Summary
Many tumors express antigens that can be specifically or selectively recognized by T lymphocytes, suggesting that T cell-mediated immunity may be harnessed for the immunotherapy of cancer. However, since tumors originate from normal cells and evolve within the context of self tissues, the immune mechanisms that prevent the autoimmune attack of normal tissues function in parallel to restrict anti-tumor immunity. In particular, the purging of autoreactive T cells and the development of immune-suppressive regulatory T cells (Tregs) are thought to be major barriers impeding anti-tumor immune responses. Here, we discuss current understanding regarding the antigens recognized by tumor-infiltrating T cell populations, the mechanisms that shape the repertoire of these cells, and the role of the transcription factor autoimmune regulator (Aire) in these processes. Further elucidation of these principles is likely to be critical for optimizing emerging cancer immunotherapies, and for the rational design of novel therapies exhibiting robust anti-tumor activity with limited toxicity.
Keywords: tumor, antigen, tolerance, regulatory T cells, Foxp3, Aire
Introduction
Seminal insights into the nature of anti-tumor immunity came in the ‘50’s and ‘60’s from studies using chemically-induced mouse models of primary sarcoma (1–3). These studies demonstrated that syngeneic mice immunized with irradiated sarcoma cells exhibited protection from subsequent challenge with live sarcoma cells (1–3). Immunity was found to be tumor-specific, as immunity to one sarcoma did not confer cross-protection to sarcomas derived from other mice. These early observations provided the framework for the concept that tumor cells express unique antigens that can be specifically recognized by T and B cells, and inspired the long-standing goal of inducing cancer regression in human patients by provoking tumor-specific adaptive immunity. In this regard, the recent clinical successes of immunotherapies for the treatment of cancer, including adoptive cell therapy (4, 5) and the blockade of co-inhibitory receptors (6, 7) have demonstrated that immune-based therapies can be translated successfully from pre-clinical mouse models to the treatment of human malignancy. However, objective clinical responses to these therapies have been observed in only a fraction of patients, and are often accompanied by adverse autoimmune side effects (8). Therefore, an improved understanding of the complexities of immune regulation and the tumor microenvironment is needed to optimize the efficacy of current approaches, and to develop new strategies for immune-based cancer therapy.
Since cancers originate from normal cells and evolve within the context of self tissues, it is thought that the immune mechanisms that impart dominant and recessive tolerance to self antigens function concurrently to restrict the development of anti-tumor adaptive immune responses. “Recessive” tolerance is imparted by the deletion or functional inactivation of lymphocytes exhibiting excessive reactivity to self antigens. Alternatively, “dominant” tolerance is enforced by suppressor cells, such as Tregs, which act in trans to suppress autoreactive lymphocytes. The thymus is a critical site for the establishment of both recessive and dominant tolerance, mediating both the deletion of autoreactive cells by negative selection, and the development of Tregs.
Historically, the study of T cell-mediated anti-tumor immunity has largely focused on the study of CD8+ effector T cells, which are capable of recognizing endogenous peptides displayed on the surface of tumor cells within the groove of HLA class I molecules, allowing CD8+ T cells to scan the interior antigenic space of a tumor cell. Importantly, the sensitivity and specificity of T cell recognition (9) allow CD8+ T cells to distinguish tumor-specific peptides bearing single amino acid changes. Once activated, CD8+ T cells can induce the cytolytic killing of target tumor cells, or promote tumor destruction via secretion of effector cytokines such as IFN-γ or TNF. A primary goal of T cell-based cancer immunotherapy is to elicit CD8+ effector T cells that are able to detect tumor-expressed antigen with high specificity and sensitivity, thereby directing potent effector function at tumor cell targets while limiting collateral damage to normal cells.
Little is known about the HLA class II-restricted antigens recognized by tumor-infiltrating CD4+ “helper” T cells, which participate in the coordination of adaptive immune responses. This is due to a number of factors, including the finding that many tumor cells do not express HLA class II molecules, the fact that CD4+ T cells do not typically exhibit robust cytolytic activity, and the technical challenges associated with identifying class II-restricted antigens (10).
In recent years, CD4+ Treg cells, characterized by expression of the transcription factor Foxp3, have garnered substantial interest in tumor immunology. Tregs are critical for the maintenance of immune homeostasis and the regulation of immune responses to foreign, self, and tumor-associated antigens (11). In many cancers, Treg density within tumor lesions correlates with either negative or positive clinical outcome (12). These findings suggest that Tregs may functionally impact tumor development in a context-dependent manner, via the suppression of anti-tumor immunity, the regulation of tumor-promoting inflammation, or other mechanisms (13). Due to their potent immune-suppressive functions, many emerging strategies for the immunotherapy of cancer aim to augment effector T cell responses by the depletion or blockade of Tregs within the tumor context (14). In this light, it will be important to identify unique aspects of the biology of tumor-associated Tregs that can be exploited for the selective modulation of Tregs in the tumor environment, leaving Tregs elsewhere in the body unaffected (15).
Many aspects of immune regulation, immune tolerance, and anti-tumor immunity have been reviewed extensively elsewhere. The intent of this review is to highlight select topics regarding the immune regulation of tumor-associated T cell responses, using recent examples from the literature and our own research experiences as a framework for our discussion. In particular, we discuss the nature of the antigens and antigen presenting cells that are recognized by tumor-infiltrating CD8+ effector T cells and CD4+ Treg cells. Additionally, we discuss endogenous mechanisms that function to limit autoimmunity and anti-tumor immunity, and the role of Aire-dependent processes that shape the repertoire of T cell subsets in the thymus.
Identification of T cell-defined tumor-associated antigens
While early work in chemically induced mouse sarcomas provided evidence of tumor-specific immunity, similar experiments using spontaneously arising mammary carcinomas failed to reveal tumor-specific immune protection (1, 16), raising critical questions regarding the generality of these principles to different types of cancer, and to the development of cancer in humans. In addition, the molecular basis underlying tumor-specific immunity remained unknown for many years. The development of methods for culturing T cell lines in vitro (17, 18) laid the groundwork for addressing these questions. Using this approach, it was demonstrated that T cell lines expanded from the tumor-infiltrating lymphocytes (TILs) of resected melanoma lesions could specifically lyse autologous melanoma cells (19), demonstrating the existence of tumor-expressed antigens that could be recognized by T cells from human cancer patients. Importantly, many T cell clones exhibited reactivity to melanoma cell lines derived from multiple HLA-matched individuals, suggesting that some of these antigens were “shared” antigens expressed by many tumors (19). Later, in a landmark study by Boon and colleagues, one of the genes encoding a T cell-defined, HLA-restricted melanoma antigen was identified (20). This gene, named MAGE-1, encoded a non-mutated antigen that was found to be expressed by a number of melanoma cell lines, but was not expressed in normal tissues, with the exception of the testes (20). These findings provided the first direct insight into the molecular nature of tumor antigens. Since this initial discovery, hundreds of tumor-associated antigenic peptides have been identified (21). The majority of these tumor antigens are non-mutated self antigens that can be classified into three major categories: 1) the “differentiation antigens”, which are also expressed by normal differentiated cells types, 2) the “cancer testis” antigens such as MAGE-1, which are normally expressed specifically in the testes but are aberrantly expressed by a number of cancer types, and 3) less restricted self antigens that are overexpressed by tumor cells. In rare cases, T cell-defined tumor antigens were found to be “neo-antigens” resulting from mutation (21, 22) or aberrant changes in post-translational modification (23). Taken together, these findings demonstrated that the infrastructure exists by which T cells can specifically or selectively recognize tumor-expressed antigens, raising the possibility of exploiting this infrastructure for the immunotherapy of cancer.
Following the identification of these antigens, progress in the field was restricted by a lack of methods for the direct quantification and phenotypic analysis of antigen-specific T cells in primary samples. Do T cells reactive to tumor-associated antigens exist at measurable frequencies in individuals with melanoma, or are tumor-reactive T cell lines simply an artifact of extensive in vitro culture, the result of multiple rounds of stimulation required to generated T cell lines? The development of peptide/HLA multimer reagents for the direct detection of antigen-specific T cells by Davis and colleagues in 1996 (24) provided a novel approach to address this question. Using multimer reagents, we demonstrated that CD8+ T cells specific for the melanocyte differentiation antigen MART-1/Melan-A were present at detectable frequencies in the peripheral blood of melanoma patients (25). Additionally, an independent study from Romero and colleagues reported the identification of MART-1/Melan-A-specific CD8+ T cells in the tumor-involved lymph nodes of melanoma patients (26), consistent with our findings. Together, these studies provided the first direct evidence that T cells specific for tumor-associated antigens are present at measurable frequencies in the peripheral blood and tumor-involved lymph node of patients with cancer.
Interestingly, the MART-1 specific CD8+ T cells isolated in our study exhibited an antigen-experienced phenotype, but were found to be functionally unresponsive when assayed directly ex vivo (25). In a subsequent study, we utilized peptide/HLA multimers to isolate and expand high-avidity or low-avidity melanoma-reactive T cell clones from bulk resected melanoma TIL cultures (27). We then found that low-avidity clones could kill target cells pulsed with exogenous MART-1/Melan-A peptide, but were unable to lyse autologous tumor cells expressing antigen at endogenous levels. In contrast, high-affinity clones were capable of lysing both peptide-pulsed targets and autologous tumor cells. These results demonstrate that the avidity of T cell recognition of tumor-associated antigens is a primary determinant of T cell sensitivity and subsequent effector response. Consistent with this idea, a recent study in a murine model of transplantable melanoma demonstrated that T cells that recognize a melanoma differentiation antigen with low avidity could be activated by challenge with an antigen-bearing dendritic cell vaccine, but failed to respond to primary tumors in vivo (10). Thus, processes that restrict the development and activation of high-avidity T cells, in this case self-reactive T cells, are likely to limit their ability to detect antigens presented at low densities on the surface of tumor cells.
The concepts discussed above have two important implications for the immunotherapy of cancer. First, the existence of shared tumor-associated antigens suggests that cancer vaccines targeting these antigens could be developed for the treatment of many patients. Second, the possible existence of unique tumor-specific antigens generated by mutation suggests that a patient’s own tumor-infiltrating lymphocytes could be harnessed to induce cancer regression. However, despite this large array of defined tumor-associated antigens, little is known about the cumulative contribution of tumor antigen-specific T cells to the repertoire of T cells infiltrating tumor lesions.
The Occupy Tumor Movement: “Who Are The 99%?”
Are tumor antigen-specific T cells abundant or rare within tumor lesions? Two recent studies provide important insights into this question (28, 29). Analyses using large panels of peptide/HLA multimers bearing defined tumor antigens representing the three major classes of non-mutated antigens discussed above revealed that the cumulative frequency of tumor antigen-specific T cells was low, generally accounting for <1% of all CD8+ T cells in melanoma TIL samples (Figure 1). If tumor antigen-specific T cells are present at low frequencies within tumors, what T cell specificities make up the remaining 99% of the repertoire? One possibility is that many CD8+ T cells may be reactive to tumor-specific “neo-antigens” that are generated by mutational processes driving tumorigenesis (Figure 1). Despite demonstrations of tumor-specific immunity and immunoediting in mouse models of chemically-induced sarcoma (30), the extent to which mutated “neo-antigens” contribute to the antigenic landscape of other murine tumor models or human cancers remains unknown. Of note, T cells specific for previously defined mutated neo-epitopes were not assayed in the aforementioned peptide/HLA multimer surveys (28, 29), primarily because mutated epitopes are expected to be unique to each tumor. Based on mutation estimates from cancer genome sequencing (31), it has been hypothesized that tumors contain numerous unique neo-antigens generated by the mutational processes driving tumorigenesis which may serve as targets for immune surveillance (32). However, until recently, it was not possible to directly test this hypothesis. In this regard, two studies provide novel insight into this issue. In one report from Rosenberg and colleagues, exome sequence data from primary melanomas was used to identify candidate antigenic epitopes encoded by mutated genes (33). The authors demonstrated that three neo-antigen-specific T cell lines, derived from three patients, could be expanded from TIL infiltrates and specifically lyse autologous tumor cells. Since the analysis involved extensive in vitro stimulation of TIL-derived T cell lines, and did not report the results for all predicted neo-antigens, it is unclear whether these neo-antigen-specific T cell clones were abundant within the primary tumor lesions from which the TIL cultures were derived. In a second study from Schumacher et al., the landscape of potential “neo-antigens” was assessed in a human melanoma that had regressed following antibody blockade of the inhibitory co-receptor CTLA-4 (34). Using tumor exome sequencing, 1,075 nonsynonymous mutations were identified in protein-coding regions. RNAseq analysis and bioinformatic prediction methods were then used to identify 448 potential CD8+ T cell neo-epitopes, which were then screened systematically using high-throughput peptide/HLA multimer-based approaches. Of these, T cells reactive to only two of the 448 epitopes were identified in expanded TIL products. Importantly, one of these antigen-specific populations comprised 3% of all CD8+ T cells, and this T cell population was found to be expanded ~5-fold in the peripheral blood following anti-CTLA4 therapy, suggesting that this population may contribute to the tumor regression observed in this patient. Together, these two studies provide an important proof-of-principle that T cells specific for mutated neo-epitopes can be detected in expanded TIL products, thereby providing direct evidence of the existence of endogenous T cells reactive to unique tumor-specific antigens. However, these initial studies also suggest that T cells reactive to mutated antigens may be of low abundance, even in human melanomas, which exhibit one of the highest mutation rates out of all human cancers analyzed thusfar (31). Future work will be required to test the generalizability of these concepts in other cancers. Moreover, it will be important to determine whether tumors exhibiting high mutation frequencies are more likely to regress following antibody-mediated blockade of the inhibitory co-receptors CTLA-4 and PD-1 (8). If this is the case, it suggests that therapies involving CTLA-4 and PD-1 blockade will be most effective for the treatment human cancers associated with the highest mutation frequencies (31).
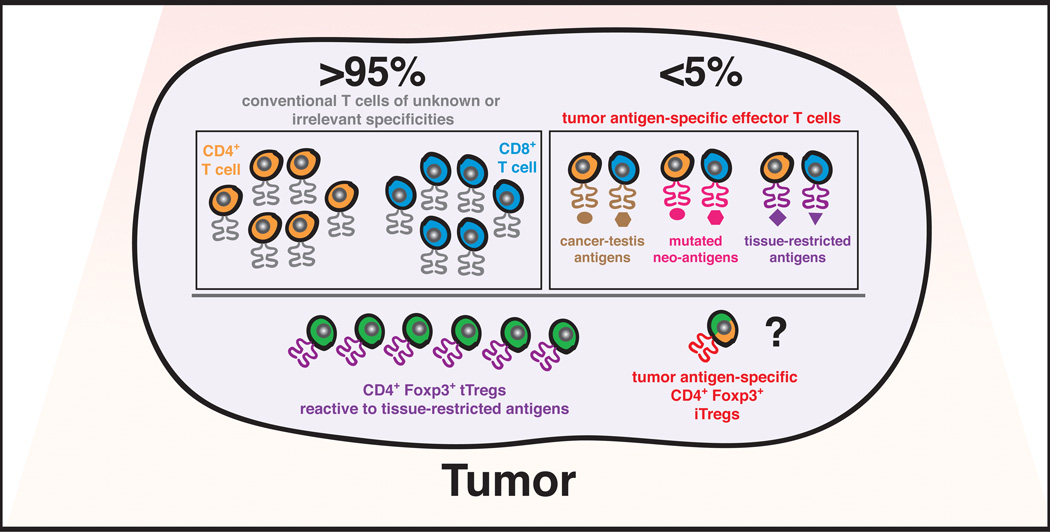
Figure 1. The tumor-infiltrating T cell repertoire.
Proposed model illustrating the relative contributions of αβ T cell populations infiltrating tumor lesions. Due to the impact of central and peripheral tolerance mechanisms on the T cell repertoire (see Figure 2), the frequency of T cells specific for tumor-associated antigens, including cancer-testis antigens, mutated neo-antigens, and tissue-restricted “differentiation” antigens is predicted to be extremely limited. Results from recent surveys of tumor-infiltrating lymphocytes from human melanomas are in agreement with such predictions, demonstrating that tumor-associated antigen specific T cells represent less than 5% of all TIL (28, 29, 34). Therefore, the remaining conventional CD4+ and CD8+ T cells are predicted to be specific for unknown or irrelevant antigens. Moreover, we hypothesize that thymic-derived Tregs (tTregs) reactive to tissue-restricted antigens associated with the organ of cancer origin are likely to be highly enriched in cancer lesions. Based on the premise that CD4+ effector T cells reactive to tumor-associated antigens are likely to be rare, the differentiation of these cells into induced Tregs (iTregs) within the tumor environment is also predicted to be negligible.
If T cell populations reactive to defined tumor-associated antigens are scarce within tumor lesions, what specificities account for the overwhelming majority of tumor-infiltrating T cells (Figure 1)? Our previous work examining endogenous CD8+ T cell responses in a mouse model of oncogene-driven prostate cancer provide potential insight into this question (35). In this study, T cell antigen receptor (TCR) repertoire analysis identified a CD8+ T cell population expressing a highly conserved TCR found recurrently enriched in murine prostate tumors. Surprisingly, these T cells exhibited reactivity to a non-mutated peptide derived from the C-terminus of a ubiquitous protein, histone H4. CD8+ T cells of this specificity were identified in tumor infiltrates using histone H4/Kb multimers, providing direct evidence that cells of this specificity are endogenously and recurrently enriched in prostate tumors, sometimes representing up to 5% of all CD8+ T cells in the tumor. The function of these histone H4-reactive T cells in the tumor environment remains unknown, although the finding that these cells are prevalent in rapidly progressing cancers implies that they do not confer functional protection against tumor development. This idea is consistent with other studies in this prostate cancer model, which demonstrated that T cells reactive to tumor-expressed antigens are tolerized in tumor-bearing mice (36–38). Regarding the nature of antigens recognized by tumor-infiltrating T cells, our findings suggest the existence of a previously unrecognized class of tumor-associated antigen: epitopes derived from widespread or ubiquitous antigens that become antigenic in the tumor environment. Although the mechanisms underlying the antigenicity of histone H4 are unknown, it is conceivable that changes in antigen processing and presentation pathways could lead to the presentation of epitopes derived from histone H4 that are not normally presented in the thymus or periphery, but are unveiled in the tumor environment. Why would our approach reveal the presence of a class of tumor-associated antigen that was not previously recognized? Classical approaches for identifying tumor antigens involve the derivation of clonal tumor cell lines grown in vitro, followed by the expansion of T cell lines or clones exhibiting reactivity to this cell line. Such approaches may not recapitulate the complexities of the tumor environment in vivo, in which heterogeneous tumors evolve in concert with a complex mixture of stromal cells. In contrast, our study involved the identification of a tumor-infiltrating CD8+ T cell population based on the direct analysis of primary tumors, bypassing the need for in vitro culture-based methods. In this way, our approach revealed the presence of a recurrently enriched T cell population that may not have been identified using classical methods. Interestingly, we found that H4/Kb multimers bound to transgenic T cells expressing the canonical histone H4-reactive TCR with extremely high stability (unpublished data), indicative of high-avidity antigen recognition. Broadly speaking, these data suggest that some tumor-infiltrating CD8+ T cells may be reactive to antigenic epitopes derived from non-mutated, widespread self proteins, and that these T cells can recognize antigen with high avidity.
What other specificities could contribute to the repertoire of tumor-infiltrating CD8+ T cells? A number of groups have described CD8+ T cell populations exhibiting suppressive or regulatory activity (38–41). While the specificities of these regulatory populations are unclear, it is possible that some of these populations exhibit reactivity to self antigens, comparable to their Foxp3+CD4+ regulatory counterparts. Finally, it is possible that many of these T cells are simply non-specific, antigen-experienced cells that are recruited by inflammatory signals into tumor lesions. This idea is supported by data from the peptide/HLA multimer analyses described above, which demonstrated that T cells specific for viral antigens were also present at detectable frequencies in TILs expanded from melanoma lesions (28, 29). Taken together, the finding that T cells specific for tumor-associated antigens are infrequent in primary tumor lesions suggests that endogenous regulatory mechanisms limit the frequency of these cells in the tumor environment.
The immunological processes that shape the repertoire of tumor-infiltrating T cells
The deletion of T cell precursors exhibiting excessive reactivity to self antigens is likely to be a major barrier limiting the frequency of tumor antigen-specific T cells in the periphery. A number of studies have attempted to estimate the fraction of developing T cells that are purged via negative selection in the thymus (42–46). However, since thymocytes undergoing apoptosis are rapidly cleared by phagocytosis, efforts to estimate this fraction have yielded conflicting results. A recent study from Hogquist and colleagues utilized Bim-deficient mice harboring a Nur77GFP reporter allele to quantify the percentage of thymocytes that undergo negative selection (47). In this system, deficiency in the pro-apoptotic molecule Bim promoted the rescue of thymocytes from clonal deletion, enabling their subsequent detection. In addition, GFP expression driven by the promoter for Nur77, an immediate early gene that is upregulated by TCR stimulation, was used as a surrogate readout of TCR signal strength (48). It was found that the thymi of Bim−/− Nur77GFP mice contain a large proportion of thymocytes expressing high levels of GFP, suggestive of autoreactive specificities that are rescued from deletion by Bim deficiency. From these data, the authors estimated that the number of cells undergoing negative selection is six times greater than the number of cells completing T cell maturation, suggesting that the majority of thymic precursors are ultimately deleted in the thymus. In addition to clonal deletion in the thymus, several studies have demonstrated that autoreactive T cell specificities can also be eliminated by deletional mechanisms in the periphery (49, 50), indicative of an additional safeguard limiting autoimmunity and immune responses to tumor-associated self antigens.
Given the elimination of autoreactive T cells by deletional mechanisms, it is important to consider the following questions. First, how failsafe are these processes in establishing tolerance to self antigens? The detection of T cells reactive to tumor-associated antigens in human melanoma patients, albeit at low frequencies, suggests that the purging of these specificities in the thymus is a “leaky” process. Second, what is the impact of deletional tolerance on the repertoire of T cells capable of responding to a tumor-associated antigen in the periphery? To address this question, Houghton and colleagues analyzed the naïve precursor frequency of mouse T cells reactive to gp100, a melanoma-associated differentiation antigen (51). The authors demonstrated that in the naïve repertoire, gp100-specific T cells were present at an extremely low frequency, roughly one in 1 million CD8+ T cells. Interestingly, the precursor frequency approached a level at which T cells of this specificity could not be expanded upon vaccination with gp100 in some mice. While the specific impact of thymic negative selection versus peripheral tolerance on the naïve precursor pool of gp100-specific cells was not assessed in this study, the finding that the precursor frequency is an order of magnitude lower than that for T cells reactive to viral antigens (52, 53) suggests that deletional tolerance limits the frequency of gp100-specific cells. In sum, these studies suggest that the endogenous pool of tumor antigen-specific T cells may be primarily limited by deletional tolerance mechanisms. However, the presence of T cells reactive to melanoma-associated antigens implies that some antigen-specific T cells escape deletional tolerance, and may be subsequently regulated by dominant tolerance mechanisms, such as Foxp3+ regulatory T cells, in the periphery.
The antigen specificity and developmental origin of tumor-associated Tregs
Tregs develop by one of two developmental pathways, generally classified based on the site of origin. Available data suggest that most Tregs originate in the thymus during T cell development (54). Although the antigens recognized by thymic-derived Tregs (tTregs) are unknown, there is considerable evidence suggesting that tTreg development is driven by the recognition of self antigens (55–57). In a second pathway, it has been shown that mature conventional T cells can differentiate into “induced” Tregs (iTregs) at extrathymic sites following recognition of environmental antigens derived from commensal bacteria, food, etc. (58). iTregs are thought to play a critical role in maintaining immune homeostasis in the gut (59, 60) and at other mucosal surfaces (61). Thus, the concepts of Treg origin and antigen specificity are closely interrelated, in that thymic origin implies specificity to self antigens, while extrathymic origin is suggestive of reactivity to antigens that are not encountered during thymic development.
Little is know about the developmental origins of tumor-infiltrating Tregs, and the nature of the antigens recognized by these cells. Conceptually, both tTregs and iTregs could contribute to the pool of tumor-infiltrating Tregs. While tumors may drive the enrichment of pre-existing, self-specific tTregs reactive to either ubiquitous or tissue-restricted antigens, it has also been hypothesized that the inflammatory milieu of a tumor may drive the differentiation of CD4+ helper T cells reactive to tumor-specific or tumor-associated antigens into Foxp3+ iTregs. This hypothesis is based on the rationale that tumors may present neo-antigens unique to the tumor environment, and that many tumors exhibit elevated levels of TGFβ, a pleiotropic cytokine that promotes iTreg differentiation (62). If iTreg differentiation contributes substantially to the enrichment of Tregs in the tumor environment, it suggests that therapeutic strategies to block or reverse this process could be used to enhance cancer immunotherapies. Due to a lack of definitive cell-surface markers to distinguish tTregs and iTregs (13), it has not been possible to directly determine the relative contribution of these Treg subsets to the tumor-infiltrating Treg repertoire. In experiments in which a model foreign antigen is ectopically expressed on tumor cells, it has been shown that a proportion of antigen-specific CD4+ Foxp3neg T cells upregulate Foxp3 expression following transfer into tumor-bearing hosts (63, 64). However, as discussed above, the introduction of a large number of antigen-specific CD4+ Foxp3neg T cells in these experiments may not recapitulate the low physiological precursor frequencies expected for CD4+ helper T cells reactive to tumor-associated self antigens.
In order to better understand the developmental origins and antigen specificities of tumor-infiltrating Tregs, we analyzed endogenous Treg populations isolated from the prostate tumors of mice bearing oncogene-driven prostate cancer (65). In this regard, sequence analysis of the TCRs expressed by polyclonal CD4+ Foxp3+ Tregs and CD4+ Foxp3neg conventional T cells isolated from prostate tumors provided insight into two critical questions. First, if iTregs were developing from Foxp3neg precursors within the tumor environment, or if the expression of Foxp3 on some Tregs was destabilized, one would expect to find some TCR clones represented in both Foxp3neg and Foxp3+ subsets. However, our data revealed that the TCRs expressed by tumor-infiltrating Treg cells were distinct from the TCRs expressed by conventional T cells (65). This finding is consistent with similar TCR repertoire analyses of T cells infiltrating transplantable or carcinogen-induced tumors (66, 67). Therefore, the available data are consistent with the idea that iTreg development and Treg instability are negligible in the tumor models examined. Second, our data revealed a number of TCRs that were recurrently expressed by Tregs within many prostate tumors. This finding suggests that prostate tumors do not simply recruit Tregs of arbitrary specificity, but instead can drive the recurrent enrichment of several distinct Treg specificities. This observation is somewhat unexpected given published studies demonstrating that the Treg TCR repertoire in the thymus and secondary lymphoid organs exhibits a high degree of diversity, comparable to that of the conventional T cell repertoire (68, 69). In light of these findings, we hypothesize that a given peripheral site, such as an inflammatory lesion or tumor, will drive the preferential recruitment or expansion of a highly restricted repertoire of Treg specificities, drawn from a diverse systemic Treg pool.
For one of the Treg clones found recurrently enriched in prostate tumors, named “MJ23”, we generated TCR transgenic mice expressing this TCR (MJ23tg mice), and used cells from these mice to characterize the development and antigen specificity of MJ23 Tregs (65). Interestingly, tumor-free male MJ23tg Rag1−/− mice, which exclusively express the MJ23 TCR, exhibit marked prostatic infiltration by activated CD4+ MJ23tg T cells. In contrast, MJ23tg T cell activation was not observed in female MJ23tg Rag1−/− mice. These results indicated that MJ23 Tregs, originally identified in prostate tumors, do not recognize a unique tumor-specific antigen generated by mutation, but instead recognize a prostate-associated antigen that is present in tumor-free mice. When MJ23tg bone marrow progenitors were seeded at low clonal frequencies into new hosts, the MJ23 TCR facilitated the development of MJ23 Tregs in the thymus. In contrast, following intravenous transfer of naïve CD4+ Foxp3neg MJ23tg T cells into tumor-bearing mice, the differentiation of MJ23tg iTregs was negligible. These results demonstrate that MJ23tg precursors encounter the ligand driving their commitment to the Treg lineage during development in the thymus, prior to their encounter with the prostate or prostate tumor environment. In all, our findings for the prostate cancer-associated MJ23 Treg specificity are consistent with a model in which a developing tumor does not generate new Treg specificities from conventional T cell precursors in the tumor environment, but instead recruits pre-existing tTregs associated with the organ of cancer origin.
Our findings that prostate tumors recruit or expand tTregs reactive to a prostate-associated antigen demonstrate the importance of studying mouse models of autochthonous cancer, in which cancer originates from and evolves within the context of the organ of origin. For example, prostate tumor cell lines injected subcutaneously may not recapitulate the natural physiology (i.e. glandular structures) of the prostate, and develop at ectopic sites that do not exist prior to tumor challenge. Thus, the T cells that are recruited into these tumors are unlikely to mimic the natural biology of T cells infiltrating the tumor lesions of human cancer patients. Given that these findings stem from analysis of a single mouse model of prostate cancer, it will be important to determine the generalizability of these findings by elucidating the development and specificity of Tregs isolated from autochthonous mouse models of different cancer types. For example, since iTregs have been shown to play an important role in maintaining immune homeostasis at various mucosal surfaces (61), iTregs may play a substantial role in cancers arising at anatomical locations such as the gut and the lungs. If so, it will be important to determine whether these iTregs recognize tumor-associated neo-antigens or environmental antigens derived from food or commensal bacteria.
Development of prostate-specific Tregs in female mice
As discussed above, we demonstrated that the MJ23 TCR facilitates Treg development in the thymus. Unexpectedly, we found that prostate-specific MJ23 Tregs developed in the thymus of both male and female mice (65). Why would female mice develop prostate-specific Tregs? In order to elucidate the mechanisms underlying this process, we considered three possibilities. First, it is possible that the antigen is abundant in the male prostate, but is not male-specific, and is present in female mice at densities that are below the level of detection of available assays. To this end, we explored the idea that the antigen may be expressed in the Skene’s gland, often referred to as the “female prostate”. However, experiments revealed that MJ23 Tregs were not found in the Skene’s gland or enriched in the periaortic LNs of female mice (authors’ unpublished data). Second, due to the plasticity / cross-reactivity inherent in TCR recognition of peptide/MHC ligands (70), it is formally possible that the antigen driving Treg development in the thymus is distinct from the prostate-associated antigen recognized in the periphery of male mice. However, this idea is inconsistent with data from Hsieh and colleagues demonstrating that Treg development in the thymus is a TCR-instructive process, in that Treg-derived TCRs facilitate Treg development, while TCRs expressed by conventional T cells do not (71). In this light, it is difficult to envision a model in which prostate-specific Tregs express TCRs exhibiting specificity for two different antigens, one that is expressed in the thymus and one that is expressed in the prostate. As a third possibility, we considered a role for Aire in promoting the development of prostate-specific Tregs in both male and female mice. In this regard, it is important to revisit available evidence regarding the role of Aire in enforcing immune tolerance.
Presentation of peripheral TRAs in the thymus
For the establishment of tolerance, the immune system faces an important logistical problem. How do thymic processes enforce dominant and recessive tolerance to antigens that are not normally expressed in the thymus, such as tissue-restricted antigens (TRAs) that are primarily expressed in peripheral organs? One potential solution is to develop mechanisms by which TRAs are transported from the periphery into the thymus, where they are displayed for recognition by developing thymocytes. In support of this idea, studies in parabiotic mice have demonstrated that a proportion of thymic dendritic cells (DCs) originate extrathymically and migrate to the thymus (72). Moreover, it has been shown that antigen-bearing circulating DCs are recruited into the thymus, where they can mediate the deletion of antigen-specific thymocytes (73). However, the extent to which DC trafficking from the periphery to the thymus contributes to the establishment of recessive and dominant tolerance remains unclear. A second possible solution invokes mechanisms by which peripheral TRAs are promiscuously expressed in the thymus via transcriptional regulators (74), thereby driving either the deletion of thymocytes reactive to TRAs or promoting the development of TRA-specific Tregs. Aire is a transcriptional regulator expressed by medullary thymic epithelial cells (mTECS) that promotes the promiscuous expression of transcripts, many of which encode TRAs (75, 76). Loss-of-function mutations in AIRE are associated with the human disease APECED/APS-1, which is characterized by mucocutaneous candidiasis, autoimmune destruction of the parathyroid and adrenal glands, and hypogonadism / infertility (77, 78). In the mouse, loss-of-function Aire mutations result in multi-organ autoimmunity (75, 79–81), the severity of which varies depending on genetic background (82). Conceptually, Aire may contribute to the establishment of immune tolerance by driving the deletion of thymocytes reactive to promiscuously expressed TRAs, and/or promoting the differentiation of such thymocytes into the Foxp3+ Treg lineage. While the role of Aire in driving the negative selection has been relatively well accepted in the field, the contribution of Aire in promoting Treg development has been controversial.
The role of Aire in negative selection
A number of studies have aimed to determine whether Aire enforces immune tolerance by driving deletional tolerance, Treg development, or a combination of both. In one study, autoimmunity was assessed following the grafting of thymi from Aire+/+ and Aire−/− mice into athymic host mice (83). The data revealed that the grafting of thymic stroma from Aire−/− mice induced organ-specific autoimmunity, confirming that Aire expression by thymic epithelial cells is required for immune tolerance. Interestingly, mice in which thymi from both Aire+/+ and Aire−/− mice were co-grafted into the same host also developed autoimmunity. From this, the authors reasoned that if the primary defect of an Aire−/− thymus is a failure to select Treg specificities, then the provision of Tregs selected on Aire+/+ thymic stroma would be expected to rescue autoimmunity. Since this effect was not observed in the co-grafting experiments, the authors concluded that Aire functions primarily to impart recessive, deletional tolerance. However, in the same series of experiments, the opposite result was observed when one Aire−/− thymus was co-grafted with four Aire+/+ thymi into athymic mice. This suggests that the outcome of the experiment may be a “numbers game”, in which one Aire+/+ thymus is not sufficient to rescue autoimmunity, but four Aire+/+ thymi are. Given that the grafting of ectopic thymi into lymphopenic adult mice may not accurately recapitulate the frequencies and development kinetics of T cells in a normal host, it is difficult to draw definitive conclusions from this numbers game.
In other lines of inquiry, multiple groups have performed studies in which a model foreign antigen is expressed as a transgene under control of the rat insulin promoter (RIP), and the fate of antigen-specific TCR transgenic T cells is assessed (83–85). These studies demonstrated that RIP-driven antigen expression can promote the thymic deletion of antigen-specific T cells. Moreover, deletion was not observed in mice on an Aire−/− background, demonstrating the Aire dependency of this effect. From these findings, it was concluded that Aire enforces immune tolerance in part by driving the negative selection of T cells reactive to TRAs. However, given that these studies relied on the transgenic expression of foreign antigens, and the tracking of TCR transgenic T cells present at supra-physiological clonal frequencies, it is unclear whether these systems recapitulate the development of naturally occurring T cell specificities, in that the antigen density, antigen “topology”, and TCR affinity for antigen may not recapitulate aspects of endogenous autoreactive T cell specificities. In this regard, little is known regarding the impact of Aire on the negative selection of naturally occurring T cell specificities reactive to peripheral TRAs. In one study, Anderson and colleagues (86) used peptide/MHC multimers to enumerate endogenous CD4+ thymocytes specific for retinal antigens. For one peptide epitope, it was found that the frequency of antigen-specific thymocytes was ~2-fold higher in Aire−/− mice relative to Aire+/+ mice, suggesting that autoreactive T cells of this specificity underwent Aire-dependent negative selection. However, the published data reveal that on average, ~15 antigen-specific T cells could be detected in the thymus of Aire+/+ mice at a given time, suggesting that negative selection of this specificity, even in the presence of Aire, is not efficient. In this light, further work will be needed to determine whether Aire is required for the thymic deletion of endogenous autoreactive T cell specificities.
To elucidate the role of Aire in shaping the repertoire of T cells reactive to tumor-associated self antigens, Su and colleagues (87) examined the impact of Aire on the thymic development of T cells reactive to a melanoma-associated differentiation antigen and the associated immune response to transplantable melanomas. Based on observations that APECED/APS-1 patients harboring loss-of-function mutations in AIRE are predisposed to autoimmune destruction of melanocytes (vitiligo), the authors hypothesized that Aire enforces deletional tolerance to melanocyte differentiation antigens, thereby restricting anti-tumor immunity to melanoma. Following tumor challenge, mice with a dominant hypomorphic mutation in Aire exhibited delayed tumor growth and increased T cell infiltration relative to wild-type controls, indicative of heightened endogenous immune responses to melanoma challenge. Additionally, the authors demonstrated that the thymic expression of the melanoma-associated antigen TRP-1 was Aire-dependent, and that the thymic deletion of transgenic T cells expressing an MHC class II-restricted TRP-1-specific TCR was abrogated in Aire-mutant mice. Taken together, these findings suggest that Aire-dependent processes restrict anti-tumor immune responses by promoting the thymic deletion of T cells reactive to tumor-associated self antigens. In other experiments, it was found that the Aire mutation did not impact the thymic development of TRP-1-specific TCR transgenic Tregs. However, since the TRP-1 TCR is derived from a conventional T cell clone, and not a naturally occurring Treg specificity (88), it is unclear whether there are endogenous niches supporting the thymic development of TRP-1-specific Tregs.
Aire and Treg Development
In addition to evidence suggesting that Aire may enforce immune tolerance in part by driving deletional tolerance, it is important to consider other evidence and rationale suggesting a role for Aire in promoting the development of Treg cells. In an elegant series of experiments, Mathis and colleagues examined the role of Aire in immune tolerance using a system in which the expression of Aire could be temporally controlled (89). It was found that Aire expression during the perinatal period was required for the prevention of autoimmunity. Furthermore, Aire expression was found to be dispensable after three weeks of age. While the role of Tregs in this effect was not extensively examined, these findings are consistent with the existence of Aire-dependent mechanisms of dominant tolerance (such as Treg development), in which Aire-dependent events within the first few weeks of life are sufficient to protect the animal from autoimmunity throughout life. In addition, the finding that Aire is dispensable after three weeks of age is inconsistent with a critical role for Aire in promoting negative selection, as putative autoreactive T cells specific for TRAs are expected to develop throughout life.
Other findings are consistent with a role for Aire-dependent processes in the development of Tregs. Tregs isolated from APECED/APS-1 patients exhibit defects in suppressive capacity in vitro and diminished Foxp3 protein expression (90, 91), demonstrating that loss-of-function AIRE mutations impact Treg development and/or function in humans. Another study demonstrated that ectopic expression of a model antigen by Aire-expressing cells can promote the thymic development of antigen-specific Tregs (92). Finally, Aire deficiency is associated with a reduction in the frequency of thymic Foxp3+ cells (83) and the density of these cells in the thymic medulla (93).
Based on this evidence, in an effort to elucidate the mechanisms underlying the thymic development of prostate-specific MJ23 Tregs in both male and female mice, we tested the hypothesis that Aire drives the promiscuous expression of transcripts encoding the prostate-associated MJ23 antigen by mTECs in the thymus, thereby promoting the development of MJ23 Tregs regardless of sex. To test this, we introduced bone marrow precursors from MJ23tg Rag1−/− mice into Aire+/+ and Aire−/− hosts and monitored the fate of MJ23 T cells (65). Consistent with our hypothesis, our data revealed that MJ23 Tregs failed to develop in Aire−/− hosts, demonstrating that Aire is critical for the thymic development of MJ23 Tregs. Importantly, the same effect was observed for a second naturally occurring prostate tumor-associated Treg specificity. Additional analysis revealed that the frequency of polyclonal Tregs in the thymus was significantly reduced in Aire−/− mice relative to Aire+/+ mice, consistent with previous reports (83, 93). Taken together, our results demonstrate that Aire is essential for the thymic development of some, but not all, Treg specificities, suggesting that Aire enforces immune tolerance in part by promoting Treg development. The fact that many Aire-dependent transcripts expressed by mTECs are thought to encode peripheral TRAs (75, 76) suggests that Aire promotes the thymic development of TRA-specific Tregs (such as the prostate-specific MJ23 Tregs), which may function to prevent organ-specific autoimmunity in the periphery (94, 95). Further work will be required to determine the relative contribution of Aire-dependent Treg specificities to the peripheral Treg pool, and the functional role of Aire-dependent Tregs in enforcing immune tolerance. In addition, it will be important to elucidate the cellular and molecular parameters that determine whether a thymocyte reactive to an Aire-dependent antigen undergoes either clonal deletion or differentiation into the Treg lineage.
Thus, the integration of available evidence suggests that Aire drives both the deletion of autoreactive T cells and the development of a subset of Foxp3+ Tregs. The finding that some, but not all, Treg specificities are dependent on Aire for thymic development suggests that the systemic Treg repertoire may be divided into Aire-dependent and Aire-independent Treg subsets, raising the possibility of a “division of labor” between these subsets (Figure 2). We hypothesize that Aire-independent Tregs preferentially recognize widespread or ubiquitous antigens, and function to maintain systemic immune homeostasis and tolerance to widespread antigens. In contrast, we hypothesize that Aire-dependent Tregs preferentially recognize tissue-restricted antigens, and specialize in the regulation of peripheral tissues and solid organs. If such a dichotomy exists, it would also suggest that Aire-dependent Tregs play a critical role in the regulation of antitumor immune responses against cancers arising in solid organs such as prostate, breast, liver, etc.
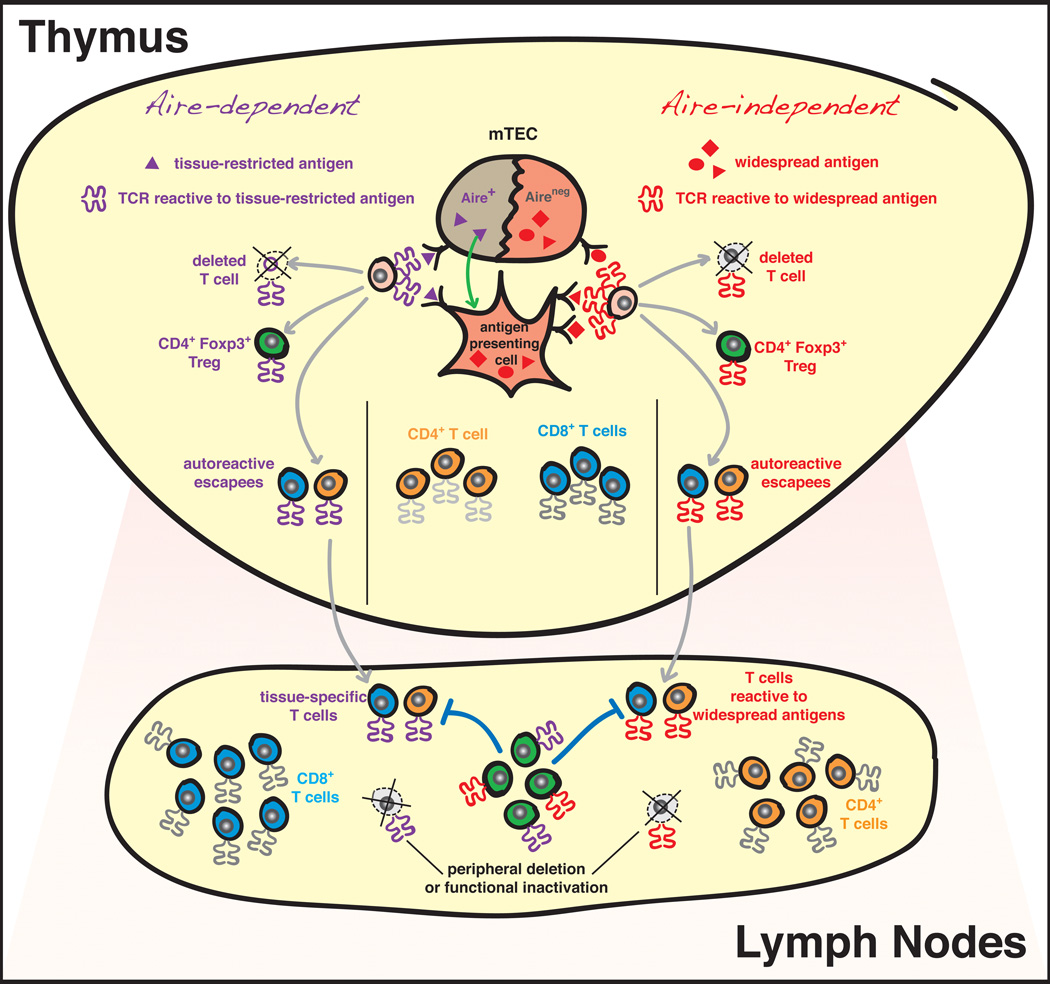
Figure 2. Central and peripheral tolerance mechanisms shaping the T cell repertoire.
Proposed model of Aire-dependent vs. Aire-independent mechanisms of central tolerance. After successful rearrangement of an αβ T cell antigen receptor (TCR), a positively selected thymocyte (shown in pink) will be exposed to a wide variety of self antigens in the thymic medulla. These antigens range from those widely expressed throughout the body, to the more tissue-restricted antigens. The expression of the transcription factor autoimmune regulator (Aire) by medullary thymic epithelial cells (mTECs) is thought to be a major mechanism driving the promiscuous expression of tissue-restricted antigens in the thymus (100). Aire-dependent antigens are thought to be presented directly by mTECs (101), or acquired and presented by neighboring antigen presenting cells, such as dendritic cells (102). Encounter with self antigen can lead to three fates, which are not mutually exclusive: (1) negative selection/deletion; (2) development into the Foxp3+ regulatory T cell (Treg) lineage; or (3) escape from deletion (“escapees”). As described in the text, we hypothesize that Tregs reactive to widespread antigens will develop in an “Aire-independent” manner, and will function to maintain systemic immune homeostasis and tolerance to widespread antigens. In contrast, we hypothesize that “Aire-dependent” Tregs preferentially recognizing tissue-restricted antigens encoded by Aire-dependent transcripts in the thymus will specialize in immune regulation in peripheral tissues and solid organs. We predict that these tissue-specific Aire-dependent Tregs are likely to be highly enriched in tumor lesions developing at peripheral sites (see Figure 1). While negative selection is thought to purge most autoreactive T cells from the repertoire, autoreactive T cell “escapees” are thought to be restricted via Treg-mediated suppression, deletion, or functional inactivation in the periphery. Finally, since T cells reactive to tumor-associated self antigens are likely to be derived from the pool of T cell escapees, these central and peripheral mechanisms are thought to restrict the repertoire of T cells specific for tumor-associated antigens in the tumor environment (Figure 1).
The contributions of dominant and recessive tolerance to the prevention of autoimmunity
What are the relative contributions of Aire-dependent clonal deletion and Treg development to the enforcement of immune tolerance? Consider the logistics of establishing tolerance to peripheral TRAs solely by deletional mechanisms. In order to fully purge the repertoire of autoreactive specificities by negative selection, all developing thymocytes would have to encounter the complete array of antigenic self peptides, both HLA class I and II restricted, during development in the thymus, for the entire lifetime of the individual. Any small lapse in diligence would result in the presence of autoreactive T cells in the periphery. Metaphorically, this is akin to continuously plugging thousands of dynamic holes in a dam, a task that seems daunting. Alternatively, consider the logistics of establishing immune tolerance using dominant mechanisms, in which Tregs reactive to TRAs are selected in the thymus and maintain order by patrolling peripheral sites. In this scenario, the thymic presentation of TRAs would drive the development of distinct Tregs specificities. These TRA-specific Tregs would then traffic to peripheral sites enriched with the relevant TRAs and would function in trans to maintain dominant tolerance throughout the lifetime of the individual. Conceivably, only one or a few Treg specificities would be required to regulate a given organ site. Consistent with this idea, our analysis of endogenous MJ23 Tregs in mouse prostate cancer revealed that in many tumors, transcripts encoding the MJ23 TCR utilize a single nucleotide sequence, suggesting that MJ23 Tregs in a given tumor may originate from a single clone (65). Thus, single Treg cells may expand substantially, and could be sufficient to regulate a given anatomical site.
The available evidence suggesting that Aire promotes both clonal deletion and Treg development suggests that these mechanisms function in an additive, complementary manner to impart immune tolerance. However, additional studies will be needed to more fully address this question. It will be important to determine the specific role of Tregs in preventing the autoimmune manifestations of Aire deficiency. In this regard, if Tregs are found to be both necessary and sufficient to prevent autoimmunity, it would suggest that Aire enforces tolerance primarily by facilitating Treg development. In addition, in order to definitively demonstrate that Aire promotes the thymic deletion of autoreactive T cells, it will be essential to identify naturally occurring autoreactive T cell specificities that are normally deleted by Aire-dependent processes, but escape negative selection in the absence of Aire.
The antigen presenting cells recognized by T cells in the tumor environment
Despite the identification of a large array of tumor-associated antigens based on in vitro stimulation assays, little is known about the cell types that present these antigens for recognition by T cells in situ. Conceptually, CD8+ T cells may recognize peptides presented by HLA class I molecules expressed by all nucleated cell types, including tumor and stromal cells such as dendritic cells, macrophages, fibroblasts, etc. Initial studies demonstrated a critical role for bone-marrow-derived antigen presenting cells (APCs) in priming and/or sustaining the anti-tumor T cell response in transplantable tumor models (96). However, for the tumor-associated antigens defined in human melanoma, it has not been possible to determine whether antigens are presented uniformly by all tumor cells within a primary tumor lesion, whether antigens are also presented by stromal APCs within the tumor, and whether the cell-surface antigen density is sufficient to enable recognition by T cells. In this regard, experiments in transplantable mouse tumor models demonstrated that high-level expression of a model antigen on tumor cells was required for the acquisition and cross-presentation of tumor-derived antigen by stromal APCs, and that this cross-presentation was critical for T cell-mediated tumor eradication and the “bystander” elimination of antigen-loss tumor variants (97, 98). Given this proof-of-concept study, it will be critical to determine whether tumor-expressed antigens are efficiently cross-presented by stromal APCs within human tumor lesions.
Similarly, little is known about the cell types that present antigen for recognition by Treg cells within the tumor environment. Conceivably, any cell type that expresses HLA class II, either constitutively or transiently, is a candidate interaction partner for Tregs. A number of different cell types have been implicated in modulating Treg function within the tumor, including DC and myeloid cell subsets. However, available data are based primarily on correlative studies or in vitro mixing experiments, lacking direct evidence of TCR-peptide/HLA dependent interactions in situ. Since APCs may choreograph many aspects of Treg activity within the tumor, including enrichment, positioning, and immune suppression, identifying the bona fide APCs recognized by tumor-infiltrating Tregs will be critical for understanding the function of Treg cells. For example, do Tregs interface with the same APCs as CD8+ effector T cells within the tumor? If not, how do Tregs regulate effector T cell responses? A recent study demonstrated that the frequency of Tregs in human ovarian cancer ascites correlated with that of plasmacytoid DCs (pDCs). In addition, the in vitro suppressive function of Tregs was found to be dependent on ICOS-L stimulation conferred by pDCs. While the functional significance of this effect on the progression of ovarian cancer is unclear, one possibility is that Tregs modulate the inflammatory milieu of ovarian cancer by regulating cytokine production by pDCs, which are a critical source of type I interferons (99). In this way, Tregs may modulate the inflammatory milieu of ovarian cancer via specific interaction with pDCs, without the need to interface directly with effector T cells or other APC types. It is important to note that the APCs that interface with Tregs in the tumor environment may change during tumor progression, and may vary for different types of cancer.
Our finding that the TCRs expressed by prostate cancer-infiltrating Treg cells are distinct from the TCRs expressed by conventional T cells (65) suggests that these T cell populations recognize different antigens within the tumor. Two possible explanations can be invoked to explain this finding: Treg and T conventional cells may recognize distinct antigen sets presented by the same APC type, or these subsets may recognize distinct antigens presented by different APC types. Further work will be required to identify the APC subsets that present antigen for recognition by Treg cells, CD4+ helper cells, and CD8+ effector cells within primary tumors, and to determine whether these T cell populations interact with the same APC types, or interface with distinct APC subsets within the tumor environment.
Concluding remarks
The development of new technologies such as genome sequencing, high-throughput TCR sequencing, in vivo imaging, and immune monitoring approaches have provided new tools for the study of tumor-associated T cell responses and immune regulation in human cancer patients and murine cancer models. In the coming years, use of these platforms is expected to further the understanding of the antigens recognized by tumor-infiltrating T cells, the function of distinct T cell subsets within tumor lesions, and the impact of immune-based therapies on these T cell populations. Further elucidation of these principles is likely to be essential for optimizing current cancer immunotherapies, and for the rational design of new therapies exhibiting robust anti-tumor activity with limited toxicity.
Acknowledgments
We thank Fabiola Rivas for critical reading of the manuscript. P.A.S. is supported by R01 (#R01CA160371-01) and a Cancer Research Institute Investigator Award.
References
- 1.Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13:835. [PubMed] [Google Scholar]
- 2.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18:769. [PubMed] [Google Scholar]
- 3.Klein G, Sjogren HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561. [PubMed] [Google Scholar]
- 4.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012 Apr;12:269. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363:711. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011 Nov;11:805. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune Modulation in Cancer with Antibodies. Annual review of medicine. 2013 Oct 30; doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 9.Davis MM, et al. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 10.Wang RF. Identification of MHC class II-restricted tumor antigens recognized by CD4+ T cells. Methods. 2003 Mar;29:227. doi: 10.1016/s1046-2023(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deleeuw RJ, Kost SE, Kakal JA, Nelson BH. The Prognostic Value of FoxP3+ Tumor-Infiltrating Lymphocytes in Cancer: A Critical Review of the Literature. Clin Cancer Res. 2012 Jun 1;18:3022. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 13.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends in immunology. 2012 Sep 19; doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajewski TF, Chesney J, Curriel TJ. Emerging strategies in regulatory T-cell immunotherapies. Clin Adv Hematol Oncol. 2009 Jan;7:1. [PubMed] [Google Scholar]
- 15.Menetrier-Caux C, et al. Targeting regulatory T cells. Targeted oncology. 2012 Mar;7:15. doi: 10.1007/s11523-012-0208-y. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976 Mar;33:241. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268:154. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 18.Gillis S, Baker PE, Ruscetti FW, Smith KA. Long-term culture of human antigen-specific cytotoxic T-cell lines. J Exp Med. 1978 Oct 1;148:1093. doi: 10.1084/jem.148.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 20.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254:1643. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 21.Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Database of T cell-defined human tumor antigens: the 2013 update. Cancer Immun. 2013;13:15. [PMC free article] [PubMed] [Google Scholar]
- 22.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008 Oct;20:276. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skipper JC, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996 Feb 1;183:527. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996 Oct 4;274:94. [PubMed] [Google Scholar]
- 25.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999 Jun;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 26.Romero P, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998 Nov 2;188:1641. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol. 1999 Feb 15;162:2227. [PubMed] [Google Scholar]
- 28.Kvistborg P, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012 Jul 1;1:409. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen RS, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012 Apr 1;72:1642. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 30.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Annals of the New York Academy of Sciences. 2013 May;1284:1. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogelstein B, et al. Cancer genome landscapes. Science. 2013 Mar 29;339:1546. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008 Feb 1;68:889. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 33.Robbins PF, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013 Jun;19:747. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooij N, et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-Cell Reactivity in an Ipilimumab-Responsive Melanoma. J Clin Oncol. 2013 Sep 30; doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage PA, et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008 Jan 11;319:215. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 36.Drake CG, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005 Mar;7:239. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007 Feb 1;178:1268. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 38.Shafer-Weaver KA, et al. Cutting Edge: Tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009 Oct 15;183:4848. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Human immunology. 2008 Nov;69:681. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 40.Shi Z, et al. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol. 2009 Aug;39:2106. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011 Dec;23:446. doi: 10.1016/j.smim.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994 Nov 3;372:100. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 43.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996 Sep 5;383:81. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 44.van Meerwijk JP, et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997 Feb 3;185:377. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkenschlager M, et al. How many thymocytes audition for selection? J Exp Med. 1997 Oct 6;186:1149. doi: 10.1084/jem.186.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997 Mar 7;88:627. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 47.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci U S A. 2013 Mar 19;110:4679. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011 Jun 6;208:1279. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardner JM, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008 Aug 8;321:843. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007 Feb;8:181. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto GA, et al. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009 Apr 13;206:849. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002 Mar 4;195:657. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008 Jun;28:859. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012 Mar;12:157. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 55.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001 Apr;2:301. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 56.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002 Aug;3:756. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 57.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012 Sep 21;37:475. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 59.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011 Sep 21; doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 61.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012 Feb 16;482:395. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends in immunology. 2010 Jun;31:220. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Getnet D, et al. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J Immunol. 2009 Apr 15;182:4675. doi: 10.4049/jimmunol.0803400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007 Feb 15;178:2155. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 65.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013 Mar 8;339:1219. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hindley JP, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011 Feb 1;71:736. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed towards public sequences. Cancer Res. 2012 May 9; doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004 Aug;21:267. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Wong J, et al. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007 Jun 1;178:7032. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 70.Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- 71.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009 Jun;10:610. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009 Mar 16;206:607. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonasio R, et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006 Oct;7:1092. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 74.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 75.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002 Nov 15;298:1395. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 76.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005 Jul 4;202:33. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aaltonen J. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997 Dec;17:399. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 78.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997 Dec;17:393. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 79.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Human molecular genetics. 2002 Feb 15;11:397. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 80.Hubert FX, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009 Mar 15;182:3902. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 81.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005 Feb 15;174:1862. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 82.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005 Sep 19;202:805. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005 Aug;23:227. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003 Apr;4:350. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 85.Su MA, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008 May;118:1712. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taniguchi RT, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A. 2012 May 15;109:7847. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013 Apr 1;73:2104. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008 Jul 15;112:362. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009 Jun 8;206:1245. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laakso SM, et al. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. J Autoimmun. 2010 Dec;35:351. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Kekalainen E, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007 Jan 15;178:1208. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 92.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007 Apr;8:351. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 93.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011 Feb 14;208:383. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KS. Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol. 2008 Apr 1;180:4366. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 95.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005 Sep 19;202:771. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang AY, et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994 May 13;264:961. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 97.Spiotto MT, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002 Dec;17:737. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 98.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004 Mar;10:294. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 99.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010 Mar;234:142. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 101.Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010 Jun;11:512. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 102.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009 Jul 6;206:1505. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]