Abstract
In 2012, John Gurdon and Shinya Yamanaka shared the Nobel Prize for the exciting demonstration that the identity of differentiated cells is not irreversibly determined but can be changed back to a pluripotent state under appropriate instructive signals. The principle that differentiated cells can revert to an embryonic state and even be converted directly from one cell-type into another not only turns fundamental principles of development on their head but also has profound implications for regenerative medicine. Replacement of diseased tissue with newly reprogrammed cells and modeling of human disease are concrete opportunities. Here, we focus on the central nervous system to consider whether and how reprogramming of cell identity may impact regeneration and modeling of a system historically considered immutable and hardwired.
The power of nuclear reprogramming
In the earliest stages of embryonic development, a handful of uncommitted cells possess the potential to differentiate into any cell type if given the right cues. During the first half of the 20th century, a major question in developmental biology was whether permanent genomic changes accompany differentiation and are in place to enable such pluripotent cells to attain and maintain terminal, cell-type specific characteristics (1). In 1962, John Gurdon first published the results of seminal experiments that challenged the commonly held belief that differentiation was unidirectional and irreversible (2, 3). He used somatic cell nuclear transfer, a technique developed a decade earlier by Briggs and King (4), to transplant the nucleus of a differentiated tadpole intestinal cell into an irradiated egg and showed that normal adult frogs could develop from these eggs. This groundbreaking work provided the first proof-of-principle demonstration that it is indeed possible to reprogram differentiated cells back to pluripotency. More recently, similar conclusions were extended to mammalian cells (5).
These experiments indicate that barriers that lock these cells into their differentiated state do not involve permanent genomic changes and that there are factors in the egg's cytoplasm that enable fully differentiated cells to “reverse development” and regain pluripotency. One set of such factors was revealed when Yamanaka's group successfully converted fibroblasts into pluripotent stem cells with a cocktail of transcription factors. The resulting cells were thereby named induced pluripotent stem cells (iPSCs) (6). Along with Gurdon, Yamanaka received the Nobel Prize for this work, and these exciting findings, more than forty years apart, contributed to establish that nuclear reprogramming is possible across a spectrum of organisms, including mammals (see (7) for an in-depth review on iPSCs and mechanisms of induced pluripotency) (Figure 1).
Figure 1. Historical perspective on nuclear reprogramming.
Selected milestone findings from experiments in amphibians first demonstrated that the nucleus of 16-cell stage cells(63) and differentiated adult cells(2) are plastic and capable of generating full organisms. Conrad Waddington is credited for theoretically conceiving the epigenetic landscape(8). More recent evidence indicates that differentiated mammalian cells are equally able to reprogram to either a pluripotent state(6, 64) or to a new differentiated cell state(65).
Conrad Hal Waddington famously likened the process of cellular differentiation and its associated epigenetic changes during development to a marble traveling along a downward slope and ending up in one of many valleys surrounded by impassable hills (8). Reverting differentiated cells back to pluripotency via nuclear reprogramming is comparable to forcibly pushing a marble from a valley back to the starting point, also known as the developmental “ground state.” However, it has become evident that it is also possible to push the marble from valley to valley in a process that turns one differentiated cell type directly into another without transitioning through a pluripotent cell state. This process has been termed transdifferentiation or direct lineage reprogramming, and various cell types have been directly reprogrammed to acquire a new differentiated identity, across organ systems and in different species (9). Direct lineage reprogramming has several attractive features including low likelihood of tumor formation and increased speed and efficiency of conversion if starting from a related cell type (10). Most notably, this approach carries great potential for applicability in vivo, a key advantage when aiming to rebuild cells of a tissue as complex as the central nervous system. Here, we examine direct lineage reprogramming into neurons and discuss the developmental programs that facilitate conversion of cell identity in the central nervous system.
Generation of development-inspired neurons
In The Greatest Show on Earth, Richard Dawkins likened embryology to a concerted, graceful flight murmuration of starling birds, each individual starling in the flock following its own local rules, with no overall goal or blueprint for what the flock should ultimately resemble (11). Like starlings in a flock, during development cells interact according to local guidance cues that converge on the activation of intrinsic programs, and ultimately allow for a collection of low-level unspecified units to self-assemble into a high-level configuration. Development is a bottom-up process that requires highly orchestrated and complex signaling mechanisms to produce a whole tissue and organism. Several studies have highlighted the amazing similarities in self-organizing properties between organogenesis in embryos and generation of complex tissues from pluripotent stem cells in a dish. In the nervous system, investigators have demonstrated that a 3D culture of pluripotent stem cells in defined differentiation media could induce self-directed organization of complex tissues (“organoids”), which developed into structures highly similar to the optic cup (12, 13) and the cerebral cortex (14). Such approach may become a useful strategy to generate the complexity of neural tissue beyond individual neurons.
Developmental studies have also identified master transcription factors that alone are able to instruct signature features of neuronal classes as they develop in the central nervous system. This has fueled top-down experiments in which researchers dictate the use of a handful of master regulators to generate specific neurons from other types of cells. Scientists are venturing into daring territories where neuronal cells in their own brand new category are generated outside the context of embryogenesis following development-inspired protocols. For the first time, protocols to generate pre-defined neuronal classes from human ESCs, fibroblasts and other cell types are rapidly expanding, and this progress is likely to have a major impact not only in the clinics but also on our understanding of human neural development, a process that cannot be studied in vivo.
Potency of developmental transcription factor modules to generate neurons
The idea of direct lineage reprogramming with transcription factors is not new, and over the years, overexpression of key transcription factors has been used to successfully convert the identity of various cell types, both in vitro and in vivo (9). One of the first indications that intrinsic modulation of transcription factors may be sufficient to generate neurons from non-neuronal cells came from experiments where Pax6 was overexpressed in young glial cells isolated from the early postnatal brain (15). These results are in line with the known developmental role of Pax6 in the cerebral cortex, where its loss results in reduced numbers of neurons generated from radial glia cells (15). Subsequent studies have demonstrated that other neurogenic factors, namely Ngn2, Ascl1, and Dlx2 can also reprogram early-postnatal astrocytes into neurons in vitro, including both GABAergic and glutamatergic fates (16–18). Neurons have been subsequently produced from many differentiated cell types, including hepatocytes (19), pericytes (20), adult astrocytes (21), and most often, fibroblasts (22) (Figure 3).
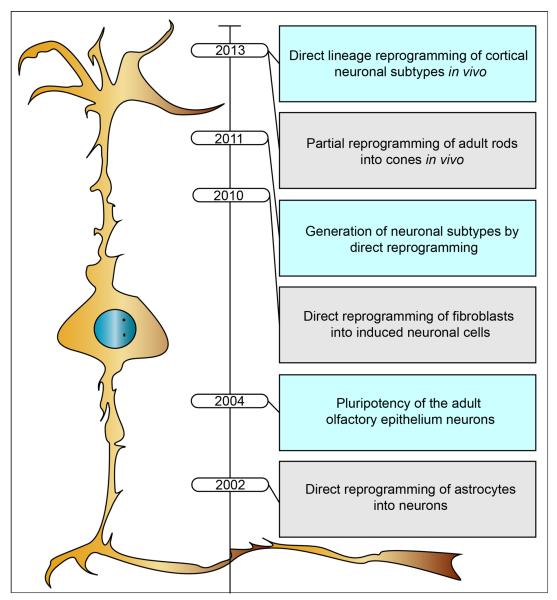
Figure 3. Historical perspective on neuronal reprogramming and reprogramming into neurons.
Selected milestone experiments that collectively supported the view that neurons are amenable to be reprogrammed and that non-neuronal cell types can be reprogrammed into neurons. Neurons could be generated from non-neuronal cells in vitro (15), and successive studies have shown that lineage-distant fibroblasts could be used as the starting cells for direct reprogramming into generic neurons (22) and specific neuronal subtypes (34, 38). Somatic cell nuclear transfer experiments have determined that adult neurons can undergo nuclear reprogramming (51, 52). Studies have induced neuronal class-switch in vivo (58–60), suggesting that some neurons can undergo lineage reprogramming, although this capacity drastically decreases with neuronal age.
Fibroblasts have been extensively used for reprogramming experiments. In a first groundbreaking study, a module of three factors - Brn2, Ascl1, Myt1l - collectively known as the BAM factors, has been used successfully to reprogram mouse embryonic fibroblasts (MEFs) and tail-tip fibroblasts into induced neuronal cells (iN cells), albeit at a low efficiency (<20%) (22). iN cells generated by these methods display neuronal morphology and gene expression, as well as functional electrophysiological properties. Integration of the BAM module with NeuroD1 extended this reprogramming capacity to human fibroblasts (23). Each of the BAM factors has demonstrated functions in neuronal development. Ascl1 in particular is necessary for neuronal differentiation in the ventral telencephalon, neurogenesis in the olfactory epithelium, and development of the sympathetic ganglia (24). In line with such a powerful developmental role, Ascl1 has also been shown to be the primary driver, among the BAM factors, of the MEFs to iN cell conversion (25). Notably, the presence of a specific trivalent chromatin signature at Ascl1 binding sites on the genome appear to alone predict the capability of different cell types to reprogram into iN cells (25). How these mechanisms relate to Ascl1 function during normal development is not known; however, it is interesting that Ascl1 DNA binding sites in MEFs and ESC-derived neural progenitor cells largely overlap. This data suggests that Ascl1 may instruct neuronal reprogramming of MEFs by binding to the same genomic loci that it occupies in neural progenitor cells during development, which in turn would make this reprogramming protocol a useful platform to explore principles of developmental neurogenesis.
Fibroblast-to-neuron conversion has enabled subsequent studies aimed at improving the efficiency and precision by which iN cells are generated. A long road lies ahead; however, it seems that manipulation of transcription factors may be combined with extrinsic, development-inspired cues to enhance direct reprogramming. Overexpression of Ascl1 and Ngn2 or Ngn2 alone was combined with small molecule inhibitors of pathways normally repressed during developmental neurogenesis to generate iN cells at a high efficiency (26, 27). The mechanisms by which patterning signals modify the fate of a differentiated cell remain unexplained. During development, these signals act at early stages of neural induction, on progenitors that are in a plastic epigenetic state. Therefore, it is plausible that Ascl1 and Ngn2 might synergistically facilitate the process of direct reprogramming of differentiated cells by inducing chromatin remodeling. Such role in chromatin remodeling by master regulators might result in the establishment of a plastic cellular environment in fibroblasts, which in turn makes them sensitive to extracellular patterning signals. In line with this concept, it was shown that Pax6 directly interacts with the chromatin remodeler Brg1-containing BAF complex to regulate a transcriptional cross-regulatory network that can reprogram glia into neurons (28). Interestingly, ectopic expression of brain-enriched microRNAs that promote the assembly of neuron-specific BAF complexes has successfully converted fibroblasts into neurons, indicating that active alteration of the epigenetic landscape of non-neuronal cells can be sufficient to generate neuron-like cells (29).
Inducing neuronal diversity
The human brain consists of approximately 100 billion neurons, which are grouped in a staggering number of neuronal classes (30). Classification of neuronal diversity in the mammalian brain is far from complete, but it is clear that defined differences exist among different classes of neurons and that susceptibility to neurological conditions is strongly neuronal class-specific. Therefore, generation of specific neuronal subtypes for the purpose of cell replacement therapy or in vitro disease modeling becomes important.
Progress has been made to generate a small number of neuronal classes by direct reprogramming. These classes were typically chosen based on contribution to disease and knowledge of the factors controlling their neuron class-specific development. Spinal motor neurons are a notable example. They are susceptible to selective degeneration in pathologies like Spinal Muscular Atrophy and Amyotrophic Lateral Sclerosis (ALS) (31) and several of the transcription factors governing spinal motor neuron development are known (32). In 2002, a pioneer study provided proof-of-principle evidence that spinal motor neurons can be made from ESCs using developmental patterning signals (33). Subsequently, a cocktail of transcription factors made of BAM and motor neuron transcription factors (Brn2, Ascl1, Myt1l, Lhx3, Hb9, Isl1, Ngn2) reprogrammed mouse fibroblasts into induced motor neurons (iMNs) (34). While these iMNs did not show clear anterior-posterior (A-P) motor neuron identities, two different sets of programming factors – NIL (Ngn2, Isl1, Lhx3) and NIP (Ngn2, Isl1, Phox2a) – succeeded at differentiating ESCs into two groups of motor neurons with spinal and cranial identity, respectively. Interestingly, by performing chromatin immunoprecipitation sequencing analysis of Isl1 genome binding in NIL and NIP programmed neurons, it was shown that Isl1 binds to different genomic loci depending on whether Lhx3 or Phox2a are co-expressed, indicating that synergistic binding of programming factors may be crucial to drive the generation of different types of motor neurons (35). Indeed, during the MEF to iMN conversion, application of Lhx3, Hb9, Isl1, and Ngn2 enhanced efficiency, while the addition of Sox1, Pax6, Nkx6.1, and Olig2 significantly decreased the efficiency rather than being neutral. These data suggest that the composition of each transcription factor module is critical to the success of reprogramming and that the most effective modules may be those composed of transcription factors that are expressed synchronically within the desired cell lineage in the embryo. In the future, deeper understanding of the synergism between individual transcription factors will clarify the mechanisms of reprogramming into specific neuronal cells. This knowledge should lead to a more informed choice of transcription factors and better predictions of reprogramming outcome to ultimately replace the current candidate screening approach.
Researchers have also extensively invested in generating midbrain dopaminergic (mDA) neurons because of their degenerative phenotype in patients with Parkinson's disease (PD) (36). Nurr1 and Lmx1a, transcription factors necessary to generate mDA neurons during development (37), were coexpressed with Ascl1 in mouse and human fibroblasts in an attempt to generate induced dopaminergic (iDA) neurons, in vitro (38). Since then, several different combinations of factors have also been used to reprogram fibroblasts to a similar neuronal fate (39–41). Notably, the resulting neuronal cells displayed functional characteristics of endogenous dopaminergic neurons, including the ability to release dopamine and class-specific electrophysiological properties. Moreover, although additional work will be required to determine the clinical utility of these iDA neurons, encouragingly, transplantation experiments showed integration into mouse striatum and a mild amelioration of Parkinsonian symptoms (39). It should be noted however that cells generated by these methods did not acquire a distinct midbrain dopaminergic neuron identity. Clear persistence of epigenetic memory of fibroblast origin was observed, and subtype-specific markers of mDA neurons were not present to the extent observed in the endogenous counterparts (38, 39). This result highlights that despite the necessary role of the reprogramming transcription factors during mDA development, these modules were not sufficient to impart the cells with class-specific traits of midbrain DA neurons. However, the data also suggests that even if lacking lineage-specific features, iDA neuronal cells may mimic key functional features of mDA neurons and therefore be clinically relevant. The fact that different combinations of factors lead to neuronal cells with similar functional properties may reflect the fundamental nature of such induced traits, which in vivo are shared by most types of dopaminergic neurons, not only mDA neurons. Alternatively, different molecular pathways may direct reprogramming to the same final cellular identity.
Significant questions and challenges remain; nonetheless, pioneering studies in both direct lineage reprogramming and directed differentiation of defined neuronal classes indicate that production of patient-specific, defined classes of human neurons with clinical value is becoming a reality.
Challenges of generating neuronal diversity
Direct lineage reprogramming is a nascent, but promising field. While both unspecialized and specialized neuronal cells have been generated, the extent of reprogramming is largely undefined, and this young field is collectively in need of better-defined criteria to classify the neurons obtained by these approaches. Some broad questions remain unanswered. How similar are reprogrammed neurons to their endogenous counterparts? Or, perhaps more relevantly, how close do these neurons need to be for applications such as disease modeling and cell replacement therapy? Do neurons obtained by direct reprogramming always maintain a memory of their original identity and how does that influence their functionality? Some of these questions are beginning to be answered while others remain a challenge for the future. We propose that some criteria for classifying reprogrammed neurons may be universal; however, others should take into account the intended use of the neurons.
Guidelines for defining neurons derived from non-neuronal cells in vitro have been suggested (10). The criteria include acquisition of neuronal morphology, expression of pan-neuronal markers, and functional synaptic inputs/outputs (10). We agree that these criteria are well suited to broadly define iN cells as they test the acquisition of fundamental, basic traits that distinguish all neurons from fibroblasts and other non-neuronal cells. iN cells with these properties are valuable cells for a variety of applications, including disease modeling and therapeutic screening. For example, direct reprogramming of fibroblasts into “generic” populations of induced neuronal cells via overexpression of the BAM factors may be sufficient to model pathologies that affect a broad spectrum of neurons, with limited class-specificity. In one example, fibroblasts isolated from human patients with a familial form of Alzheimer's disease have been used to generate iN cells, which in turn could model some features of the disease, including modified amyloid precursor protein processing (42).
However, within the brain and spinal cord, neurons differ greatly from each other and numerous subtypes can be recognized. Neuronal classification is based on many distinguishing features, which include global molecular identity, morphology, ultrastructural traits, electrophysiological properties and connectivity. All of these traits collectively (and not in isolation) allow class-distinction. Some neuronal subtype-specific traits, most prominently morphology, target-specific connectivity, and the ability to functionally integrate into circuitry can only be examined in vivo and remain largely untested for reprogrammed neurons. Transplantation into developing or early postnatal brain, when the corresponding endogenous neuronal classes are acquiring defining traits, should allow for a clean assessment of the true potential of reprogrammed neurons to acquire class-specific features and developmentally “behave” like their endogenous counterparts. In particular, axonal connectivity to specific targets is a key, defining feature of many classes of neurons in the central nervous system and a prime predictor of functional integration. The trajectory of axons of reprogrammed neurons could easily be determined in vivo using genetic labels and standard retrograde tracing experiments. Reporter labeling of reprogrammed neurons would also allow for morphological measurements including the establishment of stereotyped dendritic trees, which differ in shape, size, complexity and position among different neuronal classes. Finally, modulation and recording of neuronal activity, facilitated by the use of optogenetic tools, could clarify the functional contribution of reprogrammed cells within a network of neurons.
Comparative analysis of the transcriptomes among reprogrammed neuron classes, endogenous neurons of the same class, and the starting non-neuronal cells (e.g. fibroblasts) has been initiated for iDA neurons and iMNs (34, 38). Transcriptional profiling and hierarchical clustering of fibroblast-derived spinal motor neurons showed that they cluster more closely to endogenous motor neurons than fibroblasts or embryonic stem cells (34). Similarly, iDA neurons resemble to some extent endogenous dopaminergic neurons (38). However, notable differences exist. The iDA neurons obtained in vitro exhibited expression profiles that were distinguishable from those of endogenous mDAs (38, 39). Furthermore, expression of several fibroblast genes was retained after reprogramming into dopaminergic neurons. It is unclear how this memory impacts the functionality of iN cells, and mechanistic studies are necessary to understand how fibroblast identity is maintained in order to inform the development of more complete reprogramming strategies. It is likely that within a dish, neurons have achieved distinct levels of reprogramming. Induced neuronal cells have thus far been profiled as populations, which may have led to underestimating differences and similarities present at the single neuron level. Major technological progress now enables single-cell RNA sequencing (43), and these methods are being rapidly interfaced with high-throughput platforms to allow the automatic sequencing of large numbers of individual cells. Single-cell molecular profiling of reprogrammed neurons should in the near future help define the molecular underpinnings that drive the acquisition of neuronal subtype-specific identity by these methods, and allow to select better reprogrammed cells for downstream applications.
For the first time, neuroscientists and stem cell biologists alike are faced with the notable challenge of classifying an ever-growing number of “man-made”, reprogrammed neurons that did not exist a mere five years ago. It is possible that incompletely reprogrammed neurons of a specific class might be functionally equivalent to their endogenous neuronal subtypes. However, when aiming to generate neurons for goals as ambitious as neurological disease modeling and circuit replacement, we propose that a useful starting point would be to determine how iN cells relate to “nature-made” neurons, and further, that the endogenous neuronal complexity should be respected and emulated to a feasible extent.
In vivo neuronal reprogramming
In organs and tissues such as the blood, the heart, and the pancreas, it is possible to directly reprogram one cell type into another in vivo by overexpression of defined factors (44–46). However, researchers have questioned whether all cells are endowed with such plasticity.
Resident non-neuronal cells of the central nervous system have been reprogrammed into induced neuronal cells in vivo. Due to the ability to divide, abundance in the brain, and proximity in lineage-distance, astrocytes have been the ideal starting candidate cell type to generate new neurons. Early work showed that in the adult mouse neocortex, OLIG2+ cells (which include oligodendrocytes, their progenitors and astrocytes) could give rise to neurons upon injury, combined with either overexpression of Pax6 or inhibition of Olig2 (47). Since then, GFAP+ cells could be turned into morphologically-identifiable neurons in the adult striatum upon expression of the BAM cocktail (48), and overexpression of Sox2 was sufficient to reprogram adult striatal astrocytes into neuroblasts, which in turn were able to form neurons (21). These studies illustrate the feasibility of directly reprogramming non-neuronal cells into neurons in situ, which may become a therapeutic option in the future.
In addition to non-neuronal cells, an important question in the field has been whether neurons themselves could be turned from one class into another and whether this could become an optimal strategy to generate neuronal subtypes susceptible to disease with enhanced precision. The plasticity of neurons has been the subject of much debate. Once generated, neurons become post-mitotic and do not change their identity for the lifespan of the organism, suggesting that neurons cannot be converted into other cell types. Much effort has been directed to generate live mice using somatic cell nuclear transfer from primary neurons, with varying results. Some studies led to conclude that post-mitotic neurons may indeed have largely lost their developmental pluripotency (49, 50). However, a first sign that neurons may be capable of reprogramming their identity came from pioneering experiments in which a live mouse was obtained from the nucleus of an olfactory epithelium neuron (51, 52). Using a similar approach, viable mice were subsequently produced by somatic cell nuclear transfer using the nuclei of postmitotic neurons from the cerebral cortex of juvenile mice (53). These experiments provided a proof-of-principle demonstration that the nucleus of at least some classes of neurons is plastic and that no irreversible genetic or epigenetic changes have taken place that preclude the acquisition of a new cellular identity. (For a historical perspective on neuronal reprogramming and reprogramming into neurons, see Figure 3).
Should neurons retain the capability to reprogram their identity, could neurons then be converted from one class into another within the central nervous system? This field is only emerging, but some studies have begun to explore this strategy to build new neurons and circuits, in vivo. Similar to the work on reprogramming non-neuronal cells, master selector genes able to drive the acquisition of class-specific neuronal identity can be powerful tools to instruct neuronal class-switch in vivo. In a first application to neurons of the cerebral cortex, we have used the transcription factor Fezf2, a master gene capable of instructing multiple features of identity of corticospinal motor neurons (CSMN), to investigate whether reprogramming other cortical neurons to become CSMN is possible within the brain. Fezf2 is developmentally required for the birth of CSMN and in its absence all CSMN fail to generate (54–56). In agreement, Fezf2 alone can cell-autonomously instruct the acquisition of CSMN-specific features when expressed in a permissive cellular context, in vivo (57). We have demonstrated that overexpression of Fezf2 is sufficient to directly reprogram embryonic and early postnatal callosal projection neurons (CPN), a class of cortical neurons making interhemispheric connections via the corpus callosum, into corticofugal neurons, including CSMN. Reprogrammed callosal neurons acquire molecular properties of CSMN, and change their axonal connectivity from interhemispheric intracortical projections to corticofugal projections directed below the cortex, including to the spinal cord (58). In line with these findings, endogenous electrophysiological features of CSMN were induced when Fezf2 was force-expressed in layer IV stellate interneurons of the cortex (59).
What is notable about callosal neuron reprogramming is that postmitotic neuronal identity could be changed at P3 and P6 when callosal neurons have already reached their layer location, have connected to their targets in contralateral cortex, and have acquired defined, class-specific features. The data indicate that young neurons retain some ability to change and that the post-mitotic nature of the cell does not per se preclude reprogramming. However, neuronal plasticity progressively declines, and reprogramming capabilities in response to Fezf2 have exhausted by P21 (58).
These results indicate that mechanisms are in place postmitotically to progressively restrict neuronal fate potential and reprogramming capabilities as neurons age. Molecular studies are now needed to extend the critical period of postmitotic neuron reprogramming to the mature brain, and this will be an important challenge for the growth of this exciting field. To this end, one long-term goal will be to investigate the mechanisms that in the first place contribute to maintain neuron class-specific identity, during brain development and maturation. Such studies will help understand normal mechanisms used by neurons to refrain from changing, but also inform strategies to facilitate direct reprogramming of neuronal identity in the adult. It is likely that beyond fate specifying transcription factors, “opening” the permissive temporal window of neuronal reprogramming will require additional manipulations. Epigenetic status, one of the major barriers to reprogramming somatic cells, may be modulated by chemical and genetic approaches. These manipulations may increase the plasticity of the target neurons and enable fate-specifying transcription factors to reprogram neuronal identity at later developmental stages.
In support for the existence of epigenetic blocks to neuronal reprogramming in vivo, work in the retina demonstrates that failure of reprogramming of adult rods into cone photoreceptors may at least be partly due to DNA methylation at key, class-specific loci (60). This study is notable, as the authors were able to induce several molecular, ultrastructural and physiological properties of cones upon conditional removal of the rod-specifying transcription factor Nrl from adult rods. Although the cells retained some rod-specific traits, the work suggests that partial conversion of diseased rods into cones may be feasible in adults.
There are advantages to neuron-to-neuron conversion that make this approach worth pursuing. Closely-related neurons could be chosen that share some pan-neuronal features, most prominently the use of the same neurotransmitter, ability to send long-distance axons, similar morphology, and location into circuit. This method is likely to facilitate the generation of highly specialized neurons, ease their integration into circuitry, and possibly reduce off-target connectivity. Because neurons do not divide, this process is also unlikely to become tumorigenic. It is generally accepted that a small percentage of new neurons of a given class can be sufficient to regain some functionality without visible effects on the behavior mediated by the starting neuronal population. Should in vivo reprogramming of adult neurons become a reality, this approach could be used to generate neurons that are affected by disease via the conversion, in situ, of a small percent of neighboring neurons that are naturally resistant to the same pathology.
Looking into the future of induced neuronal cells
This is an exciting time for the field of cellular reprogramming in the central nervous system. Generation of neurons by direct reprogramming holds great promise for both cell replacement therapy and disease modeling. However, challenges remain. This field is just beginning to understand how differentiated cells are turned into neuronal cells. Mechanistic studies will be of fundamental importance to be able to predict the effect of different transcription factors and of the starting cellular context on the success of direct reprogramming into neuronal cells. This knowledge should help the field move away from current strategies that screen multiple factor permutations. In addition, better criteria to classify induced neuronal cells are needed, especially when aiming to obtain specific classes of neurons. We propose that as more “realistic” and complex replica of endogenous central neurons and tissue are generated, successful reprogramming in the central nervous system will require that expertise in reprogramming and embryology meet those in fundamental neuroscience to drive the choice of neurons to generate and to classify and functionally test the final neuronal products.
Given the speed of reprogramming and the ease of access to patient-derived fibroblasts, direct reprogramming of neurons is a manageable, alternative approach to generating neurons by directed differentiation of human iPSCs. We foresee that comparative characterization of neuronal cells obtained by these two methods will soon define similarities and differences between these sources. Newer approaches to achieve genome editing have also emerged over the past few years that will enable large-scale genome modification of different cell types to insert (or repair) disease-associated mutations (61, 62). These state-of-the-art technologies allow for scalable, highly-specific insertion of somatic point mutations in the genome and could ultimately add a new dimension to modeling and understanding human disease using iN cells. For neuroscientists in particular, generation of an unlimited supply of human neuronal cells – neurons are notoriously difficult to obtain from patients and cannot be expanded in culture – was merely an ambition, far from reality until this opportunity emerged a few years ago. It is now possible to design clinical trials in the dish that may revolutionize preclinical screening of therapeutic compounds by testing them in high-throughput fashion on human neuronal cells, in parallel to mouse modeling.
One exciting application of direct lineage reprogramming in the nervous system is the generation of new neurons in situ by the direct conversion of cells that are resident within the central nervous system. Given the highly specialized nature of neurons and the complexity of the connections they make and receive, it may be advantageous to generate new neurons by direct conversion of other classes of neurons. The starting neurons may have already acquired basic pan-neuronal features that are functionally critical. However, a major challenge will be to identify and overcome barriers that currently hamper reprogramming of neurons in the adult nervous system. The mechanisms that maintain neuronal class-specific identity throughout the lifespan of an organism are largely unknown. While speculative at this stage, we propose that neurons might maintain their identity using unique mechanisms. It is intriguing that the closure of the temporal window of nuclear plasticity of neurons loosely corresponds to their integration into circuit. This suggests the provocative possibility that elements of neuronal identity are sustained by the network in which each neuron integrates.
It remains to be determined whether and how local (or even long-distance) circuitry would react in response to a change in neuronal class-specific identity induced by reprogramming. Should adult neuronal reprogramming become a reality, this in vivo application could be informative in elucidating aspects of circuit plasticity and understanding some of the rules that shape circuit maintenance in vivo. With the knowledge of development and cell identity of all neurons present in the nervous system of C.elegans, this organism may be a perfect first model system to determine whether circuit remodeling accompanies the process of direct reprogramming in vivo. Investigation of invertebrate organisms that are endowed with natural reprogramming capabilities will also facilitate understanding of reprogramming in mammals.
As evidenced by the presentation of the Nobel Prize in 2012, nuclear reprogramming is an exciting, rapidly growing field with the potential to transform basic science and clinical research. Direct reprogramming from one cell into another may be particularly advantageous for the central nervous system because of its in vivo applicability, in addition to neuronal production in the dish. While the progress in the field has generated as many unresolved questions as answers, direct reprogramming has shown promises to revolutionize the way the field thinks about neuronal stability and repair.
Figure 2. Direct reprogramming of various cell types into induced neuronal cells in vitro.
(A) Cultured pericytes (20), (B) astrocytes (15–18), (C) hepatocytes (19), and (D) fibroblasts (22, 23) are reprogrammed into induced neurons by defined factors. (E) Fibroblasts are reprogrammed into iDA neurons (38, 39) and (F) iMNs (34). Here, we illustrate selected methods for the direct conversion into these neuronal subtypes. Blue box = mouse reprogramming factors. Red box = human reprogramming factors.
Figure 4. In vivo direct reprogramming of various cell types into neurons.
(A) Endogenous callosal projection neurons of early postnatal mice are directly reprogrammed into corticofugal projection neurons (58). (B) Layer IV spiny neurons are reprogrammed into neurons with electrophysiological properties of corticofugal neurons (59). (C) Adult striatal astroctyes are reprogrammed into induced neuronal cells by overexpression of the BAM factors (48). (D) Cortical OLIG2+ glial cells give rise to neuronal cells upon injury combined with either inhibition of Olig2 or overexpression of Pax6 (47).
Acknowledgements
We thank Benedikt Berninger, Matthias Stadtfeld, Hynek Wichterle and members of the Arlotta laboratory for their valuable comments on the manuscript. We apologize to colleagues whose work we could not include due to space limitations. Work in the Arlotta Lab is funded by the US National Institute of Health. P.A. is a New York Stem Cell Foundation-Robertson Investigator.
References
- 1.Gurdon J. From nuclear transfer to nuclear reprogramming: the reversal of cell differentiation. Annual review of cell and developmental biology. 2006;22:1. doi: 10.1146/annurev.cellbio.22.090805.140144. [DOI] [PubMed] [Google Scholar]
- 2.Gurdon J. Adult frogs derived from the nuclei of single somatic cells. Developmental biology. 1962;4:256. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- 3.Gurdon J, Uehlinger V. “Fertile” intestine nuclei. Nature. 1966;210:1240. doi: 10.1038/2101240a0. [DOI] [PubMed] [Google Scholar]
- 4.Briggs R, King T. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs' Eggs. Proceedings of the National Academy of Sciences of the United States of America. 1952;38:455. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes & development. 2010;24:2239. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waddington C. The Strategy of the Genes. Allen Unwin t957; London: 1957. p. 262. [Google Scholar]
- 9.Ladewig J, Koch P, Brüstle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nature reviews. Molecular cell biology. 2013;14:225. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- 10.Yang N, Ng Y, Pang Z, Südhof T, Wernig M. Induced neuronal cells: how to make and define a neuron. Cell stem cell. 2011;9:517. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawkins R. The greatest show on earth: The evidence for evolution. Simon and Schuster. 2009 [Google Scholar]
- 12.Aoki H, Hara A, Niwa M, Yamada Y, Kunisada T. In vitro and in vivo differentiation of human embryonic stem cells into retina-like organs and comparison with that from mouse pluripotent epiblast stem cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2266. doi: 10.1002/dvdy.22008. [DOI] [PubMed] [Google Scholar]
- 13.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster M, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013 doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heins N, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nature neuroscience. 2002;5:308. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 16.Berninger B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8654. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrich C, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrich C, et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nature protocols. 2011;6:214. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- 19.Marro S, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell stem cell. 2011;9:374. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell stem cell. 2012;11:471. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Niu W, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature cell biology. 2013;15:1164. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang Z, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand N, Castro D, Guillemot F. Proneural genes and the specification of neural cell types. Nature reviews. Neuroscience. 2002;3:517. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 25.Orly LW, et al. Hierarchical Mechanisms for Direct Reprogramming of Fibroblasts to Neurons. Cell. 2013;155 doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladewig J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nature methods. 2012;9:575. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 27.Liu M-L, et al. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nature communications. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ninkovic J, et al. The BAF Complex Interacts with Pax6 in Adult Neural Progenitors to Establish a Neurogenic Cross-Regulatory Transcriptional Network. Cell stem cell. 2013 doi: 10.1016/j.stem.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun A, Crabtree G, Yoo A. MicroRNAs: regulators of neuronal fate. Current opinion in cell biology. 2013;25:215. doi: 10.1016/j.ceb.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muotri A, Gage F. Generation of neuronal variability and complexity. Nature. 2006;441:1087. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- 31.Kanning K, Kaplan A, Henderson C. Motor neuron diversity in development and disease. Annual review of neuroscience. 2010;33:409. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 32.Jessell T. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature reviews. Genetics. 2000;1:20. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 33.Wichterle H, Lieberam I, Porter J, Jessell T. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 34.Son E, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell stem cell. 2011;9:205. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzoni E, et al. Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nature neuroscience. 2013 doi: 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulzer D, Surmeier D. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alavian K, Scholz C, Simon H. Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Movement disorders : official journal of the Movement Disorder Society. 2008;23:319. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- 38.Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell stem cell. 2011;9:413. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell research. 2012;22:321. doi: 10.1038/cr.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfisterer U, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10343. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiang L, et al. Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Tang F, Lao K, Surani M. Development and applications of single-cell transcriptome analysis. Nature methods. 2011;8:11. doi: 10.1038/nmeth.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton D. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buffo A, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18183. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torper O, et al. Generation of induced neurons via direct conversion in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7038. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makino H, et al. Mouse embryos and chimera cloned from neural cells in the postnatal cerebral cortex. Cloning and stem cells. 2005;7:45. doi: 10.1089/clo.2005.7.45. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki Y, et al. Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14022. doi: 10.1073/pnas.231489398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eggan K, et al. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Ishii T, Feinstein P, Mombaerts P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature. 2004;428:393. doi: 10.1038/nature02433. [DOI] [PubMed] [Google Scholar]
- 53.Osada T, et al. Developmental pluripotency of the nuclei of neurons in the cerebral cortex of juvenile mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8368. doi: 10.1523/JNEUROSCI.1591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen B, Schaevitz L, McConnell S. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17184. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J-G, Rasin M-R, Kwan K, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17792. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molyneaux B, Arlotta P, Hirata T, Hibi M, Macklis J. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 57.Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nature neuroscience. 2010;13:1345. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nature cell biology. 2013;15:214. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De la Rossa A, et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nature neuroscience. 2013;16:193. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- 60.Montana C, et al. Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1732. doi: 10.1073/pnas.1214387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cong L, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science (New York, N.Y.) 2013 doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nature biotechnology. 2011;29:149. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spemann H. Embryonic Development and Induction. Yale University Press; New Haven: 1938. p. 401. [Google Scholar]
- 64.Wilmut I, Schnieke A, McWhir J, Kind A, Campbell K. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 65.Davis R, Weintraub H, Lassar A. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]