Abstract
Background: Racial disparities in breast cancer outcomes persist, with differential adverse outcomes in African American women. Although research has examined possible genetic differences, there has been little research on potentially modifiable characteristics such as health promoting behaviors. The purpose of this article is to describe the characteristics and to compare the differences by race in lifestyle factors and inflammatory biomarkers in African American and Caucasian women with breast cancer.
Methods: This is a baseline descriptive analysis from an ongoing randomized controlled trial that includes 124 women diagnosed with early stage breast cancer prior to chemotherapy. Data sources included medical records, self-report questionnaires and a blood sample for measures of inflammation. The statistical analysis included descriptive statistics and ANOVA models to determine differences between the two groups.
Results: Overall, both groups had low levels of health promoting behaviors. African Americans had a significantly higher body mass index. Caucasian women consumed more alcohol. Levels of C-reactive protein and MIP-1β were significantly higher in African Americans.
Conclusion: Potentially modifiable factors such as nutrition, physical activity and levels of inflammation warrant further attention.
Keywords: Breast cancer, Biomarkers, lifestyle, Disparities, Creactive protein
Introduction
The American Cancer Society projected that in the United States, there will be 232,340 women diagnosed with breast cancerand that breast cancer will be the second largest cause for cancer deaths, following lung and bronchial cancer.1 Breast cancer has been widely studied with respect to factors that contribute to its risk, incidence, progression, treatment response and recurrence. As of January 2012, the American Cancer Society (ACS) has estimated that there are more than 2.9 million women in the US who have a past history of invasive breast cancer.1 The National Cancer Center database reports the 5-year survival rate for breast cancer as 88%, 81%, 74%, 67%, 41% and 49% for stages I, IIA, IIB, IIIA, IIIB and IIIC, respectively.2 Some of the factors affecting breast cancer outcomes include socio-economic and environmental conditions, genetic predisposition and family history. 3 Over the years, significant advances have been made in early detection and screening of breast cancer patients. However, these steps have not resulted in uniform beneficial effects for all women. The National Cancer Institute defines "cancer health disparities" as adverse differences in cancer incidence (new cases), cancer prevalence (all existing cases), cancer death (mortality), cancer survivorship, and burden of cancer or related health conditions that exist among specific population groups in the United States.4
African American women have the highest mortality rates and lower 5-year survival rates from breast cancer, in contrast to Caucasian women who have the highest incidence and better survival rates. One of the reasons for this inequality could be that African Americans are diagnosed in their later stages compared to Caucasians due to various reasons.5 African American women had more aggressive triple negative breast cancers than Caucasian women and this could probably be attributed to the heterogeneity of genetic marker expression of breast cancer in different races.6 Studies show the existence of defective insulin secretion and a significant association between a specific polymorphism and type 2 diabetes in African Americans when compared to Caucasians. African Americans have higher serum insulin levels and insulin resistance.7-9 These metabolic factors could play a role in cancer disparities. However, there are potentially modifiable risks that warrant greater exploration in breast cancer outcomes.
Background and Literature Review
In recent years, advanced and efficient screening practices and research have made possible early detection of breast cancer patients. Susceptibility to cancer is determined by various factors such as family history, genetic predisposition, environmental factors and the presence of other illnesses or disorders.10-11 In addition, some modifiable risk factors for breast cancer such as body mass index (BMI), level of physical activity, nutrition intake, alcohol consumption, smoking and health awareness have been identified. Biologically, inflammation is one of the hallmarks of cancer and plays a role in various facets of tumor biology including initiation, progression, angiogenesis and metastasis.12 Meta-analysis of studies analyzing the role of physical activity on breast cancer incidence shows a 25% decrease in the incidence when comparing women with most physical activity to those with the least activity.13 There is also some evidence that the synergistic effects of nutrition and physical activity may reduce the reoccurrence of breast cancer.14 Psychological well-being is also important in the survivorship of women with breast cancer. Almost 2% of women recently diagnosed with localized breast cancer reported symptoms of posttraumatic stress after the diagnosis.11 Moreover, increased spiritual well-being strongly correlates with reduction in pain, anxiety and hopelessness and improvements in overall health and quality of life in cancer patients.15-16 From a biological perspective, a tumor microenvironment presents with inflammation and an increase in the levels of pro-inflammatory cytokines. Cell-based, pre-clinical and clinical evidence show that inflammation in the breast could play a role in the development of aggressive tumors.17 Cytokines are important molecules that are secreted as an immune response to inflammation, carcinogens and infections. Cytokines could be either pro-inflammatory (Interleukin-1 [IL-1], Interleukin-6 [IL-6], Interleukin-15 [IL-15], Interleukin-17 [IL-17], Interleukin-23 [IL-23], Tumor Necrosis Factor- α [TNF-α]) or anti-inflammatory (Interleukin-4 [IL-4], Interleukin-10 [IL-10], Interleukin-13 [IL-13], Tumor Growth Factor [TGF]) and can be categorized into various sub-families such as interleukins, interferons [IFN], colony stimulating factors, growth factors, TNFs and TGFs.18 However, the relative proportions of pro-inflammatory and anti-inflammatory cytokines collectively dictate the overall inflammatory state of an individual. Increased concentrations of serum IL-6 is associated with advanced stages in various cancers such as prostate, breast and ovarian cancers.19 IL-6 plays a role in promoting proliferation and survival of premalignant cells.20 Similarly, IL-8 plays a role in the progression and reoccurrence of cancer in nasopharyngeal carcinoma and ovarian cancer respectively21 IL-10 on the other hand acts as an inhibitory factor and suppresses the production of IL-1β, IL-6, IL8 and TNF-α.22 C-reactive protein (CRP) is a protein synthesized in the liver and is found to increase in blood in response to inflammation. CRP is associated with tumor stage and the pathological features of colorectal and breast cancers and is also a predictor of poor survival rates in patients with different malignancies.23-27 In spite of advances in treatment, disparities in the risk and incidence of breast cancer in have been observed.28 Concern associated with the existing trends has led the American Cancer Society to set elimination of cancer disparities as one of their challenge goals for 2015.29
Research to examine the interrelationships of potentially modifiable biological factors such as levels of inflammation and potentially modifiable variables focusing on health promoting behaviors in different racial and ethnic groups is surprisingly lacking. Therefore, the current study sought to (a) describe the health promoting lifestyle behaviors, demographic characteristics, and inflammatory status of women with early stage breast cancer prior to chemotherapy and (b) to compare these variables in African American and Caucasian women.
Materials and Methods
Participants and Procedures
This study was approved by the University’s Institutional Review Board after obtaining approval by the institution’s Protocol Review and Monitoring System Committee. The sample for this study was from a randomized controlled trial to determine the effects of a portable, electrical device on the symptom experience of women receiving chemotherapy for early stage breast cancer. Participants were recruited from multiple sites affiliated with a National Cancer Institute Designated Cancer Center. Enrollment began in 2009 and was completed in 2013. Participants were greater than 18 years of age and were diagnosed with early stage breast cancer. All women had a performance score less than 2 according to Eastern Cooperative Oncology Group criteria and were scheduled to receive neo-adjuvant or adjuvant chemotherapy. Potential participants were identified by their healthcare providers and were approached in the clinical setting or by telephone to ascertain interest in participation.
Interested participants were assessed for eligibility using information provided by physicians and self-report. Individuals were consented and assessed prior to chemotherapy initiation. Women were not enrolled in the study if they had dementia or active psychosis, a history of a seizure disorder, had an implanted electrical device or had begun taking antidepressants or other psychotropic medications within 30 days of beginning chemotherapy as this may have skewed questions regarding feelings of sadness as well as confounded inflammation results.
Design
This was an analysis of the baseline data from a randomized controlled trial. Participants were enrolled from a breast clinic in a university health science center with an NCI-designated Cancer Center. Participants were enrolled in the major clinic as well as three affiliate sites located across the State. Trained research personnel conducted participant enrollment, consent and data collection. The demographic information for this analysis was collected from participants and medical records. The behavioral data was self-report using a validated measure and was administered to participants by trained study personnel or completed by the participant after receiving instructions from trained study personnel.
Measures
Demographic and disease profile: Information regarding patient demographic characteristics was ascertained using a standardized questionnaire.
Health Promoting Lifestyle Profile: Information regarding patient lifestyle behaviors were assessed using the Health Promoting Lifestyle Profile II (HPLP-II). The HPLP-II is a 52-item scale, developed to measure health promoting behavior which was conceptualized as a multi-dimensional concept which includes initiation of actions and perceptions by an individual to maintain or enhance one’s own wellness.30 Each item is rated on a four point scale from 1-4 and items are rated as “0” for never, “1” for sometimes, “2” often, and “3” for routinely. The HPLP scale has six domain including health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations and stress management. The total scores of the 6 subscales of health promoting behaviors were calculated by adding the 4-point Likert scale scores for all items within each subscale. The total score was calculated as the sum of all subscale scores. Lower scores indicate less health promoting behaviors. Sample items from the scale include items that ask respondents to indicate the frequency with which they engage in health behaviors in positive ways.” The alpha coefficient of internal consistency for the total scale was 0.9; alpha coefficients for the subscales ranged from 0.8 to 0.9. The 3-week test-retest stability coefficient for the total scale was 0.9.30
Inflammatory Biomarkers: Inflammation was measured by serum cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, IFN- γ, Monocyte Chemotactic Protein-1 [MCP-1], Macrophage Inflammatory Protein-1β [MIP-1β], and TNF-α) and C-reactive protein (CRP). Blood was drawn by a trained healthcare professional in the clinical setting via venipuncture or from an implanted access device. Blood was collected in an 8 ml vacutainer and transported for processing. Plasma was separated by centrifugation, and all specimens were aliquoted immediately, frozen, and stored in an –80°C freezer. Plasma concentrations of the cytokines were measured with a 17-plex bioplex assay (Biorad, Hercules, CA, USA). After incubation, contents of each microplate well were drawn into the Bio-Plex array reader (BioPlex Pro® (Bio-Rad; Hercules, CA, USA), and precision fluidics aligned the beads in a single file through a flow cell where two lasers excited the beads individually. High-speed digital signal processors and Bio-Plex Manager software (Bio-Rad) recorded the fluorescent signals simultaneously for each bead. Levels of CRP were determined with a high-sensitivity enzyme-linked immunosorbent assay (DRG International Inc, NJ, USA).
Data analysis
Descriptive statistics were computed for demographic and disease characteristics and two-sample t-tests were calculated to determine the differences between the groups for continuous demographic and lifestyle variables and a likelihood ratio test for categorical variables. Biological markers were log transformed in an attempt to satisfy the normality of the ANOVA models. The replacement values for biological markers less than the limit of detection were chosen to be 1/2 the distance between the smallest observed cytokine value and 0. The smallest observed cytokine value was determined from looking at multiple datasets from multiple populations. MANOVA was performed to analyze differences in the cytokine levels in African Americans and Caucasians. Individual ANOVA models were run subsequent to the MANOVA for each cytokine and CRP to determine differences in race. All computations were completed using the JMP 10 statistical discovery software from SAS. Level of significance was set at P = 0.05.
Results
Table 1 presents the demographics and disease characteristics data. Health characteristics showed that the mean weight and BMI of the participants were 178 pounds and 31 respectively and 61% were post-menopausal. Caucasian women had higher educational attainment (87%) compared to 73 % among the African American counterpart (P<0.05). A marked difference was also observed in their marital status, especially in the subgroups of ‘never married’ and ‘currently married’. Twenty seven percent of the African American population had never been married versus 5% amongst Caucasians. Fifty-one Caucasians were currently married compared to 19 African American women (P<0.002). Caucasians had a higher proportion of alcohol consumers (57%) (P=0.04), whereas African American women superseded them on the smoking parameter (82%). Forty-seven percent African American women were employed full time and 27% were unemployed compared to 18% unemployment amongst Caucasians.
Table 1. Demographics and Sample Characteristics .
| Caucasian (n=79) | African American (n=45) | Total (n=124) | P-value | |
| Age (mean ± SD) | 52.7 ±10.13 | 51.1 ±8.39 | 52.2 ±9.47 | 0.3412* |
| BMI (mean ± SD) | 28.8 ±7.47 | 34.0 ±9.59 | 30.7 ±8.69 | 0.0012* |
| Height (mean ± SD | 64.4 ±2.67 | 63.5 ±3.09 | 64.0 ±2.90 | 0.0895* |
| Weight (mean ± SD) | 169.7±43.37 | 193.8 ±52.32 | 178.4 ±47.99 | 0.0105* |
| Ethnicity (count (%) | 0.6565† | |||
| Not-Hispanic or Latino | 74 (93.7%) | 43 (95.6%) | 117 (94.4%) | |
| Hispanic or Latino | 5 (6.3%) | 2 (4.4%) | 7 (5.6%) | |
| Education (count (%) | 0.0466†‡ | |||
| Any education High School and less | 10 (12.7%) | 12 (27.3%) $ | 22 (17.9%) $$ | |
| Any education beyond High School | 69 (87.3%) | 32 (72.7%) | 101 (82.1%) | |
| Marital Status (count (%) | 0.0018† | |||
| Never Married | 4 (5.1%) | 12 (26.7%) | 16 (12.9%) | |
| Currently Married | 51 (64.6%) | 19 (42.2%) | 70 (56.5%) | |
| Previously Married | 24 (30.4%) | 14 (31.1%) | 38 (30.6%) | |
| Employment (count (%) | 0.7020†ǂ | |||
| Employed Full-Time | 41 (51.9%) | 21 (46.7%) | 62 (50.0%) | |
| Employed Part-time | 15 (19.0%) | 7 (15.6%) | 22 (17.7%) | |
| Retired | 9 (11.4%) | 5 (11.1%) | 14 (11.3%) | |
| Unemployed | 14 (17.7%) | 12 (26.7%) | 26 (20.9%) | |
| Menopausal Status (count (%) | 0.7648†# | |||
| Pre-menopausal | 32 (40.5%) | 17 (37.7%) | 49 (39.5%) | |
| Post-menopausal | 47 (59.5%) | 28 (62.3%) | 75 (60.5%) | |
| Currently Smoking (count (%) | 0.5063† | |||
| Yes | 18 (22.8%) | 8 (17.8%) | 26 (21.0%) | |
| No | 61 (77.2%) | 37 (82.2%) | 98 (79.0%) | |
| Cigarettes per day (mean ± SD) | 2.7 ±6.04 | 1.6 ±4.23 | 2.3 ±5.46 | 0.2264* |
| ETOH Intake | 0.0392† | |||
| Yes | 45 (57.0%) | 17 (37.8%) | 62 (50.0%) | |
| No | 34 (43.0%) | 28 (62.2%) | 62 (50.0%) | |
| Drinks per week (mean ± SD) | 3.2 ±8.09 | 1.5 ±4.16 | 2.6 ±6.90 | 0.1096* |
SD: Standard Deviation/* Using a two-sample, t-test./† Using the likelihood ratio test for the r x 2 contingency table./‡ Categories for “Didn’t finish High School” and “High School Diploma” were collapsed as ‘Any education High School and below’ to satisfy test assumptions./ǂ Categories for “Unemployed”, “Disabled” and “Student” were collapsed as ‘Unemployed’ to satisfy test assumptions./# Categories for “Pre-menopausal” and Peri-menopausal” were collapsed as ‘Pre-menopausal’ to satisfy test assumptions./$ N= 44 $$ N=123
Table 2 summarizes responses to the subscales for health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations and stress management. The overall lifestyle score had little variability among participants. The participants had high levels of Lifestyle Interpersonal Relations score (sub-scale mean=31), followed by spiritual growth (sub-scale mean=29) and lower levels of physical activity (sub-scale mean=17). The overall lifestyle score as well as the mean score for different scales did not reach significance between races except for nutrition with African Americans having lower scores on the subscalewhen compared to Caucasian women (P = 0.007).
Table 2. Subscales for Lifestyle Behavior .
| Lifestyle Domains | Caucasians (n=79) | African Americans (n=45) | All (n=124) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | t | df | P | |
| Total Lifestyle score | 148.50 ± 19.73 | 148.64± 21.33 | 148.55 ± 20.27 | -0.04 | 122 | 0.971 |
| Health Responsibility | 23.92 ± 5.33 | 25.27± 4.76 | 24.41 ± 5.23 | -1.45 | 122 | 0.149 |
| Physical Activity | 17.42 ± 5.42 | 16.69± 5.37 | 17.15 ± 5.46 | 0.73 | 122 | 0.469 |
| Nutrition | 26.06 ± 4.80 | 23.98± 4.29 | 25.31 ± 4.68 | 2.48 | 122 | 0.014 * |
| Spiritual Growth | 28.87 ± 4.18 | 29.62± 4.90 | 29.15 ± 4.45 | -0.86 | 122 | 0.389 |
| Interpersonal Relations | 30.73 ± 3.91 | 30.29± 4.02 | 30.57 ± 3.90 | 0.59 | 122 | 0.555 |
| Stress Management | 21.49 ± 4.18 | 22.8± 4.56 | 21.97 ± 4.34 | -1.58 | 122 | 0.116 |
P-Value based on independent t-test considering equal variancesfor comparing Caucasians and African Americans/*: Significant difference /
SD: Standard Deviation
Levels of CRP and Cytokines: Blood was drawn from 118 participants and the levels of cytokines and CRP was analyzed using a 17-plex bioplex assay. The pro-inflammatory and anti-inflammatory cytokines analyzed comprised of CRP, Interleukins, Granulocyte-CSF (G-CSF), Granulocyte Macrophage- CSF (GM-CSF), IFN-γ, MCP-1, MIP-1β and TNF-α. Table3 shows the values for each of these cytokines in picograms/ml (median (minimum/maxi-mum) and also states the percentage of samples that had values below the level of detection. IL-2, IL-10, IL-17 and GM-CSF had more than 50% of the samples that had undetected values. Biomarkers were compared in 75 Caucasians and 43 African Americans. Levels of CRP were generally elevated, with a maximum of 35.31 pg/ml and a median of 3.76pg/ml. The African American group showed higher median for all the cytokines measured and CRP when compared to the Caucasian participants, except for IL-7 and MCP-1. It is of note that two anti-inflammatory cytokines IL-4 and IL-13 also showed higher median values (0.58 and 0.46 pg/ml respectively) in African Americans than Caucasians (Table 3).
Table 3. Levels of CRP and Cytokines .
| Caucasians (n=75) | African Americans (n=43) | All (n=118) | P-value | ||||
| Median (Min/Max) | % BLD | Median (Min/Max) | % BLD | Median (Min/Max) | % BLD | ||
| CRP | 2.81(0.04/24.90) | 0 | 6.36(0.06/35.31) | 0 | 3.76(0.04/35.31) | 0 | 0.02 |
| IL-1ß | 0.15 (0.01/2241.53) | 29 | 0.39 (0.01/73.35) | 28 | 0.20 (0.01/2241.53) | 29 | 0.30 |
| IL-2 | 0.01 (0.01/2821.84) | 72 | 0.01 (0.01/27.88) | 79 | 0.01 (0.01/2821.84) | 75 | 0.25 |
| IL-4 | 0.22 (0.01/1400.00) | 23 | 0.58 (0.01/11.23) | 19 | 0.33 (0.01/1400.00) | 21 | 0.32 |
| IL-5 | 0.33 (0.01/1034.36) | 25 | 0.36 (0.01/2.87) | 26 | 0.35 (0.01/1034.36) | 25 | 0.61 |
| IL-6 | 2.07 (0.01/3132.48) | 27 | 3.69 (0.01/20.52) | 19 | 2.60 (0.01/3132.48) | 24 | 0.14 |
| IL-7 | 4.73 (0.01/3072.16) | 1 | 4.15 (0.14/23.83) | 2 | 4.54 (0.01/3072.16) | 1 | 0.32 |
| IL-8 | 5.15 (1.40/104.10) | 0 | 7.03 (1.63/446.50) | 0 | 5.83 (1.40/446.50) | 0 | 0.33 |
| IL-10 | 0.01 (0.01/3867.50) | 67 | 0.01 (0.01/197.38) | 70 | 0.01 (0.01/3867.50) | 68 | 0.77 |
| IL-12 | 1.73 (0.01/14071.40) | 27 | 3.39 (0.01/122.89) | 23 | 1.95 (0.01/14071.40) | 25 | 0.60 |
| IL-13 | 0.16 (0.01/2490.48) | 47 | 0.46 (0.01/88.71) | 37 | 0.21 (0.01/2490.48) | 43 | 0.70 |
| IL-17 | 0.01 (0.01/771.19) | 68 | 0.01 (0.01/98.43) | 53 | 0.01 (0.01/771.19) | 63 | 0.19 |
| G-CSF | 8.01 (0.10/3155.28) | 8 | 10.83 (0.10/33.83) | 9 | 9.30 (0.10/3155.28) | 8 | 0.93 |
| GM-CSF | 0.01 (0.01/8562.68) | 60 | 0.01 (0.01/180.51) | 60 | 0.01 (0.01/8562.68) | 60 | 0.86 |
| IFN-γ | 11.93 (0.10/29359.56) | 31 | 23.70 (0.10/524.14) | 14 | 17.88 (0.10/29359.56) | 25 | 0.09 |
| MCP-1 | 31.04 (5.35/2715.62) | 0 | 28.15 (5.42/106.03) | 0 | 30.39 (5.35/2715.62) | 0 | 0.06 |
| MIP-1ß | 74.14 (28.24/165.87) | 0 | 87.04 (28.24/335.37) | 0 | 76.21 (28.24/335.37) | 0 | 0.01 |
| TNF-α | 2.47 (0.01/24874.18) | 19 | 3.35 (0.01/22.36) | 16 | 2.83 (0.01/24874.18) | 18 | 0.92 |
P-Value based on individual ANOVAs between Caucasians and African Americans/ BLD: Below levels of detection
Figure 1 shows the cytokine means and standard errors (in log picograms per ml) that illustrate the differences between the Caucasian and African American women with early stage breast cancer. P-values from the ANOVA of each cytokine and CRP are listed in Table 3. They indicate that the levels of CRP (P= 0.02) and MIP-1β (P= 0.01) were significantly higher and MCP-1 (P= 0.06) and IFN-γ (P = 0.09) were marginally significant in African Americans when compared to Caucasians.
Fig. 1 .
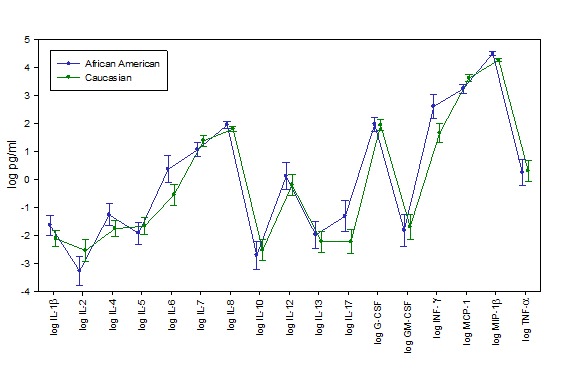
Differences in Levels of Cytokines in African American and Caucasian women with Early Stage Breast Cancer
Discussion
The purpose of the current study was to understand the health promoting lifestyle behaviors, demographic information, and inflammatory status of women with early stage breast cancer prior to chemotherapy and further compare these variables in African American and Caucasian women. BMI was elevated in the majority of the sample, irrespective of race (Table 1) and levels of physical activity were low (Table 2). Both obesity and physical activity are interrelated and is an avenue that should be addressed to enhance outcomes in women with breast cancer.31-32
In this study, African American women had lower scores on the nutritional subscale and this could have contributed toan adverse inflammatory state in the balance between the pro-inflammatory and the anti-inflammatory cytokines.33 Inflammation could also contribute to insulin resistance and disruptions in leptin levels. Women of all races benefitted from physical activity; however, a particularly strong effect on breast cancer risk has been observed in non-Caucasian women.13 It has also been speculated that this association may be attributed to various biological pathways such as adiposity, insulin resistance and chronic inflammation.34 Altogether, this calls for a careful assessment of nutritional status and body composition of women in general, and particularly, in women with early stage breast cancer. In this study, African Americans had a higher median level for multiple cytokines when compared to the Caucasian participants, except for IL-7 and MCP-1 (Table 3). We also observed significantly higher levels of CRP and MIP-1β in African American women than in Caucasian women with breast cancer. CRP is an important biomarker of inflammation that may have prognostic value in breast cancer.35 MIP-1β guides mononuclear phagocytes to form mature macrophages that serve as the main source of growth factors and cytokines and also stimulates production of proteolytic enzymes such as matrix metalloproteinases (MMPs).12
Conclusion
This study highlights potentially modifiable health promoting behaviors that, if addressed, could lead to more optimal self-management strategies for women with breast cancer. This study also identified several differences in inflammatory biomarkers hat warrant further research. With the prediction that the number of breast cancer survivors willreachthe level of 3.4 million individuals by the year 2015, more research is needed to improve the quality of life of this growing group of individuals.36 Addressing potentially modifiable health promoting behaviors early in treatment may set a better stage for optimal long-term outcomes of survivors and may decrease some of the disparities in breast cancer outcomes.
Competing interests
There is no conflict of interest to declare.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures 2013. Atlanta: American Cancer Society; 2013.
- 2.American Cancer Society. Cancer treatment and survivorship facts and figures 2012-2013. Atlanta: American Cancer Society; 2012.
- 3.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institue. Cancer Health Disparities[internet]. 2013[updated 2013cited 2013]. Available at: http: //www.cancer.gov/ cancertopics/factsheet/disparities/cancer-health-disparities
- 5.Loerzel VW, Bushy A. Interventions that address cancer health disparities in women. Fam Community Health. 2005;28:79–89. doi: 10.1097/00003727-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan HC, Oprea-Ilies G, Adams AL, Page AJ, Kim S, Wang J. et al. riple-negative Breast Carcinoma in African American and Caucasian Women: Clinicopathology, Immunomarkers, and Outcome. Appl Immunohistochem Mol Morphol. 2014;22:17–23. doi: 10.1097/PAI.0b013e318281148e. [DOI] [PubMed] [Google Scholar]
- 7.Gower BA, Fernández JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 8.Higgins PB, Fernández JR, Goran MI, Gower BA. Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res. 2005;58:850–854. doi: 10.1203/01.PDR.0000182583.92130.08. [DOI] [PubMed] [Google Scholar]
- 9.Tsai C-J, Giovannucci EL. Hyperinsulinemia, insulin resistance, vitamin D, and colorectal cancer among whites and African Americans. Dig Dis Sci. 2012;57:2497–2503. doi: 10.1007/s10620-012-2198-0. [DOI] [PubMed] [Google Scholar]
- 10.Shannon KM, Chittenden A. Genetic testing by cancer site: breast. Cancer. 2012;18:310–319. doi: 10.1097/PPO.0b013e318260946f. [DOI] [PubMed] [Google Scholar]
- 11.Vin-Raviv N, Hillyer GC, Hershman DL, Galea S, Leoce N, Bovbjerg DH. et al. Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J Natl Cancer Inst. 2013;105:563–572. doi: 10.1093/jnci/djt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenreich CM. The role of physical activity in breast cancer etiology. Semin Oncol. 2010;37:297–302. doi: 10.1053/j.seminoncol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Magné N, Melis A, Chargari C, Castadot P, Guichard JB, Barani D. et al. Recommendations for a lifestyle which could prevent breast cancer and its relapse: Physical activity and dietetic aspects. Crit Rev Oncol Hematol. 2011;80:450–459. doi: 10.1016/j.critrevonc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Garlick M, Wall K, Corwin D, Koopman C. Psycho-spiritual integrative therapy for women with primary breast cancer. J Clin Psychol Med Settings. 2011;18:78–90. doi: 10.1007/s10880-011-9224-9. [DOI] [PubMed] [Google Scholar]
- 16.Yanez B, Edmondson D, Stanton AL, Park CL, Kwan L, Ganz PA. et al. Facets of spirituality as predictors of adjustment to cancer: relative contributions of having faith and finding meaning. J Consult Clin Psychol. 2009;77:730–741. doi: 10.1037/a0015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury-and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamidullah Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 23.Allin KH, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13:R55. doi: 10.1186/bcr2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011;41:510–513. doi: 10.1007/s00595-009-4297-x. [DOI] [PubMed] [Google Scholar]
- 25.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 26.Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer AM. et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- 27.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Diseasese. Vital signs: racial disparities in breast cancer severity-United States, 2005-2009. Morbidity and Mortality Weekly Report . 2012;61:922–926. [PubMed] [Google Scholar]
- 29.Byers T, Mouchawar J, Marks J, Cady B, Lins N, Swanson GM. et al. The American Cancer Society challenge goals. How far can cancer rates decline in the U.S. by the year 2015? Cancer. 1999;86:715–727. [PubMed] [Google Scholar]
- 30.Walker SN, Sechrist KR, Pender NJ. The health-promoting lifestyle profile: Dev-elopment and psychometric cha-racteristics. Nurs Res. 1987;36:76–81. [PubMed] [Google Scholar]
- 31.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer. 2008;112:2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 32.Smith SG, Chagpar AB. Adherence to physical activity guidelines in breast cancer survivors. Am Surg. 2010;76:962–965. [PubMed] [Google Scholar]
- 33.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–788. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 35.Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S. et al. Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2011;26:209–215. doi: 10.5301/JBM.2011.8872. [DOI] [PubMed] [Google Scholar]
- 36.Ellsworth RE, Valente AL, Shriver CD, Bittman B, Ellsworth DL. Impact of lifestyle factors on prognosis among breast cancer survivors in the USA. Expert Rev Pharmacoecon Outcomes Res. 2012;12:451–64. doi: 10.1586/erp.12.37. [DOI] [PubMed] [Google Scholar]


