scores (especially, the degree of visible blood in stools and the presence of nocturnal diarrhea) compared to the control.21,22 At baseline 10 patients had reported diarrhea while the episodes of diarrhea decreased in all patients and was eliminated in two cases after treatment. Solemn et al. showed that CRP elevation was significantly associated with severe clinical activity in UC patients.23 In our study, GBF intervention could significantly reduce CRP levels. Therefore, the observed favorable trend in the reduction of clinical signs after GBF consumption may be due to the reduction in CRP concentration. Although the exact mechanism of GBF action is not understood, it seems that it is due to butyrate production by the administration of GBF. GBF or its fiber content significantly increases butyrate production in the lower intestinal tract.24 The previous studies showed butyrate ameliorates inflammation in ulcerative colitis.25
Conclusion
This study showed the consumption of GBF along with routine medication in attenuates the inflammation and appears to be an effective and safe treatment in UC. On the other hand, it can prolong the remission course with an improvement in clinical signs in these patients. The present study was designed as a pilot study and the results may provide a basis for more future clinical trials to obtain the details of molecular and biological anti-inflammatory mechanism of GBF action. Further studies are required to examine the effect of GBF on different inflammatory factors.
Acknowledgments
We thank the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences for financial support, Liver and Gastrointestinal Disease Research Centre, Tabriz University of Medical Sciences and all patients who participated in the study.
Competing interests
The authors declare that there is no conflict of interests.
References
- 1.Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S. et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 2.Blais Lecours P, Marsolais D, Cormier Y, Berberi M, Haché C, Bourdages R. et al. Increased prevalence of methanosphaera stadtmanae in inflammatory bowel diseases. PLoS One. 2014;9:e87734. doi: 10.1371/journal.pone.0087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 4.Head KA, Jurenka JS. Inflammatory bowel disease Part 1: ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247–283. [PubMed] [Google Scholar]
- 5.Head K, Jurenka JS. Inflammatory bowel disease. Part II: Crohn's disease--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2004;9:360–401. [PubMed] [Google Scholar]
- 6.Pizzi LT, Weston CM, Goldfarb NI, Moretti D, Cobb N, Howell JB. et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:47–52. doi: 10.1097/01.mib.0000191670.04605.e7. [DOI] [PubMed] [Google Scholar]
- 7.Kühbacher T, Schreiber S, Fölsch UR. Ulcerative colitis: conservative management and long-term effects. Langenbecks Arch Surg. 2004;389:350–353. doi: 10.1007/s00423-004-0477-8. [DOI] [PubMed] [Google Scholar]
- 8.van Hogezand RA, Verspaget HW. Selective immunomodulation in patients with inflammatory bowel disease--future therapy or reality? Neth J Med. 1996;48:64–67. doi: 10.1016/0300-2977(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 9.Heilpern D, Szilagyi A. Manipulation of intestinal microbial flora for therapeutic benefit in inflammatory bowel diseases: review of clinical trials of probiotics, pre-biotics and synbiotics. Rev Recent Clin Trials. 2008;3:167–184. doi: 10.2174/157488708785700302. [DOI] [PubMed] [Google Scholar]
- 10.Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- 11.MacDermott RP. Alterations of the mucosal immune system in inflammatory bowel disease. J Gastroenterol. 1996;31:907–916. doi: 10.1007/BF02358624. [DOI] [PubMed] [Google Scholar]
- 12.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I. et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanauchi O, Agata K. Protein, and dietary fiber-rich new foodstuff from brewer's spent grain increased excretion of feces and jejunum mucosal protein content in rats. Biosci Biotechnol Biochem. 1997;61:29–33. doi: 10.1271/bbb.61.29. [DOI] [PubMed] [Google Scholar]
- 14.Kanauchi O, Agata K, Fushiki T. Mechanism for the increased defecation and jejunum mucosal protein content in rats by feeding germinated barely foodstuff. Biosci Biotechnol Biochem. 1997;61:443–448. doi: 10.1271/bbb.61.443. [DOI] [PubMed] [Google Scholar]
- 15.Kanauchi O, Nakamura T, Agata K, Fushiki T. Preventive effect of germinated barley foodstuff on diarrhea induced by water-soluble dietary fiber in rats. Biosci Biotechnol Biochem . 1997;61:449–454. doi: 10.1271/bbb.61.449. [DOI] [PubMed] [Google Scholar]
- 16.Vermeire S, Van Assche G, Rutgeerts P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:580–586. doi: 10.1038/ncpgasthep0359. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn's disease. Dig Dis Sci. 2007;52:2063–2068. doi: 10.1007/s10620-006-9691-2. [DOI] [PubMed] [Google Scholar]
- 18.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: preliminary report on a therapeutic trial. Br Med J. 1954;2:375–378. doi: 10.1136/bmj.2.4884.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuyama K, Saiki T, Kanuachi O, Iwanaga T, Tomiyasu N, Nishiyama T. et al. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12:1225–1230. doi: 10.1046/j.1365-2036.1998.00432.x. [DOI] [PubMed] [Google Scholar]
- 20.Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I. et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643–647. [PubMed] [Google Scholar]
- 21.Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T. et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37 Suppl 14:67–72. doi: 10.1007/BF03326417. [DOI] [PubMed] [Google Scholar]
- 22.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A. et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med . 2003;12:701–704. [PubMed] [Google Scholar]
- 23.Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 24.Araki Y, Andoh A, Koyama S, Fujiyama Y, Kanauchi O, Bamba T. Effects of germinated barley foodstuff on microflora and short chain fatty acid production in dextran sulfate sodium-induced colitis in rats. Biosci Biotechnol Biochem. 2000;64:1794–1800. doi: 10.1271/bbb.64.1794. [DOI] [PubMed] [Google Scholar]
- 25.Zapolska-Downar D, Siennicka A, Kaczmarczyk M, Kołodziej B, Naruszewicz M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: the role of NF-kappaB and PPARalpha. J Nutr Biochem. 2004;15:220–228. doi: 10.1016/j.jnutbio.2003.11.008. [DOI] [PubMed] [Google Scholar]