Abstract
Purpose
To assess the predictive value of examinations of tissue adherent to multitined electrodes on local tumor progression-free survival (LPFS) and overall survival (OS) after liver tumor radiofrequency ablation (RFA).
Methods
An institutional review board–approved, Health Insurance Portability and Accountability Act–compliant review identified 68 liver tumors treated with RFA in 63 patients with at least 3 years’ follow-up. Tissue adherent to the electrode after liver tumor RFA was evaluated with proliferation (Ki-67) and apoptotic (caspase-3) markers. LPFS and OS were evaluated by Kaplan–Meier methodology and the log-rank test. Multivariate analysis assessed the effect of tumor size, pathology, and post-RFA tissue characteristics on LPFS and OS.
Results
Post-RFA tissue examination classified 55 of the 68 tumors as completely ablated with coagulation necrosis, with cells positive for caspase-3 and negative for Ki-67 (CN). Thirteen had viable Ki-67-positive tumor cells. Mean liver tumor size was larger in the viable (V) group versus the CN group (3.4 vs. 2.5 cm, respectively; P = .017). For the V and CN groups, respectively, local tumor progression occurred in 12 (92 %) of 13 and 23 (42 %) of 55 specimens. One, 3-, and 5-year LPFS was 8 %, 8 %, and 8 %, and 79 %, 47 %, and 47 % (P <.001) for the V and CN groups, respectively. During a 63-month median follow-up, 92 % of patients in the V group and 58 % in the CN group died, resulting in 1-, 3-, and 5-year OS of 92 %, 25 %, and 8 % vs. 92 %, 59 %, and 33 % (P = .032), respectively.
Conclusions
Ki-67-positive tumor cells on the electrode after liver tumor RFA is an independent predictor of LPFS and OS. Size, initially thought to be an independent risk factor for local tumor progression in tumors 3–5 cm, does not hold its significance at long follow-up.
Primary hepatocellular carcinoma (HCC) and metastatic colorectal carcinoma (CRC) are the most common malignant liver tumors. HCC is diagnosed in approximately 1 million people worldwide every year, and it is the most common solid carcinoma in the world.1,2 Annually, 700,000 people develop CRC worldwide, and as many as 50 % of these patients will develop liver metastases during the course of their disease.3 Hepatectomy is considered the treatment of choice for malignant liver tumors, but the majority of patients with liver malignancies are not candidates for surgery.2,3 In the last decade, there has been an increasing use of radiofrequency (RF) ablation (RFA) for liver tumors, and its efficacy has been demonstrated in several studies.2–10
Local tumor progression (LTP) after liver tumor RFA remains an important limitation of RFA with relatively short local progression-free survival (LPFS) in substantial percentages of patients.11–20 There is a need for the utilization of prognostic tools and markers to identify patients at risk for LTP and short LPFS after RFA. Prior studies of hepatic RFA demonstrated that histopathologic analysis of tissue adherent to multitined RFA electrodes was feasible.21 The use of Ki-67 proliferation and caspase-3 apoptosis markers can classify specimens from ablated tumors as viable or necrotic.22 Caspase-3 regulates downstream activators, which cleave cytoskeletal and nuclear proteins, inducing apoptosis and irreversible cell death.23 Ki-67 is a proliferation antigen that is expressed during all cell cycle phases except the G0 phase and is indicative of a cell that maintains the ability to proliferate and thus is viable.24
Our hypothesis was that the identification of even a single Ki-67-positive tumor cell adherent to the RFA electrode is indicative of incomplete ablation and is associated with a higher probability for LTP and shorter patient survival. The aim of this study was to examine the predictive value of histopathologic findings on LPFS and overall survival (OS). This study was designed to assess whether the proliferation marker Ki-67 can be used as an independent predictor of oncologic outcomes, particularly survival, after liver tumor RFA.
MATERIALS AND METHODS
We collected and examined the tissue adherent on multi-tined RF electrodes used for ablation of 68 liver tumors applying proliferation (Ki-67) and apoptotic (caspase-3) markers. An institutional review board waiver was obtained for retrospective review. All specimens with Ki-67-positive tumor cells were classified as viable; all the rest were classified as coagulation necrosis (CN). We reviewed all medical records and relevant imaging studies to determine long-term clinical outcomes and assess the prognostic value of the histopathologic features of tissue adherent to the electrode after RFA of liver malignancies. A detailed description of methodology and analysis was made in a preliminary study.22
Inclusion Criteria
Patients with up to three primary or metastatic liver tumors (≤ 5 cm in diameter) and limited to no more than three lesions outside the liver were included in the study. A minimum follow-up of 3 years after ablation was required for inclusion in this cohort.
Exclusion Criteria
We excluded patients with noncorrectable coagulopathy (international normalized ratio of >1.5 or platelet count of <50,000 mm3); a tumor location of <1 cm from a major bile duct, gastrointestinal tract, or major blood vessel; and patients who were unable to undergo general anesthesia.
Subjects
Between March 20, 2003, and March 9, 2006, we ablated 68 consecutive hepatic tumors in 63 patients (32 women, 31 men, age 27–88 years) by using two multitined RF electrodes as described below. Twenty-nine tumors were metastatic CRC, 19 were HCCs, and the rest were diverse metastatic tumors (Table 1). In order to plan the procedure, all patients were evaluated by dynamic computed tomography (CT) within 30 days from the ablation. Patient demographics in the CN and viable (V) groups were of comparable size (P >.05) (Table 1).
TABLE 1.
Demographics of 63 patients with 68 malignant hepatic tumors
| Characteristic | CN group | V group | CN and V groups |
|---|---|---|---|
| Age (y), median (range) | 64.94 (27–88) | 59.67 (45–75) | 63.94 (27–88) |
| Sex, M:F | 24:27 | 7:5 | 31:32 |
| Tumor size (cm), mean (range) | 2.5 (.6–5.0) | 3.4 (2.0–5.0) | 2.7 (.6–5.0) |
| Tumor type | |||
| HCC | 17 | 2 | 19 |
| Colorectal cancer | 20 | 9 | 29 |
| Breast cancer | 7 | 0 | 7 |
| Cholangiocarcinoma | 3 | 0 | 3 |
| Lung cancer | 2 | 0 | 2 |
| Neuroendocrine cancer | 2 | 0 | 2 |
| Ovarian cancer | 1 | 0 | 1 |
| Retroperitoneal cancer | 1 | 0 | 1 |
| Duodenal cancer | 1 | 0 | 1 |
| Round cell tumor | 1 | 0 | 1 |
| Leiomyosarcoma | 0 | 2 | 2 |
| Adjuvant chemotherapy | 22 | 8 | 30 |
| Prior hepatic surgery | 16 | 7 | 23 |
| Total no. of lesions | 55 | 13 | 68 |
| Extrahepatic disease (n = 18)a | |||
| Lung | 6 | 5 | – |
| Lymph node | 4 | 4 | – |
| Bone | 3 | 2 | – |
| Peritoneum | 1 | 0 | – |
| Total | 10/51 (20 %) | 8/12 (67 %) | 18/63 (29 %) |
| No. of patients with disease at two sites | 4/51 (8 %) | 3/12 (25 %) | 7/63 (11 %) |
Seven patients had two sites
Treatment
All tumors were treated with CT-guided RFA. Patients were sedated and monitored by an anesthesiologist. A pro-phylactic antibiotic (1 g Ancef; GlaxoSmithKline, Research Triangle Park, NC) was administered intravenously just before the procedure. RFA was performed with the Radio-therapeutics LeVeen (n = 54) or the RITA Starburst XL (n = 14) device, depending on tumor size and geometry, and operator preference. The goal was to create an area of CN at least 1 cm larger than the tumor’s largest diameter to achieve a minimum ablation margin of 5 mm uniformly around the tumor. Needle placement and accurate tumor targeting was always evaluated with CT and when necessary with sonography. As needed, overlapping ablations were performed in order to completely treat the tumor with a sufficient margin.25 Fifty-five tumors were treated with more than one overlapping ablations. Of these, 10 were treated with three overlapping ablations. No tumor was treated with more than three overlaps. We applied and completed the manufacturer’s recommended protocol in all cases. Detailed description of these protocols has been previously described.22
Histopathologic and Immunohistochemical Analysis
A detailed methodology has been previously described.22 Tissue was identified in all multitined electrodes. In brief, after all tissue fragments were collected from electrodes, they were fixed in formalin and stained with hematoxylin and eosin, then analyzed for the apoptotic marker caspase-3 and for the cell proliferation marker Ki-67.23,24 Specimens positive for Ki-67 alone or for both markers were classified as viable tumor. The remaining specimens included thermal artifact/CN without identifiable cells or cells negative to Ki-67, and were classified as CN.
Imaging Follow-up
Dynamic CT was performed within 25–42 days of RFA to evaluate response. A defect covering the target tumor with lack of enhancement of the ablated area was considered evidence of complete and effective ablation. Irregular peripheral or nodular enhancement within 1 cm of the ablated area was considered untreated (residual) tumor and a technical failure.26,27 Thereafter, radiologic follow-up continued at 2–4-month intervals for 5 years (or until death) and was used to evaluate LTP at the site of prior ablation . Evidence of irregular or nodular enhancement within 1 cm of the previously treated tumor was considered LTP.26,27
Definitions
We have adhered to the guidelines regarding terminology and definitions as described by Goldberg et al.27 These are as follows. Technical success of RFA is documented when tumor is treated according to protocol and covered completely as seen on the immediately postablation CT. Technique effectiveness is defined as an ablation defect covering the entire tumor at a 4–8-week post-RFA CT. LTP is defined as enhancement or focal growth of tumor tissue within 1 cm of the ablated tumor developing in subsequent follow-up CT, performed every 2–4 months. Primary LPFS is defined as the time interval between the initial RFA and the first radiologic evidence of LTP at the site or within 1 cm from the ablated tumor. Intervention-assisted LPFS is defined as the cumulative time between initial RFA and the latest radiologic follow-up that shows LTP. This interval includes all ablations performed for the treatment of LTP within 1 cm from the treated tumor. Finally, OS time is defined as the cumulative time between RFA and patient’s death or most recent follow-up.
Statistical Analysis
Tumor size was expressed as mean ± standard deviation and compared between the V and CN groups by the two-sample t test. The Fisher’s exact test was used to determine the association between overlapping ablations and presence of Ki-67-positive tumor on the electrode. Overall and local progression-free survival probabilities were estimated by the Kaplan–Meier method and compared with the log-rank test. The effect of overlapping ablations on the LTP-free survival was also estimated by the Kaplan–Meier method and compared between the V and CN groups with the log-rank test. We also repeated a bivariate analysis with viable tissue and tumor size simultaneously, with 3 cm used as the cutoff, as previously established.22
RESULTS
We treated 63 patients with 68 hepatic tumors up to 5 cm in largest diameter with RFA. Fifty-five specimens had CN and 13 had viable tumor. There was no difference in overlapping ablations performed to completely treat the tumors in the two groups: 44 (80 %) of 55 tumors in the CN group and 11 (85 %) of 13 tumors in the V group were treated with at least one overlapping ablation (P = 1.0000). Mean tumor size ± standard deviation before treatment was larger in the V (3.4 ± 1 cm) than in the CN group (2.5 ± 1.1 cm) (P = .017).22 Disease and patient characteristics for the CN and V groups are summarized in Table 1.
LTP and Primary LPFS
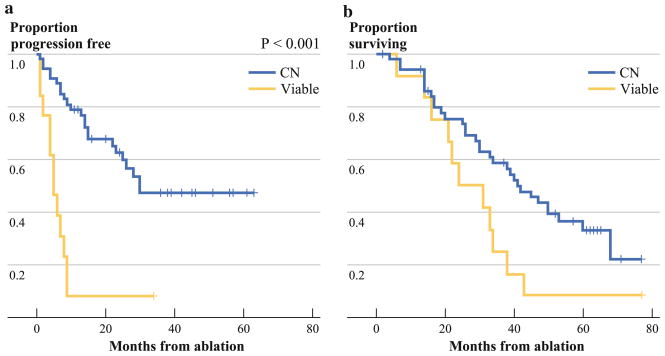
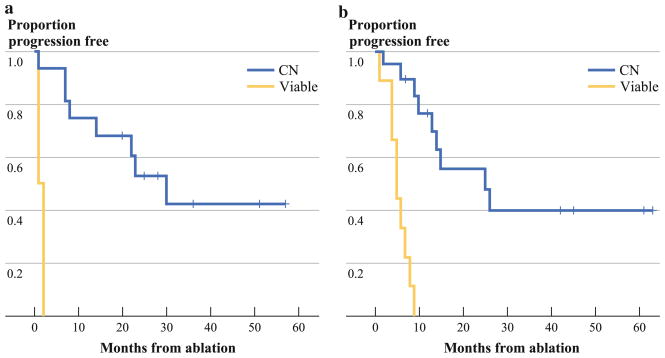
Median follow-up for the cohort was 63 (range 37–77) months and median LPFS was 25 (range 1–63) months. LTP occurred in 12 (92 %) of 13 lesions in the V group and 23 (42 %) of 55 in the CN group (P <.001). Figure 1a shows a significantly prolonged LPFS for the CN when compared to the V group. The primary LPFS rates for V and CN groups were 8 % vs. 79 %, 8 % vs. 47 %, and 8 % vs. 47 % at 1, 3, and 5 years after RFA, respectively (P <.001). There was no difference in LPFS between HCC and CRC patient groups (P = .78). For both HCC and CRC, there were no LTP-free patients within the V group after only 6 months. For both the HCC and the CRC populations, significantly prolonged LPFS in the CN groups was recorded (Fig. 2) (P <.001 for both).
FIG. 1.
Kaplan-Meier curves for V and CN groups (yellow line, viable tumor; blue line, CN). a LPFS by groups. b OS by groups
FIG. 2.
LPFS on the basis of the viable tumor or CN status of tissue (yellow line, viable tumor; blue line, CN). a LPFS for HCC lesions by groups. b LPFS for CRC lesions by groups
Intervention-assisted LPFS
During this study, five lesions in the CN group and three in the V group underwent a second RFA to treat LTP. Two lesions, one in the CN group and one in the V group, underwent a third RFA for additional LTP. The 1-, 3-, and 5- year intervention-assisted LPFS probabilities were 31 % vs. 85 %, 8 % vs. 66 %, and 8 % vs. 51 % in the V and CN groups, respectively (P <.001).
Analysis of Factors Related to LTP
We evaluated the effect of tumor size, ablation technique, and viability after RFA on LTP (Table 2). The performance of overlapping ablations did not affect LTP. The 2-year LTP-free survival rates were 52 % for tumors treated without overlaps and 51 % for tumors treated with at least one overlap (P = .445). Lesion size, when used as a continuous variable in univariate analysis, was a significant predictor of LTP with each additional centimeter in tumor size, increasing the risk of LTP by 40 % (P = .04). In a multivariate analysis including size and viability, size was not significant (hazard ratio [HR] 1.25, 95 % confidence interval [CI] .89–1.76, P = .20), while viability retained its significance (HR 5.1, 95 % CI 2.4–10.6, P <.001). These findings confirm that prognostic significance of tumor viability is sustained at 5-year follow-up. In contrast, size is no longer an independent risk factor for LTP for all lesions up to 5 cm in largest diameter.
TABLE 2.
Multivariate analysis of factors related to LTP
| Factor | HR | 95 % CI | P |
|---|---|---|---|
| All lesions | |||
| Size (cm) | 1.25 | .89–1.76 | .20 |
| Viability | 5.1 | 2.4–10.6 | <.001 |
| Lesions <3 cm | |||
| Size (cm) | .72 | .37–1.4 | .33 |
| Viability | 5.1 | 1.6–15.9 | <.001 |
Patient Survival
Of 63 patients in the study, 51 were in the CN group and 12 were in the V group. During the study, there were 44 deaths, 32 in the CN group and 12 in the V group, with a median OS of 34 (range 2–77) months. After the RFA procedure, the overall 1-, 3-, and 5-year survival rates for all 63 patients were 92 %, 49 %, and 25 %, respectively (Fig. 1b). Median and 1-, 3-, and 5-year OS rates for the CN group were 41 months, and 94 %, 59 %, and 33 %, respectively. These were significantly longer than in the V group, which reached a median OS of 24 months, and 1-, 3-, and 5- year OS of 92 %, 25 %, and 8 %, respectively (P = .032) (Fig. 1b). We evaluated whether size and viable tissue are both independent predictors in a multivariate model. Size was not significant (P = .38, HR 1.13), leaving viable (Ki-67 positive) tumor cells as the only predictor of OS (P = .037, HR 2.11, 95 % CI 1.05–4.25).
Separate analysis of the HCC and CRC groups was performed on the basis of the viable tumor or CN status of the tissue. The median OS for HCC was 25 and 30 months for the V and CN groups, respectively (P = .24). The 1-, 3-, and 5-year survival rates for the HCC group were 94 %, 50 %, and 36 %, respectively. The median OS for CRC was 23 and 47 months for the V and CN groups, respectively (P = .18). The 1-, 3-, and 5-year survival rates for the CRC group were 88 %, 57 %, and 32 %, respectively.
DISCUSSION
Primary HCC and metastatic CRC are the two most common malignant liver tumors. Hepatic resection is the ideal treatment for hepatic malignancies; however, only 5–15 % of patients with HCC and 20–25 % of those with liver metastasis are candidates for resection at diagnosis.2 RFA is a safe and effective treatment of unresectable hepatic tumors.28–31 Unfortunately, incomplete tumor treatment and local tumor recurrence or progression (LTP) are common after percutaneous RFA.14,15,31,32 Percutaneous ablation techniques are designed to destroy tumor locally without tissue removal. At the end of the procedure, the ablated tumor remains in the body and, unlike in surgery, there is no tissue examination to confirm tumor death and sufficient margin creation. This limitation and the lack of evaluation of regional lymph nodes represent the relative disadvantages of percutaneous ablation when compared to open surgery.
This study reports 5-year follow-up data for patients who had examinations of tissue adherent to multitined RF electrodes after ablation of liver malignancies. On the basis of the findings from our latest work, the identification of Ki-67-positive tumor cells adherent on the RF electrode was considered an independent predictor of LTP after liver tumor ablation.22,33 Ki-67 seems to be the most relevant marker to evaluate the ability of tumor cells to proliferate and eventually result in LTP.22 In this 5-year follow-up study, we confirmed our initial results showing that Ki-67 analysis of tissue adherent to electrodes can reliably predict LTP after RFA of liver malignancies. When comparing the median progression-free survival of the CN (30 months) and V (5 months) groups, it is evident that the V group (Ki-67-positive tumor cells) carries a significantly higher risk for LTP and shorter LPFS (P <.001). On the contrary—size, initially thought to be an independent risk factor for LTP in tumors 3–5 cm, does not hold its significance. Specifically, the new hazard ratio attributed to tumor size is 1.25, compared to 1.4 in the initial study. This and a relatively high hazard ratio for tumor viability (Ki-67-positive tumor cells) (5.1) are responsible for the lack of tumor size significance and the added importance of the Ki-67 as predictors of LTP after ablation of liver malignancies. Despite these changes, the current results are consistent in the sense that the updated estimates of the hazard ratios (1.25 for size and 5.1 for viability) are contained within the 95 % CIs from the preliminary study.22 The conclusions for the analysis of the subset of lesions <3 cm in size are unchanged from the preliminary results to the current analysis.
Prior publications have demonstrated that Ki-67 can be used as an independent predictor of disease-free survival and OS in patients with different types of cancer, including HCC and colon cancer.28,34–39 High levels of Ki-67 were also shown to be an independent predictor of survival after resection of CRC hepatic metastases.40 In another study, patients who underwent hepatic resection along with Ki-67 immunohistochemical analysis demonstrated a significantly improved 5-year survival rate when the Ki-67 labeling index was less than 50 % (49 % survival) as opposed to a Ki-67 labeling index above 50 % (16 % survival).41 This work did not perform quantitative analysis of the Ki-67. Instead, tissue samples positive for Ki-67 and negative for caspase-3 were classified as viable, while samples negative for Ki-67 were classified as CN. If both Ki-67 and caspase-3 were positive in a single sample, this was labeled viable, using the logic that after ablation, even a single tumor cell positive for proliferation can cause local recurrence.22
A recent study by Snoeren et al. analyzed the viability of tissue adherent on the electrode at the end of ablation of liver tumors by the autofluorescence method and by glucose-6-phosphate diaphorase staining. Similarly, in that cohort, viable tissue was an independent risk factor for LTP.42 The local recurrence rate in that study was high: 36 %, within a median follow-up time of 34 months. Comparably, in our preliminary study we observed a LTP rate of 41 % within a median follow-up time of 28 months. The additional follow-up time allowed for the development of 7 more local recurrences, resulting in a 51 % rate of LTP within a median follow-up of 63 months in the current study. This may more accurately represent the long-term rate of LTP after ablation. This rate is on the high end but still within the reported range of LTP after RFA. Although we assumed that all of these progressions were the result of residual tumor left behind at the ablation zone, it is possible that some of the progressions are the result of new tumor development and are not related to the originally ablated tumor. This may be particularly true in the patients in whom no Ki-67 tumor cells were identified and in whom diffuse progression was noted in addition to LTP (20 patients), as well as for originally ablated tumors (7 tumors) that developed LTP more than 2 years after ablation. Notably, all the progressions in the Ki-67 group occurred within a year of ablation. The relatively high LTP is a serious limitation of ablation.11–14 The identification of prognostic factors and biomarkers that can predict outcomes is critical for the evaluation of percutaneous ablation therapies. Such markers may allow for treatment modifications (repeat ablation or other locoregional treatment or adjuvant chemotherapy) of the patients at risk in order to improve clinical outcomes.
Our 5-year follow-up demonstrated a clear survival benefit in the group of patients who had only coagulated tissue found on the electrode at the end of RFA. This indicates that Ki-67-positive tumor cells adherent on the electrode after RFA is a strong independent predictor of relatively short OS. In a multivariate analysis, no other factor was an independent predictor of survival after RFA of liver malignancies.
There were a number of limitations to our study. The most important limiting factor affecting the significance of our data was the relatively small number of enrolled patients (n = 63). Similarly, the number of patients who were found to have Ki-67-positive tumor cell on tissue adherent on the RF electrode was only 13. Although the differences between the V and CN groups in terms of LPFS and OS are striking, these may not be an accurate reflection of the overall population of patients with liver malignancies treated with RFA. Another limitation was the lack of sampling of the entire ablated tumor similar to what happens with the evaluation of the margins after surgery. Although a complete tissue evaluation of the ablation margin is likely impossible, additional sampling might offer information about the ablation and its effect on tumor. Finally, our described methodology is limited to the cases when tissue was adherent on the electrode. This method cannot be universally applied in all ablation devices. With regard to our immunohistochemical analysis, it appears that the evaluation of specimens with caspase-3 was of little value because specimens that were positive for both markers (Ki-67 and caspase-3) were classified as viable. Although our immunohistochemical analysis can be performed in fixed specimens any time after the procedure, it cannot provide an immediate assessment of the ablated tumor that could modify the treatment. Our current efforts toward modifying the technique and improving clinical outcomes after tumor ablation include sampling the ablation zone by biopsy and investigating viability stains that may enable immediate assessment of tumor death and viability during ablation.
Ki-67 positivity might be used in the decision-making process for additional therapy in a patient-tailored manner with additional locoregional or systemic therapy. To our knowledge, this is the first time that Ki-67-positive tumor cells from ablated liver tumors have been associated with OS. This important information demonstrates the value of tissue characteristics as surrogate biomarkers of outcomes, and in particular survival, in the treatment of liver malignancies and cancer in general.
In conclusion, we were able to confirm our hypothesis that the presence of Ki-67-positive tumor cells adherent on the electrode is an independent predictor of LPFS and OS after RFA of liver tumors. Although we continue our investigation in terms of defining the best biomarkers of outcomes after tumor ablation, it seems that Ki-67 is a reliable predictor of long-term oncologic outcomes, and in particular LPFS and OS after liver tumor RFA.
Acknowledgments
Supported in part by National Institutes of Health grant 5R21CA131763.
Footnotes
Presented at the 2011 Society of Interventional Radiology annual meeting.
References
- 1.Motola-Kuba D, Zamora-Valdes D, Uribe M, Mendez-Sanchez N. Hepatocellular carcinoma. An overview. Ann Hepatol. 2006;5:16–24. [PubMed] [Google Scholar]
- 2.Hildebrand P, Kleemann M, Roblick UJ, et al. Radiofrequency-ablation of unresectable primary and secondary liver tumors: results in 88 patients. Langenbecks Arch Surg. 2006;391:1–6. doi: 10.1007/s00423-006-0024-x. [DOI] [PubMed] [Google Scholar]
- 3.Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer. 2004;4:92–100. doi: 10.3816/ccc.2004.n.012. [DOI] [PubMed] [Google Scholar]
- 4.Gillams AR. The use of radiofrequency in cancer. Br J Cancer. 2005;92:1825–9. doi: 10.1038/sj.bjc.6602582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curley SA, Izzo F. Radiofrequency ablation of primary and metastic liver tumors. Surg Technol Int. 2002;10:99–106. [PubMed] [Google Scholar]
- 6.Chen MH, Yang W, Yan K, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395–401. doi: 10.3748/wjg.v11.i40.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. Am J Roentgenol. 2007;189:883–9. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 8.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation—intermediate and long-term survival rates. Radiology. 2009;253:861–9. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhim H, Lim HK, Choi D. Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg. 2010;2:128–36. doi: 10.4240/wjgs.v2.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobs TF, Hoffmann RT, Schrader A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32:38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181–90. doi: 10.1001/archsurg.141.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41–52. doi: 10.1016/j.suronc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radiofrequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–26. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 14.Harrison LE, Koneru B, Bahramipour P, et al. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–64. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 15.White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256–63. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 16.Livraghi T, Solbiati L, Meloni MF, Gazelle EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R, Crocetti L, Cioni D, Della Pina C, Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol. 2004;39:689–97. doi: 10.1097/00004424-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 18.White TJ, Roy-Choudhury SH, Breen DJ, et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases—initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg. 2004;21:314–20. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- 19.Siperstein A, Garland A, Engle K, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors. Technical considerations. Surg Endosc. 2000;14:400–5. doi: 10.1007/s004640000067. [DOI] [PubMed] [Google Scholar]
- 20.Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–9. doi: 10.1001/archsurg.137.12.1332. [DOI] [PubMed] [Google Scholar]
- 21.Snoeren N, Jansen MC, Rijken AM, et al. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg. 2009;26:56–62. doi: 10.1159/000194946. [DOI] [PubMed] [Google Scholar]
- 22.Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–74. doi: 10.1148/radiol.2491071752. [DOI] [PubMed] [Google Scholar]
- 23.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Ramnath N, Moysich KB, et al. Prognostic significance of MCM2, Ki-67 and gelsolin in non–small cell lung cancer. BMC Cancer. 2006;6:203. doi: 10.1186/1471-2407-6-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–82. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 26.Choi H, Loyer EM, DuBrow RA, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics. 2001;21(Spec No):S41–54. doi: 10.1148/radiographics.21.suppl_1.g01oc08s41. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–39. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graur F, Vlad L, Furcea L, Miclaus D, Osian G. Radiofrequency ablation of liver tumors: technique and preliminary results. Chirurgia (Bucur) 2006;101:159–67. [PubMed] [Google Scholar]
- 29.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–47. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–22. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 31.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–73. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 32.Pulvirenti A, Garbagnati F, Regalia E, et al. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516–7. doi: 10.1016/s0041-1345(00)02577-x. [DOI] [PubMed] [Google Scholar]
- 33.Sofocleous CT, Klein KM, Hubbi B, et al. Histopathologic evaluation of tissue extracted on the radiofrequency probe after ablation of liver tumors: preliminary findings. AJR Am J Roentgenol. 2004;183:209–13. doi: 10.2214/ajr.183.1.1830209. [DOI] [PubMed] [Google Scholar]
- 34.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 35.Tollefson MK, Thompson RH, Sheinin Y, et al. Ki-67 and coagulative tumor necrosis are independent predictors of poor outcome for patients with clear cell renal carcinoma and not surrogates for each other. Cancer. 2007;110:783–90. doi: 10.1002/cncr.22840. [DOI] [PubMed] [Google Scholar]
- 36.King KL, Hwang JJ, Chau GY, et al. Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13:273–9. doi: 10.1111/j.1440-1746.1998.01555.x. [DOI] [PubMed] [Google Scholar]
- 37.Garrity MM, Burgart LJ, Mahoney MR, et al. Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes’ B2 or C colon cancer: a North Central Cancer Treatment Group study. J Clin Oncol. 2004;22:1572–82. doi: 10.1200/JCO.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Toquet C, Le Neel JC, Guillou L, et al. Elevated (>or = 10 %) MIB-1 proliferative index correlates with poor outcome in gastric stromal tumor patients: a study of 35 cases. Dig Dis Sci. 2002;47:2247–53. doi: 10.1023/a:1020187211376. [DOI] [PubMed] [Google Scholar]
- 39.Vilar E, Salazar R, Pérez-García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14:221–32. doi: 10.1677/ERC-06-0074. [DOI] [PubMed] [Google Scholar]
- 40.Weber JC, Nakano H, Bachellier P, et al. Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? Am J Surg. 2001;182:81–8. doi: 10.1016/s0002-9610(01)00656-0. [DOI] [PubMed] [Google Scholar]
- 41.Yu HC, Cheng JS, Lai KH, et al. Factors for early tumor recurrence of single small hepatocellular carcinoma after percutaneous radiofrequency ablation therapy. World J Gastroenterol. 2005;11:1439–44. doi: 10.3748/wjg.v11.i10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18:3702–10. doi: 10.1245/s10434-011-1762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]