Abstract
Background
The genotoxic effect of cigarette smoke is routinely measured by treating cells with cigarette Particulate Matter (PM) at different dose levels in submerged cell cultures. However, PM exposure cannot be considered as a complete exposure as it does not contain the gas phase component of the cigarette smoke. The in vitro γH2AX assay by High Content Screening (HCS) has been suggested as a complementary tool to the standard battery of genotoxicity assays as it detects DNA double strand breaks in a high-throughput fashion. The aim of this study was to further optimise the in vitro γH2AX assay by HCS to enable aerosol exposure of human bronchial epithelial BEAS-2B cells at the air-liquid interface (ALI).
Methods
Whole mainstream cigarette smoke (WMCS) from two reference cigarettes (3R4F and M4A) were assessed for their genotoxic potential. During the study, a further characterisation of the Borgwaldt RM20S® aerosol exposure system to include single dilution assessment with a reference gas was also carried out.
Results
The results of the optimisation showed that both reference cigarettes produced a positive genotoxic response at all dilutions tested. However, the correlation between dose and response was low for both 3R4F and M4A (Pearson coefficient, r = -0.53 and -0.44 respectively). During the additional characterisation of the exposure system, it was observed that several pre-programmed dilutions did not perform as expected.
Conclusions
Overall, the in vitro γH2AX assay by HCS could be used to evaluate WMCS in cell cultures at the ALI. Additionally, the extended characterisation of the exposure system indicates that assessing the performance of the dilutions could improve the existing routine QC checks.
Keywords: GammaH2AX, DNA damage, In vitro, Cigarette smoke, Genotoxicity, High content screening
Background
Cigarette smoke is a complex aerosol mixture consisting of more than 6,000 identified compounds that can be divided between the particulate phase, accounting for 4.5% of the total aerosol mixture mass, and the gas phase, accounting for 95.5% of the total aerosol mixture mass [1].
Testing and understanding the toxicity of cigarette smoke in vitro is a key step in the characterisation of modified tobacco products with potentially reduced harm. Adopting such strategies are in line with recommendations published by the Institute of Medicine “Clearing the Smoke” [2] and the World Health Organisation Framework convention on Tobacco Control (WHO FCTC) “The scientific basis of tobacco product regulation” [3].
Johnson and colleagues published a thorough review on the in vitro systems used to evaluate the toxicity of cigarette smoke [4]. In this review, the authors highlighted that the majority of tobacco-related in vitro toxicology studies are carried out in non-human cell models which are poorly validated for tobacco product comparison. They also concluded that better methods are needed, especially in relation to regulation and health claims. In the field of in vitro genotoxicity, the authors described that the evaluation of cigarette smoke has been carried out using mainly cigarette smoke condensate (CSC). However, CSC contains primarily particulate phase components compared to whole mainstream cigarette smoke (WMCS) which contains both particulate and gas phase components. We consider WMCS a more comprehensive exposure system to study toxicological effects in vitro (Table 1). Moreover, the in vitro genotoxicity data has been mainly obtained from animal-derived cell systems which are functionally very different from human-derived cells.
Table 1.
Physical forms of cigarette smoke used in in vitro testing
| Name | Description |
|---|---|
| Cigarette smoke condensate (CSC) |
Comprises the particulate phase along with some vapour phase components. Generated by cold-trapping and condensation of smoke at extremely low temperatures. The condensed ‘tar’ is then typically extracted and diluted using acetone. |
| Cigarette smoke particulate matter (PM) |
Comprises the particulate phase only. Particulates are typically collected by passing cigarette smoke through a Cambridge filter pad and are subsequently eluted from the filter pad using a solvent such as dimethylsulphoxide. |
| Cigarette smoke extract (CSE) |
Comprises the particulate phase along with some vapour phase components. Generated by bubbling smoke through a liquid (e.g. phosphate-buffered saline or cell culture medium). |
| Whole mainstream cigarette smoke (WMCS) | Cells are directly exposed to smoke at the air-liquid interface. This is more representative of human exposure conditions, as cells are exposed to the gas and vapour phase components in an aerosol [5]. |
Table adapted from [6].
There are different in vitro genotoxicity assays that have been widely used in the assessment of tobacco products [4]. Some of the assays described such as the micronucleus or the mouse lymphoma assay focus on fixed DNA damage like chromosomal damage and mutations, their strengths and limitations have been previously summarised [7]. The comet assay is the only assay described by Johnson and colleagues that specifically detects DNA strand breaks. Although the assay is widely accepted and considered a mature method [8], it does not discriminate between single or double strand breaks and has shown high inter- and intra-experimental variation [9]. The in vitro γH2AX assay, on the other hand, is an emerging method for DNA damage detection. The phosphorylation of H2AX (named γH2AX) in response to DNA double strand breaks (DSB) was first described in 1998 [10] and has since been extensively investigated [11]. Some applications in which γH2AX has been used as a biomarker of DNA damage are pre-clinical drug development and clinical studies [12]. More recently, γH2AX has been suggested as a potential complement to the current battery of in vitro genotoxicity assays with applications in the assessment of cigarette smoke [7,13].
The aim of this study was to optimise the novel in vitro γH2AX assay by High Content Screening (HCS) that we had previously developed [14], in order to adapt it for the assessment of aerosols and to evaluate the genotoxic effect of two reference cigarettes in human lung-derived BEAS-2B cells at the air-liquid interface (ALI). The optimisation employs the Borgwaldt RM20S® smoking machine (RM20S®) as part of the exposure system that delivers WMCS to cells at the ALI [5].
The in vitro γH2AX assay has been previously used in the assessment of cigarette smoke using mainly CSC and indirect exposure to WMCS i.e. cell cultures that had a layer of media covering the cells continuously or intermittently during smoke exposure and therefore not considered true ALI exposure [15-19]. In general, flow cytometry has been the main method for γH2AX detection and analysis. In this study, we selected a microscopy-based automated scoring system known as HCS to acquire and quantify the γH2AX response after WMCS exposure to BEAS-2B cells at the ALI. WMCS was tested from two different cigarettes, 3R4F a reference cigarette from the University of Kentucky [20] and M4A a historical control used as internal reference in genotoxicity studies by British American Tobacco [21].
Overall, the results show that the in vitro γH2AX by HCS can be used as a high throughput tool to assess the genotoxic effect of WMCS in cultures exposed at the ALI. The results can be used to compare the genotoxic responses of different tobacco products. Furthermore, the optimised in vitro γH2AX assay for aerosol exposure could be a useful high content screening tool to assess the genotoxic potential of toxicants in gaseous form.
Methods
Cell culture
The human bronchial epithelial cell line BEAS-2B was purchased from ATCC (United States). Normal bronchial epithelium cells obtained from autopsy of non-cancerous individuals had been isolated, then infected with a replication-defective 12-SV40/adenovirus hybrid (Ad12SV40) and cloned to create an immortalised phenotype [22]. Cells were seeded into culture vessels that had been pre-coated with 0.03 mg/mL PureCol® bovine collagen solution (Nutacon, The Netherlands). Cells were then maintained in Bronchial Epithelial Growth Medium (BEGM®) at 37°C and 5% CO2 in a humidified incubator. BEGM® was prepared by supplementing Bronchial Epithelial Basal Medium with growth supplements provided in the manufacturer’s BEGM® SingleQuot® kit (Lonza Group Ltd., Belgium) containing: bovine pituitary extract, hydrocortisone, human epidermal growth factor, epinephrine, insulin, triiodothyronine, transferrin, gentamicin /amphotericin-B and retinoic acid. BEAS-2B cells were cultured and expanded in-house, the cells were used between passages 3 and 12 only. All cultures were negative for mycoplasma. Additionally, the cells were authenticated using the short tandem repeat profiling to confirm the nature of the cell cultures (LGC Standards, United Kingdom) [23].
Smoking system
The selection of the RM20S® 8-syringe smoking machine as the WMCS exposure system was based on previous in vitro studies [5,24] and thorough evaluations of precision, accuracy, repeatability and reproducibility [25,26]. The smoking exposure system is schematically represented in Figure 1. The RM20S® employs a dilution system that mixes WMCS with different proportions of air to generate a dilution ratio represented as 1 : X (smoke volume : air volume). Cigarettes are automatically loaded into cigarette holders (Figure 1A) where WMCS is drawn directly into the glass syringe and diluted with air taken from the laboratory environment (Figure 1B) following a multi-step process operated by a plunger (Figure 1C). The diluted WMCS is then delivered to an exposure chamber (Figure 1D) containing four Transwell® inserts with BEAS-2B cells seeded on top of the insert’s membrane (Figure 1E). At the time of the exposure the cells are directly exposed to WMCS at the air-liquid interface (ALI).
Figure 1.
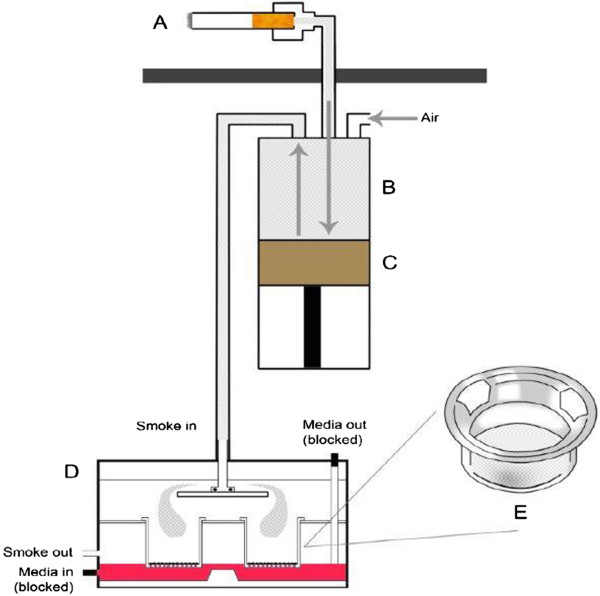
Schematic representation of a single RM20S® syringe combined with British American Tobacco’s exposure chamber (UK patent publication WO 03/100417/ A1) (not to scale). The RM20S® can smoke up to eight cigarettes simultaneously. [A] Cigarette holder with cigarette in place; [B] 150 mL glass syringe where cigarette smoke dilution in air is prepared; [C] Plunger; [D] Exposure chamber containing porous membrane inserts with cells seeded on top at the ALI [E] Transwell® insert representation. Figure adapted from [26].
Dilution performance evaluation
A range of dilutions were selected for this study from 1:25 to 1:20000 (smoke volume : air volume) (Table 2). The methodology employed by Kaur and colleagues used methane (CH4) as a reference gas standard with known parts per million (PPM) to compare syringe performance and has been adapted here to assess dilution performance [25]. For our experiments, three different methane reference standards in nitrogen were purchased from Air Products PLC (United Kingdom), 10% containing 100,000 PPM methane, 50% containing 500,000 PPM methane and 99.95% containing 1,000,000 PPM methane. The relevant reference gas was loaded into a sealed bag and connected directly to the smoking machine cigarette holder (Figure 1A). The dilution to be tested was then programmed into the RM20S® and then gas diluted following International Standard Organization (ISO) 3308:2012 puffing profile consisting of 35 mL puff volume, 2 sec puff duration, and 60 sec puff interval [27]. A second empty sealed bag was connected to the exhaust position in the place of the exposure chamber to collect the diluted gas (Figure 1D). Quantification of methane in PPM was performed with a 3010 MINIFID portable heated flame ionization detector total hydrocarbon analyser (Signal Group Ltd, United Kingdom) as per manufacturer’s instructions. Table 2 summarises details about dilutions, reference gas standard used per dilution and expected PPM. The laboratory environment was conditioned at 22 ± 2°C and 60 ± 5% Relative Humidity (RH).
Table 2.
Range of dilutions, details of methane reference gas and expected PPM
| Dilutions | Expected PPM with 10% methane | Expected PPM with 50% methane | Expected PPM with 100% methane |
|---|---|---|---|
| 1:25 |
4000 |
|
|
| 1:50 |
2000 |
|
|
| 1:100 |
1000 |
|
|
| 1:250 |
400 |
|
|
| 1:500 |
200 |
|
|
| 1:1000 |
100 |
|
|
| 1:1500 |
67 |
|
|
| 1:2000 |
|
250 |
|
| 1:3000 |
|
167 |
|
| 1:4000 |
|
125 |
|
| 1:6000 |
|
83 |
|
| 1:8000 |
|
63 |
|
| 1:16000 |
|
|
63 |
| 1:20000 | 50 |
Smoke exposure
Cigarettes were conditioned for a minimum of 48 hours prior to use (60 ± 3% relative humidity, 22 ± 1°C according to ISO 3402:1999) [28] and smoked continuously throughout the exposure on a RM20S® smoking machine (Borgwaldt KC, Germany) using a 35 ml puff volume drawn over 2 seconds, once every minute according to ISO 3308:2012 [27]. The smoking environment was conditioned at 22 ± 2°C and 60 ± 5% RH.
In this study two reference cigarettes were used to test whether the in vitro γH2AX assay by HCS could discriminate between products. The reference cigarette 3R4F supplied by the University of Kentucky, is a “US style” blended cigarette that delivers 9.4 mg tar and 0.7 mg nicotine under ISO conditions for cigarette smoking (ISO 3308:2012) [27]. Internal reference cigarette M4A is a flue cured cigarette that delivers 10 mg of tar and 1.0 mg nicotine under ISO conditions for cigarette smoking (ISO 3308:2012) [27].
Controls
Etoposide (1 mM final) was used as a positive control during the experimentation (Sigma-Aldrich, United Kingdom). Etoposide is a well-known DNA-damaging compound and has previously been used in the in vitro γH2AX assay by HCS as a reference compound and positive control respectively [14,29]. Two different negative controls were used in this study; air control and incubator control. The air control was generated by the smoking machine to evaluate the quality of the air used to dilute the WMCS and mimic the exposure conditions. The incubator control evaluated the incubation conditions used to generate the positive controls.
WMCS treatment, γH2AX immunostaining and imaging analysis
The methodology used during this study to detect and quantify γH2AX by HCS was previously described [14] with variations for the ALI exposure. In this study, cells were seeded on top of the membrane of collagen pre-coated 24-Transwell® plate (Corning Incorporated Life Sciences, Unites States) at a concentration of 1.2 × 105 cells/mL and 500 μL of BEGM® were added underneath to keep the cells hydrated. The plates were then incubated overnight at 37°C in an atmosphere of 5% CO2 in air. At the time of treatment, the culture media was removed from the Transwell® membrane so the cells could be exposed directly at the ALI. Then, four inserts were transferred to each exposure chamber containing 25 mL of Dulbecco’s Modified Eagle Medium supplemented with 1% L-Glutamine and 0.5% penicillin/streptomycin (10000 IU/mL – 10000uG/mL). The exposure chambers were then placed in an incubator at 37°C and connected with plastic tubing to the smoking machine as represented in Figure 1D (smoke in/smoke out connectors). The smoking machine pre-programmed with the appropriate dilutions was set for a 3 hour exposure. We selected a 3 hour exposure as it is the minimum recommended in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH guidelines) [30]. Following exposure, the inserts were placed in clean pre-labelled 24-well plates where the cells were fixed with 4% paraformaldehyde (100 μL/insert) and incubated for 15 minutes at room temperature. The fixed samples were processed for γH2AX immunostaining following manufacturer’s recommendation (ThermoScientific, United States).
Image acquisition was performed using the Cellomics ArrayScan® VTI platform (ThermoScientific, USA). Image analysis used the Target Activation Bioapplication software v.6.6.1.4. The protocol was set to count a minimum of 500 cells per insert, giving a minimum of 2000 cells per concentration tested. Nuclear DNA staining (Hoechst dye) was used to identify viable cells nuclei. These nuclei were used as the target areas for the measurement of γH2AX specific fluorescence intensity represented as absolute intensity units. Viable cell counts from negative controls were defined as 100% cell viability. The viable cell counts in the WMCS and etoposide treated samples were then compared to those in the negative control, and the percentage cell viability was calculated and referred to as Relative Cell Counts (RCC).
Data analysis and criteria
Dilution performance evaluation
1-sample t-test was used to compare the results obtained in PPM for each dilution with the expected PPM. A variability of ±10% over the expected PPM was included afterwards as accepted measurement variation [31]. Repeatability and Reproducibility statistics were computed for all data points according to ISO 5725–2:1994 [32]. Experiments were replicated 3 times, with 6 repeats per dilution per experiment. Data analysis and graphical representations were performed with Minitab software v.16.
WMCS genotoxicity evaluation
The evaluation criteria used in this study (Table 3) was first described by Smart et al. for the analysis of γH2AX by flow cytometry [13] and recently applied by Garcia-Canton et al. for the analysis of γH2AX by HCS [14]. Experiments were replicated at least three times, with 4 repeats per dilution per experiment and graphical representation was performed using GraphPad Prism software v.6.01.
Table 3.
Genotoxicity evaluation criteria for the in vitro γH2AX assay by HCS
| γH2AX response | Classification |
|---|---|
| > 1.5-fold γH2AX @ RCC > 25% |
Genotoxic (+) |
| < 1.5-fold γH2AX @ RCC 100-0% |
Non-genotoxic (-) |
| > 1.5-fold γH2AX @ RCC < 25% |
“False” positive; Cytotoxic-driven genotoxicity (C) |
| 1.5-fold γH2AX @ RCC ≥ 25% | Equivocal (±) |
Table adapted from [13].
Results
The Borgwaldt RM20S® smoking machine combined with British American Tobacco’s chamber were used as an exposure system during the optimisation of the novel in vitro γH2AX assay by HCS for the evaluation of aerosols.
The initial steps in this optimisation included extending the QC checks of the RM20S® to include 14 dilution performance evaluations (Table 2). From the 10 dilutions generating accurate deliveries, 6 smoke dilutions were selected for further experiments based on range finder experiments (data not shown). The tested smoke dilutions covered a wide range of WMCS dilutions to assess the genotoxicity effect of two reference cigarettes (3R4F and M4A).
Dilution performance evaluation
An initial range of 14 dilutions from 1:25 to 1:20,000 were selected to evaluate the actual dilution delivery in PPM units using reference methane gases (Table 2). The data in Figure 2 graphically represents the results from the statistical 1-sample t-test analysis performed comparing PPM obtained per dilution (box plot) against the expected PPM (red dot), the analysis did not incorporate the ±10% tolerance accepted for machinery measurement variation and was, therefore, added to the expected PPM value afterwards [31]. Results indicate that in the majority of the cases (10 out of 14 dilutions) the dilution delivery was as expected when the ±10% tolerance was included in the analysis. There were four dilutions where the statistical analysis (1-sample t-test) showed a statistically significant difference between measured and expected PPM (including ±10% measurement variation), those dilutions are identified in Figure 2 with a hash (#) (1:1,000, 1:6,000, 1:8,000 and 1:20,000) and were not taken into consideration for the assessment of WMCS in the in vitro γH2AX assay.Figure 3 represents the repeatability and reproducibility results indicating the precision of the smoking machine dilution performance within the same experiment and in different experiments respectively. The repeatability and reproducibility increased linearly with concentration as expected.
Figure 2.
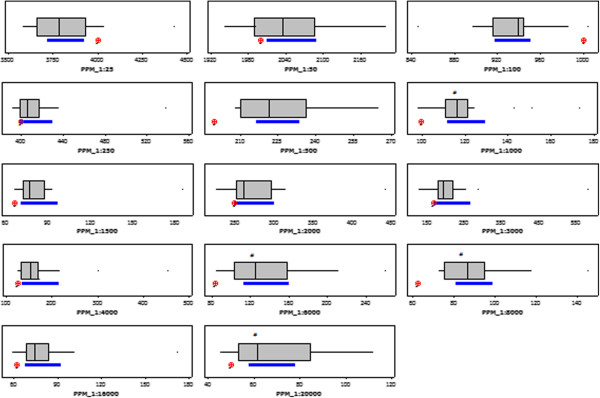
Test dilutions t-test boxplots. Expected PPM (red dot), 95% confidence interval from PPM results (blue line). The asterisk (*) indicates outliers while hash (#) indicates dilutions producing a significant different PPM than expected.
Figure 3.
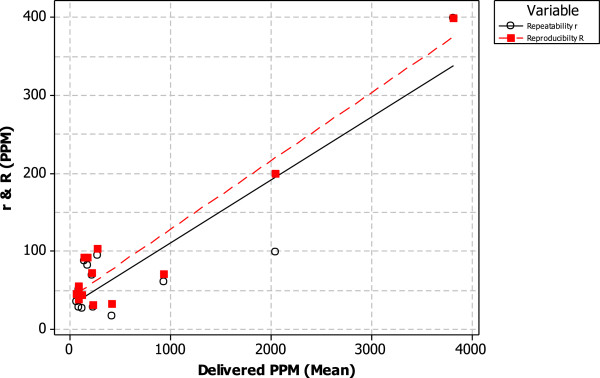
Scatterplot of repeatability (r) (white circle) and reproducibility (R) (red square).
WMCS genotoxicity assessment
Initial range finder experiments showed that 3 hour exposures to WMCS from 3R4F cigarettes at dilutions more concentrated than 1:500 produced tar depositions, this effect was considered equivalent to precipitation. Only dilutions greater than 1:500 were included in further experiments. Both reference cigarettes 3R4F and M4A produced a significant increase in γH2AX frequency (above 1.5-fold increase) compared to the air-treated control in all the dilutions tested (Figure 4). In all experiments the positive control etoposide produced an increase greater than 1.5-fold compared to the air-treated control (Figure 4A and B). Relative Cell Counts (RCC) for all results presented were above the acceptance limit of toxicity (RCC > 25%) and therefore no cytotoxic-driven genotoxicity was observed (Table 3).Figure 4A illustrates the response produced after 3 hour exposure to 3R4F WMCS. A variation in the response can be observed between the most concentrated WMCS (1:500) and the most diluted WMCS dilution (1:16,000). However, the linear regression model indicates a low correlation between the dose and the response (Pearson coefficient, r = -0.53). Figure 4B showed the results obtained after 3 hour exposure to M4A WMCS. In this case, a variation in the response can only be observed at the most diluted WMCS dilution tested (1:16,000). The linear regression model produced a low correlation between the dose and the response (Pearson coefficient r = -0.44). Figure 4C graphically represents the fold-induction results from both reference cigarettes. In general, 3R4F WMCS exposure seems to have a more potent genotoxic effect compared to M4A WMCS exposure, especially at the most concentrated dilution 1:500.
Figure 4.
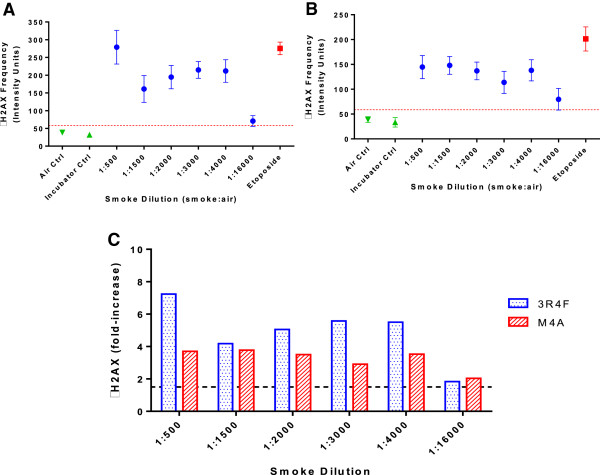
γH2AX frequency mean ± SD after 3 h exposure to WMCS from reference cigarette. [A] 3R4F, [B] M4A. Circle (-●-) represents WMCS results, square (-■-) represents positive control etoposide (1 mM final), triangles (-▼- and -▲-) represents negative controls, air and incubator controls respectively and dotted red line represents the 1.5-fold increase over the air control indicating the threshold of genotoxic response. [C] γH2AX fold-induction for both reference cigarettes 3R4F (blue) and M4A (red), dotted line indicates genotoxic level (>1.5-fold γH2AX response).
Discussion
The main objective of this study was to optimise the novel in vitro γH2AX by HCS for the genotoxicity assessment of aerosols. During the optimisation, the genotoxic potential in the form of γH2AX induction from various dilutions of WMCS of two reference cigarettes were tested and differences in the response evaluated.
The cell system selected was the BEAS-2B cell line, a human-derived cell line from the lung, the first target tissue of inhaled aerosols. The non-tumorigenic human-derived BEAS-2B cell line was isolated from normal human epithelium and immortalised by virus infection [22]. The normal phenotype and wild-type p53 status support the use of this cell line in DNA damage studies [33-35]. BEAS-2B cells, however, lack normal metabolic activity for the majority of cytochrome P450 family, an essential factor for the phase I bioactivation of some cigarette smoke toxicants such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) [36]. The limitation in the metabolic capability of the cell line would need to be considered in future experimental designs i.e. including an external source of metabolic activation in part of the experiments to have a more comprehensive genotoxicity evaluation of the WMCS.
The Borgwaldt RM20S® smoking machine has been extensively used for the in vitro evaluation of WMCS [5,24,37]. Although, some QC analyses have been reported for the accurate performance of the syringes [25,26] further QC tests for the accurate performance of the programmed dilutions have proved necessary. Our results in this study indicate that not all of the programmed dilutions deliver the expected amount of reference gas in PPM (Figure 2). We have observed that more diluted dilutions seem to produce less accurate deliveries; this effect could be caused by the smoking machine dilution programming. The smoking machine performs a multistep process to dilute WMCS with laboratory conditioned air, the process requires the programming of more dilution steps for more diluted dilutions, hence, the potential for more variation. The discrepancy between expected and delivered aerosol could affect the exposure to the cell cultures and ultimately the outcome of the assay. The same approach could be applied in the future to the particulate phase expected in the different dilutions employing Quartz Crystal Microbalances (QCM) previously described for this aerosol exposure system [38]. Nevertheless, the smoking machine performance has shown an overall good reproducibility and repeatability from dilutions delivering 50 PPM or above as can be seen in Figure 3. The performance of syringes and dilutions can be carried out using the same methodology and apparatus already in place for the standard QC checks. Moreover, the extended QC check could easily be incorporated into the routine service of the Borgwaldt RM20S® smoking machine.
The γH2AX results obtained during the assessment of two reference cigarettes seem to indicate that the in vitro γH2AX assay by HCS was able to detect the genotoxic potential of WMCS, however the correlation between the dose and the response was low for both reference cigarettes evaluated in this study across all the tested dilutions (Figure 4). Nevertheless, the γH2AX response obtained after BEAS-2B cells were exposed to a range of 3R4F WMCS dilutions for 3 hours was in general more potent than the response obtained for M4A WMCS and can be visually observed in Figure 4C. If the genotoxicity response was primarily associated to the gas phase we would have expected a better γH2AX dose–response correlation with the different dilutions tested. Therefore, we have considered that the particulate phase may have a significant effect in driving the genotoxic potential. This could be further investigated by characterising the particulates deposited at different dilution levels with tools such as the QCM balance mentioned earlier in this discussion.
It is important to notice that 3 hour continuous exposure as recommended by ICH guidelines [30] could be the longest exposure time a submerged monolayer culture might be exposed at the ALI. In our experiments, the cell cultures were immediately fixed after the exposure to evaluate the DNA damage in the form of γH2AX. Pilot experiments were conducted where the cell cultures were left to recover for a further 24 hours submerged in media to evaluate potential DNA repair after the acute 3 hour exposure. The proliferation of the BEAS-2B cells was greatly affected in WMCS and air control samples. Interestingly, the same effect was not observed in incubator control cultures where the humidity is maintained at a higher level (data not shown). We concluded that for in vitro assays using submerged cultures as cell systems, 3 hour exposure at the current conditions of ALI exposure system would cause irreversible damage due to drying as opposed to aerosol exposure.
Following the optimisation described in this study, further investigations employing different exposure times, a larger number of products and an external source of metabolic activation would be necessary to support the applicability of the in vitro γH2AX assay for the evaluation of tobacco products in aerosol exposure. Future work could also carry out an in-depth characterisation on the effect that product variations such as different tobacco blends have in γH2AX induction to understand the differences in response.
Nevertheless, the optimisation performed here could also be applied to the genotoxicity evaluation of other aerosols such as aerosolised drugs, pollutants and cigarette smoke toxicants present in the gas phase (e.g. benzene).
Conclusions
Overall, the in vitro γH2AX assay by HCS could be used to evaluate WMCS in cell cultures at the ALI. Additionally, the extended characterisation of the exposure system indicates that assessing the performance of the dilutions could improve the existing routine QC checks.
Abbreviations
ALI: Air liquid interfase; BEGM: Bronchial epithelial growth medium; CSC: Cigarette smoke condensate; CSE: Cigarette smoke extract; DSB: Double strand break; HCS: High content screening; ISO: International standard organization; PM: Particulate matter; PPM: Parts per million; QCM: Quartz crystal microbalance; RCC: Relative cell count; RH: Relative humidity; SD: Standard deviation; WHO FCTC: World health organisation framework convention on tobacco control; WMCS: Whole mainstream cigarette smoke.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
CGC, GE and CM designed the study. CGC conducted all the experiments. Data analysis was performed by CGC and GE. CGC wrote the manuscript with GE and CM support. CM and AA provided scientific support. All authors approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Carolina Garcia-Canton, Email: Carolina_Garcia-Canton@bat.com.
Graham Errington, Email: Graham_Errington@bat.com.
Arturo Anadon, Email: Anadon@vet.ucm.es.
Clive Meredith, Email: Clive_Meredith@bat.com.
Acknowledgements
This work was supported by Group Research and Development of British American Tobacco (Investments) Ltd as part of its research programme focusing on reducing the health impact of tobacco use. C. Garcia-Canton, G. Errington and C. Meredith are employees of British American Tobacco. A. Anadón is employee of the University Complutense of Madrid and has not received any funding for this research.
The authors thank Mr N. Asquith, Mr M. Barber and Mr D. Azzopardi for their technical help and support during the experimental phase; and Dr E Minet for his scientific advice while writing this manuscript.
References
- Rodgman A, Perfetti TA. In: The Chemical Components of Tobacco and Tobacco Smoke. 2. CRC Press, editor. Florida (USA); 2013. [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S. In: Clearing the smoke: Assessing the science base for tobacco harm reduction. National Academies Press, editor. Washington, DC (USA); 2001. [PubMed] [Google Scholar]
- World Health Organization. The Scientific Basis Of Tobacco Product Regulation. WHO; 2008. technical report series; no. 951. http://www.who.int/tobacco/global_interaction/tobreg/publications/9789241209519.pdf. [PubMed] [Google Scholar]
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidem Biomar. 2009;18:3263–3304. doi: 10.1158/1055-9965.EPI-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Kluss B, Richter A, Massey E. Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. ATLA. 2005;33:239–248. doi: 10.1177/026119290503300310. [DOI] [PubMed] [Google Scholar]
- Breheny D, Oke O, Faux SP. The use of in vitro systems to assess cancer mechanisms and the carcinogenic potential of chemicals. ATLA. 2011;39:233–255. doi: 10.1177/026119291103900301. [DOI] [PubMed] [Google Scholar]
- Garcia-Canton C, Anadon A, Meredith C. GammaH2AX as a novel endpoint to detect DNA damage: applications for the assessment of the in vitro genotoxicity of cigarette smoke. Toxicol In Vitro. 2012;26:1075–1086. doi: 10.1016/j.tiv.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Sasaki JC, Elespuru R, Jacobson-Kram D, Thybaud V, De Boeck M, Aardema MJ, Aubrecht J, Benz R, Dertinger SD, Douglas GR, White PA, Escobar PA, Fornace A Jr, Honma M, Naven RT, Rusling JF, Schiestl RH, Walmsley RM, Yamamura E, van Benthem J, Kim JH. New and emerging technologies for genetic toxicity testing. Environ Mol Mutagen. 2011;52:205–223. doi: 10.1002/em.20614. [DOI] [PubMed] [Google Scholar]
- Zainol M, Stoute J, Almeida GM, Rapp A, Bowman KJ, Jones GD. Introducing a true internal standard for the comet assay to minimize intra- and inter-experiment variability in measures of DNA damage and repair. Nucleic Acids Res. 2009;37:e150. doi: 10.1093/nar/gkp826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, Bonner WM. H2AX: functional roles and potential applications. Chromosoma. 2009;118:683–692. doi: 10.1007/s00412-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart DJ, Ahmedi KP, Harvey JS, Lynch AM. Genotoxicity screening via the gammaH2AX by flow assay. Mutat Res. 2011;715:25–31. doi: 10.1016/j.mrfmmm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Canton C, Anadon A, Meredith C. Assessment of the in vitro gammaH2AX assay by high content screening as a novel genotoxicity test. Mutat Res. 2013;757:158–166. doi: 10.1016/j.mrgentox.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Albino AP, Huang X, Jorgensen E, Yang J, Gietl D, Traganos F, Darzynkiewicz Z. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle. 2004;3:1062–1068. [PubMed] [Google Scholar]
- Albino AP, Huang X, Jorgensen ED, Gietl D, Traganos F, Darzynkiewicz Z. Induction of DNA double-strand breaks in A549 and normal human pulmonary epithelial cells by cigarette smoke is mediated by free radicals. Int J Oncol. 2006;28:1491–1505. [PubMed] [Google Scholar]
- Albino AP, Jorgensen ED, Rainey P, Gillman G, Clark TJ, Gietl D, Zhao H, Traganos F, Darzynkiewicz Z. GammaH2AX: a potential DNA damage response biomarker for assessing toxicological risk of tobacco products. Mutat Res. 2009;678:43–52. doi: 10.1016/j.mrgentox.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Huang X, Jorgensen E, Gietl D, Traganos F, Darzynkiewicz Z, Albino AP. ATM activation accompanies histone H2AX phosphorylation in A549 cells upon exposure to tobacco smoke. BMC Cell Biol. 2007;8:26. doi: 10.1186/1471-2121-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Albino AP, Jorgensen E, Traganos F, Darzynkiewicz Z. DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry. Cytometry A. 2009;75:840–847. doi: 10.1002/cyto.a.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Kentucky. 3R4F reference cigarette. http://3r4f.com, (accessed September 2013)
- Combes R, Scott K, Dillon D, Meredith C, McAdam K, Proctor C. The effect of a novel tobacco process on the in vitro cytotoxicity and genotoxicity of cigarette smoke particulate matter. Toxicol In Vitro. 2012;26:1022–1029. doi: 10.1016/j.tiv.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- Nims RW, Sykes G, Cottrill K, Ikonomi P, Elmore E. Short tandem repeat profiling: part of an overall strategy for reducing the frequency of cell misidentification. In Vitro Cell Dev Biol Anim. 2010;46:811–819. doi: 10.1007/s11626-010-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey E, Aufderheide M, Koch W, Lodding H, Pohlmann G, Windt H, Jarck P, Knebel JW. Micronucleus induction in V79 cells after direct exposure to whole cigarette smoke. Mutagenesis. 1998;13:145–149. doi: 10.1093/mutage/13.2.145. [DOI] [PubMed] [Google Scholar]
- Kaur N, Lacasse M, Roy JP, Cabral JL, Adamson J, Errington G, Waldron KC, Gaca M, Morin A. Evaluation of precision and accuracy of the borgwaldt RM20S((R)) smoking machine designed for in vitro exposure. Inhal Toxicol. 2010;22:1174–1183. doi: 10.3109/08958378.2010.533840. [DOI] [PubMed] [Google Scholar]
- Adamson J, Azzopardi D, Errington G, Dickens C, McAughey J, Gaca MD. Assessment of an in vitro whole cigarette smoke exposure system: the borgwaldt RM20S 8-syringe smoking machine. Chem Cent J. 2011;5:50. doi: 10.1186/1752-153X-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO. ISO 3308: 2012 - routine analytical cigarette-smoking machine -- definitions and standard conditions. 2012.
- ISO. ISO 3402:1999 - tobacco and tobacco products -- atmosphere for conditioning and testing. 2010.
- Garcia-Canton C, Anadon A, Meredith C. Genotoxicity evaluation of individual cigarette smoke toxicants using the in vitro gammaH2AX assay by high content screening. Toxicol Lett. 2013;223:81–87. doi: 10.1016/j.toxlet.2013.08.024. [DOI] [PubMed] [Google Scholar]
- ICH. Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use S2(R1) 2011. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf. [PubMed]
- Supplier Quality Requirements Taskforce, Daimler Chrysler Corporation, Ford Motor Company, General Motors Corporation. Measurement Systems Analysis, Reference Manual. Michigan, United States: General Motors; 2002. [Google Scholar]
- ISO. ISO 5725–2:1994 - accuracy (trueness and precision) of measurement methods and results -- part 2: basic method for the determination of repeatability and reproducibility of a standard measurement method. 2013.
- Svetlova MP, Solovjeva LV, Tomilin NV. Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks. Mutat Res. 2010;685:54–60. doi: 10.1016/j.mrfmmm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- IARC. TP53 database. http://p53.iarc.fr/, (accessed June 2013)
- Garcia-Canton C, Minet E, Anadon A, Meredith C. Metabolic characterization of cell systems used in in vitro toxicology testing: lung cell system BEAS-2B as a working example. Toxicol In Vitro. 2013;27:1719–1727. doi: 10.1016/j.tiv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Thorne D, Wilson J, Kumaravel TS, Massey ED, McEwan M. Measurement of oxidative DNA damage induced by mainstream cigarette smoke in cultured NCI-H292 human pulmonary carcinoma cells. Mutat Res. 2009;673:3–8. doi: 10.1016/j.mrgentox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Adamson J, Hughes S, Azzopardi D, McAughey J, Gaca MD. Real-time assessment of cigarette smoke particle deposition in vitro. Chem Cent J. 2012;6:98. doi: 10.1186/1752-153X-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]


