Abstract
Purpose
This study was designed to evaluate the relationship between the minimal margin size and local tumor progression (LTP) following CT-guided radiofrequency ablation (RFA) of colorectal cancer liver metastases (CLM).
Methods
An institutional review board-approved, HIP-PA-compliant review identified 73 patients with 94 previously untreated CLM that underwent RFA between March 2003 and May 2010, resulting in an ablation zone completely covering the tumor 4–8 weeks after RFA dynamic CT. Comparing the pre- with the post-RFA CT, the minimal margin size was categorized to 0, 1–5, 6–10, and 11–15 mm. Follow-up included CT every 2–4 months. Kaplan–Meier methodology and Cox regression analysis were used to evaluate the effect of the minimal margin size, tumor location, size, and proximity to a vessel on LTP.
Results
Forty-five of 94 (47.9 %) CLM progressed locally. Median LTP-free survival (LPFS) was 16 months. Two-year LPFS rates for ablated CLM with minimal margin of 0, 1–5 mm, 6–10 mm, 11–15 mm were 26, 46, 74, and 80 % (p < 0.011). Minimal margin (p = 0.002) and tumor size (p = 0.028) were independent risk factors for LTP. The risk for LTP decreased by 46 % for each 5-mm increase in minimal margin size, whereas each additional 5-mm increase in tumor size increased the risk of LTP by 22 %.
Conclusions
An ablation zone with a minimal margin uniformly larger than 5 mm 4–8 weeks postablation CT is associated with the best local tumor control.
Keywords: Ablation, Radiofrequency ablation, Minimal margin, Local tumor progression, Colon cancer liver metastasis, Image guided, CT guided, Ablation margin evaluation method
Introduction
Radiofrequency ablation (RFA) has been used increasingly for the treatment of hepatic malignancies in selected unresectable patients [1–4]. Several studies have analyzed risk factors and patterns of local tumor progression (LTP) in an effort to improve local disease control and oncologic outcomes after RFA [1, 5–7]. The ablation margin is considered one of the key factors associated with local tumor control [6, 8–12].
Although current societal guidelines for liver tumor ablation provide a general definition of the ablation margin [13, 14], there is still no universally accepted definition of the ablation margin. Some authors defined the postablation margin as the difference between the maximum diameter of the postablation zone and the maximum pretreatment tumor diameter divided by 2 [11]. This metric assumes a centrally located tumor within the ablation zone and does not consider a possible eccentric spatial relationship between the two. Efforts have been made to characterize the minimal ablation margin, but inadequate description of the methodology makes the reproducibility of these techniques questionable [6, 9, 10]. Most previous publications have evaluated the minimal margin after ablation of primary liver tumors, in particular HCC [6, 8–11, 15, 16]. A small, previous series demonstrated no advantage in 3D volumetric evaluation of the ablation defect compared with traditional CT for the detection of LTP after CLM RFA [12]. Another study showed that incomplete ablation defect margin on immediate post-RFA CT was associated with LTP after treatment of CLM [17]. A recent publication evaluated the feasibility of a new technique for registration of post- to preablation CT images for the assessment of the margin after RFA [18]. This method requires dedicated registration software and the technique was only applied to selected patients with HCC tumors treated with RFA.
The goals of this study are: 1) the explicit description of a method to measure the minimal margin after thermal ablation using anatomical landmarks on pre- and postablation CT; and 2) the correlation of the minimal ablation margin size to local tumor progression (LTP) after RFA of CLM.
Materials and Methods
Subjects
Institutional review board waiver was obtained for retrospective review. All patients signed informed consent for ablation before treatment. A retrospective review of our prospectively created HIPAA registered RFA database identified 171 consecutive colorectal hepatic metastases (CLM) in 116 patients treated by percutaneous RFA between March 2003 and May 2010. Ninety-four tumors with largest diameter ranging between 0.5 to 4 (median, 1.75) cm in 73 patients (39 males, 34 females; mean age, 60.86 ± 13) were deemed eligible for enrollment in this study using the following inclusion criteria: 1) well-defined CLM with no previous locoregional treatment by ablation, embolization, or resection; and 2) CT evidence of effective ablation: ablation defect completely covering the target CLM on 4–8 weeks after RFA contrast enhanced CT [19].
Seventy-seven of 171 tumors were excluded from this study, including 17 previously ablated lesions, 8 complex, fused or irregular lesions, 1 lesion involving the abdominal wall, 3 lesions with incomplete ablation, and 48 lesions with pre- or postablation follow-up imaging that did not meet the inclusion criteria (baseline or first followup imaging only by MRI or PET or first follow-up CT beyond the 8-week window).
In our department, CT-guided RF ablation for CLM is generally offered to patients with up to three metastatic liver tumors (≤5 cm in diameter) with limited or no extrahepatic disease. The decision to perform ablation rather than resection or radiotherapy is made by a multi-disciplinary colon cancer or hepatobiliary disease management team on an individual basis to provide the best possible treatment for each patient.
Treatments
All tumors were treated by percutaneous CT-guided RFA under general anesthesia. A prophylactic antibiotic (1 g Ancef, GlaxoSmithKline, Research Triangle Park, NC) was administered intravenously immediately before the procedure. RFA was performed with the Radiotherapeutics LeVeen (n = 33), the RITA (n = 26), or the Covidien/Valley Lab/Radionics (n = 35) device. RF device choice was based on tumor size, shape, and location as well as the operator’s preference. The goal of each treatment was to create a zone of ablation at least 1 cm larger than the tumor’s largest diameter to achieve a minimum ablation margin of at least 5 mm uniformly all around the tumor. We applied and completed the manufacturer’s recommended protocol in all cases. Overlapping ablations were performed whenever needed to provide the desired minimal margin as previously described [5, 8].
Imaging Protocol
Portal venous phase contrast-enhanced CT (with 5-mm axial slices) was used for treatment planning and was available within 6 weeks from the RFA. In general, at our institution we do not offer ablation for lesions situated less than 5 mm to a vessel measuring more than 7 mm in diameter. Index tumors were characterized according to the size, subcapsular location (≤5 mm from the liver capsule), and proximity (within 10 mm) to any measurable vessel (3–6 mm).
The first post-RFA CT was performed within 4–8 weeks and was used according to SIR guidelines for the evaluation of treatment efficacy [19]. An ablation defect completely covering the target CLM was considered a complete and technically effective RFA. Irregular peripheral or nodular enhancement within 1 cm of the ablated area on this postprocedure CT was considered untreated (residual) tumor and a technical failure [19–21].
Subsequent radiologic follow-up continued at 2–4-month intervals for at least 1 year and was used to evaluate LTP. Evidence of irregular or nodular enhancement within 1 cm of the previously treated tumor was considered LTP [19, 20].
Description of semiquantitative evaluation of the minimal margin based on CT anatomic landmarks
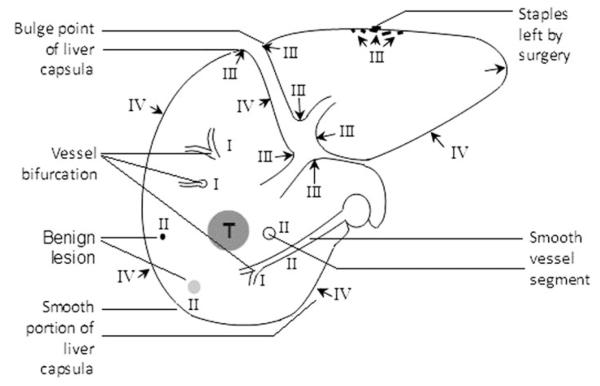
Using a PACS workstation with split monitor capabilities, the pre- and postablation portal venous phase CT images were reviewed side by side to compare the index tumor and the ablation zone/defect. Anatomic landmarks present on both pre- and postablation CT images were classified into four categories according to their reliability and reproducibility (Table 1; Fig. 1).
Table 1.
Landmarks choice according to level of reproducibility/reliability
| Category level |
landmarks |
|---|---|
| I | Point of intrahepatic vessel bifurcations |
| II | Intrahepatic smooth vessel segment; small benign lesions (calcification, cyst, etc.) |
| III | Indentation or bulge points of liver capsula or ligament; surgical staples |
| IV | Smooth portion of liver capsula or ligament |
Fig. 1.
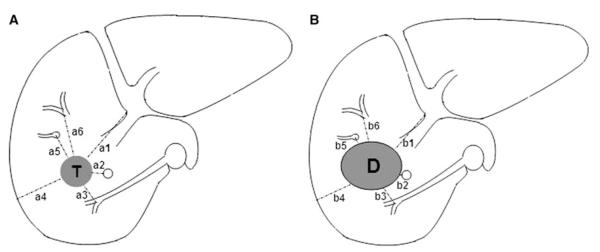
Minimal margin evaluation method. Minimal margin (M) is the minimum of n margin values (m): m1 = a1–b1, a2–b2, a3–b3, etc. In this demonstrated case, “a3–b3” represents the minimal margin
The landmark choice in each case was based on the following criteria: 1) higher level reliability landmark was preferred over lower level landmark; and 2) the nearest landmark to the tumor contour was preferred over a distant landmark.
On the basis of selected landmarks at the same CT axial image level, the distance values “an” between the selected “n” landmarks and the periphery of the ablation defect in at least four directions (medial, lateral, anterior, and posterior) were measured. Similarly, the distance values “bn” between the same “n” landmark and the periphery of the tumor in the same directions were recorded. Subtracting the corresponding value of an from bn yields mn, the margin measurements for the specific landmark. The minimal margin (M) for each ablated CLM is the minimum value of mn set or margins (Fig. 2). To determine the margin at the craniocaudal aspects of the tumor, the CT slice position with identified defect above and below the CLM were counted.
Fig. 2.
Landmark classifications according to reliability
The clock position of the minimal margins locations was recorded [8]. Given the limited spatial resolution of CT image and the potential error in subcentimeter calculations, the minimal margin measurements were stratified into four groups: 0; 1–5; 6–10; 11–15 mm. In cases in which one or more areas of the margin were <5 mm, we categorized the margin(s) as “close.” We subsequently categorized the close margin into multiple or single as defined in Table 2. The site concordance between the minimal margin location and the site of LTP was recorded as defined in Table 2.
Table 2.
Definitions related to minimal margin
| Margin terms | Definitions |
|---|---|
| Margin size | 0, 1–5, 6–10, 11–15 mm |
| Single close margin (<5 mm) | Margin extending less than four consecutive hour points |
| Multiple close margin (<5 mm) | At least two noncontinuous parts of the margin or one consecutive margin extending for more than four consecutive hour points |
| The site concordance | The concordance of at least one hour point on the “round the clock” coordination system between the minimal margin and the LTP site |
The minimal margins were evaluated individually by two experienced radiologists who were blinded to the oncologic outcomes.
Statistical Analysis
The LTP-free survival (LPFS) probabilities were estimated by using Kaplan-Meier survival methodology and compared by using the log-rank test. Factors considered included the size of the minimal margin, the tumor size and location, the patient’s age and sex, and the time interval between the primary tumor resection to the development of liver metastases (DFI1), the time interval from initial diagnosis of liver metastasis to ablation (DFI2), previous chemotherapy for liver metastasis, previous hepatectomy, and multiplicity of the minimal margin. Multivariate analysis was conducted using Cox’s regression, including those risk factors that were found to be significant on univariate analysis. Chi-square test was used to compare the site concordance rate of LTP between different margin sizes. Two-sided p < 0.05 was considered significant.
Results
Local Tumor Progression
The median follow-up period was 20 (range, 2–87) months. Overall 45 of 94 (47.9 %) tumors progressed. Median hepatic local tumor progression free survival (LPFS) was 16 months (95 % confidence interval (CI), 6.6–25.41 months; Fig. 3). The 1-, 2-, and 3-year cumulative LPFS rates were 59, 46, and 38 %, respectively.
Fig. 3.
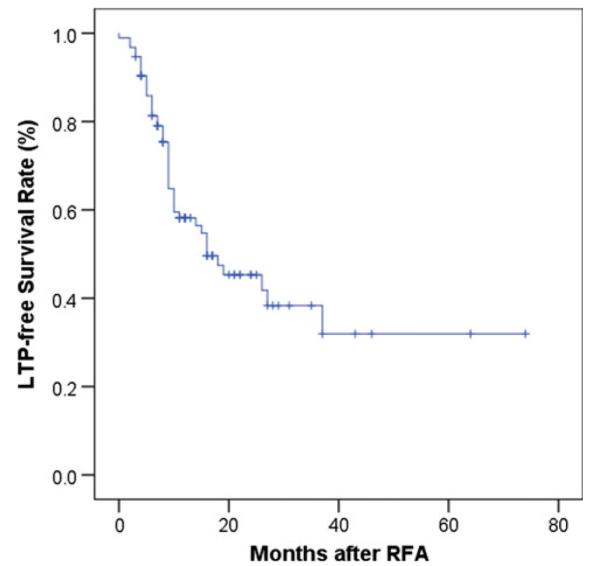
Total local tumor progression-free survival after ablation
Minimal Margin and LTP
Of the 94 ablated CLM, the minimal margin as evaluated at the 4–8 weeks after RFA CT was 0 in 30 (31.9 %), 1–5 mm in 41 (43.6 %), 6–10 mm in 15 (16 %), and 11–15 mm in 8 (8.5 %) tumors, respectively. Two-year recurrence rate for tumors with 0, 1–5, 6–10, or 11–15 mm minimal margin were 74, 54, 26, and 20 %, respectively (p = 0.011; Figs. 4,5, 6). The corresponding site concordance rates for LTP were 21/21 (100 %), 16/19 (84.2 %), 1/4 (25 %), and 0/1 (0 %) for 0, 1–5, 6–10, and 11–15 minimal margin category (p < 0.001), respectively (Table 3) Fig. 5.
Fig. 4.
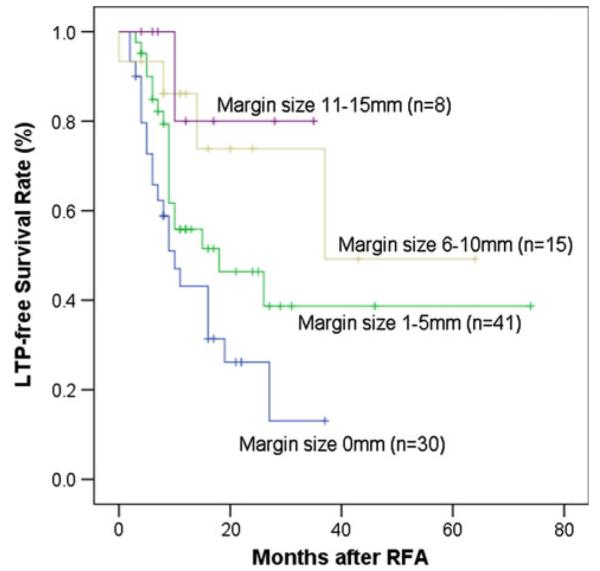
LTP-free survival curves for different minimal margin
Fig. 5.
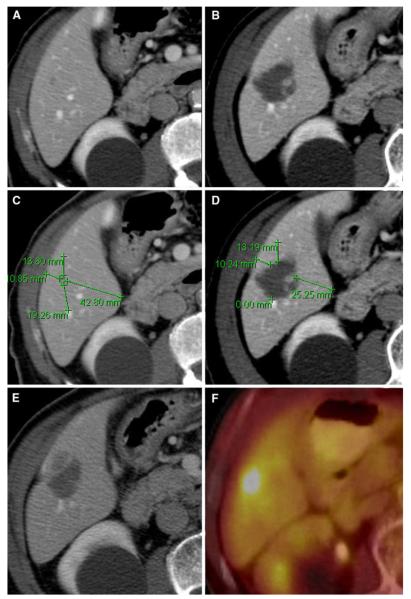
LTP of CLM after ablation from the site of the minimal margin (82-year-old male). A Preablation CT scan shows a small tumor located in the right hepatic lobe.
B Postablation CT shows the expected changes within the ablation zone. C Measuring the distance between the tumor edge and nearest reliable landmark in different directions on pre-CT. D Measuring the distance between the above same landmarks and ablation defect on post-CT; minimal margin was acquired to be 1–5 mm located at approximately 11 o’clock. E, F Five months later, CT and PET all showed local tumor progression at the site of this minimal margin
Fig. 6.
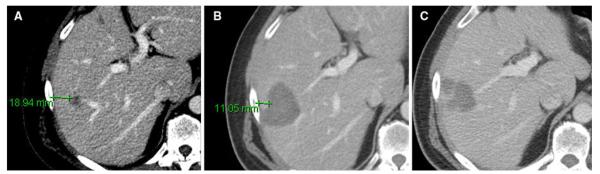
Concordant LTP of CLM after ablation at the site of minimal margin (55-year-old male). A Preablation CT scan showed a tumor located in right lobe. B Postablation CT showed the expected changes within the ablation zone; minimal margin was calculated as 6–10 mm located at approximately 9 o’clock. C Eight months later, concordant LTP occurred at the site of minimal margin
Table 3.
Local tumor recurrence and site concordance for different margin size
| Margin size (mm) |
No. | No. with LTP |
2-yr LTP rate (%) |
Site concordance (%) |
|---|---|---|---|---|
| 0 | 30 | 21 | 74 | 21/21 (100) |
| 1–5 | 41 | 19 | 54 | 16/19 (84.2) |
| 6–10 | 15 | 4 | 26 | 1/4 (25) |
| 11–15 | 8 | 1 | 20 | 0/1 (0) |
Seventy-one of 94 (75.5 %) CLM had a close margin (<5 mm). Of those, 35 of 71 (49.3 %) tumors had a single close margin and 36 of 71 (50.7 %) tumors had multiple close margins (Fig. 7). The presence of multiple close margins was associated with a higher risk for LTP compared with a single close margin, with median LPFS of 9 (95 % CI, 7.27–10.73) versus 19 months (95 % CI, 13.52–24.48), respectively (p = 0.03; Fig. 8).
Fig. 7.
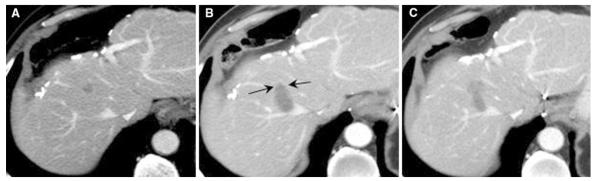
LTP associated with multiple margin after RFA (69-year-old man). A, B Multiple “0” margin (arrow) based on 1-month follow-up CT was demonstrated. C Seven-month follow-up CT showed concordant LTP at the site of the minimal margin
Fig. 8.
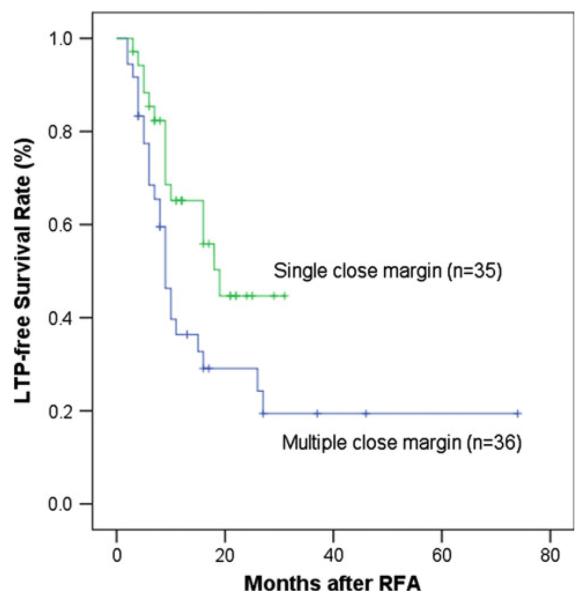
LTP-free survival curves for single and multiple close margin (<5 mm)
Univariate and multivariate analysis of factors associated with local tumor progression
In the univariate analysis, minimal margin (p = 0.011), tumor size (p = 0.023), and multiple close margins (p = 0.03) were significant risk factors associated with LTP. Age, sex, subcapsular tumor location, proximity to measurable vessel (3–6 mm), DFI1, DFI2, previous chemotherapy, and previous hepatectomy were not statistically significant predictors of LTP (Table 4).
Table 4.
Univariate analysis of local tumor recurrence risk factors
| Variable | Total | No. LTP | p value |
|---|---|---|---|
| Gender | 0.722 | ||
| Male | 51 | 21 | |
| Female | 43 | 24 | |
| Age (yr) | 0.414 | ||
| <60 | 53 | 27 | |
| >60 | 41 | 18 | |
| DFI1 | 0.904 | ||
| <12 M | 80 | 38 | |
| >12 M | 14 | 7 | |
| DFI2 | 0.916 | ||
| <12 M | 19 | 9 | |
| >12 M | 75 | 36 | |
| Tumor size (cm) | 0.023 | ||
| <2.5 | 70 | 29 | |
| ≥2.5 | 23 | 16 | |
| Subcapsular | 0.454 | ||
| No | 50 | 26 | |
| Yes | 44 | 19 | |
| Adjacent to vessel | 0.432 | ||
| No | 63 | 30 | |
| Yes | 31 | 15 | |
| Prior chemo for liver mets | 0.712 | ||
| No | 7 | 4 | |
| Yes | 87 | 41 | |
| Prior hepatectomy | 0.948 | ||
| No | 28 | 14 | |
| Yes | 66 | 31 | |
| Minimal margin (mm) | 0.011 | ||
| 0 | 30 | 21 | |
| 0–5 | 41 | 19 | |
| 5–10 | 15 | 4 | |
| 10–15 | 8 | 1 | |
| Single vs. Multiple close margin | 0.03 | ||
| Single | 35 | 15 | |
| Multiple | 36 | 25 |
Significant factors from the univariate analysis (tumor size, margin size, and multiplicity) were used in multivariate analysis. Multiple margins was not significant (p = 0.18) and was removed, resulting in the final model presented in Table 5.
Table 5.
Multivariate Cox regression analysis of factors related to LTP*
| Variable | Hazard ratio | 95 % Confidence interval | p value |
|---|---|---|---|
| Size (for each 5-mm increase) | 1.22 | 1.02–1.49 | 0.028 |
| Margin (for each 5-mm increase) | 0.54 | 0.36–0.79 | 0.002 |
Tumor size, margin size, and multiplicity of close margin were included in the initial multivariate analysis; multiple close margin lost its significance (p = 0.18) and therefore was removed, resulting in this “final” model
The risk of LTP decreased by 46 % for each 5-mm increase in margin size. Each additional 5 mm in tumor size increased the risk of local recurrence by 22 %.
Discussion
Factors associated with relatively lower recurrence rates after surgical resection of malignant liver tumors include absence of extrahepatic disease, small number of resected tumors, and the ability to achieve a tumor-free resection margin [22, 23]. RFA has been used increasingly as an alternative locoregional treatment for primary and secondary hepatic malignancies and has been frequently used in patients who are poor candidates for surgery or deemed unresectable [1–4, 24]. Local tumor control of ablation for CLM varies significantly between published series, with local tumor recurrence rates of 2–60 % [4, 25–34].
Several studies have analyzed risk factors and patterns of LTP in an effort to improve local disease control and oncologic outcomes after RFA [1, 5–7]. Like the surgical margin [35–37], the ablation margin is one of the factors associated with local tumor control [6, 8–12] after ablation. The minimal surgical margin represents the thickness of normal tissue interposed between the tumor edge and the margin of the resected tissue. Unlike the resected specimen, the evaluation of the ablation zone and corresponding margin is very challenging when it depends solely on imaging. To overcome the limitations of standard CT imaging, Kim et al. used fused CT imaging to quantitate the ablation margin size after RFA of HCC [8]. Although this technique allows for the direct assessment of margin size, it has several limitations: the minimal margin is calculated from measurements of the distance of the ablation defect periphery from the edge of the tumor. The method presumes that the ablation zone represents coagulation necrosis. Accurate fusion based on tumor center is questionable, and up to 18 % of cases could not be analyzed due to architectural distortion and displacement, patient position, and respiratory phase difference. Moreover, the fusion workstation is not widely available and thus cannot be proposed as a standard technique for margin evaluation after ablation. We described a method for the evaluation of the ablation margin, based on anatomic landmarks on contrast-enhanced CT imaging. As such, this methodology can be performed in any center that has CT and PACS workstations.
The surgical margin after resection of CLM has been associated with LTP and overall survival [35–37]. It was shown that all 10-year survivors after resection of CLM were those patients where a surgical margin of at least 10 mm was achieved [38]. Our study has similar results. Multivariate analysis showed that the minimal margin (p = 0.002) was an independent risk factor for LTP. A 46 % decrease in risk of LTP was achieved for each 5-mm increase in margin. This finding is consistent with the theory that LTP is attributed to the growth of microscopic viable tumor at the site of ablation [5, 8]. This is supported by previous work, which showed that tumors cells may be detected on the RF electrode by pathologic examinations and that detection of proliferation or viability in these tumor cells was a strong independent predictor of LTP after hepatic malignant tumor ablation [5, 39–41]. As a matter of fact in a recent publication [42], Ki67 + tumor cells adherent on the electrode were a strong independent predictor of patient survival after liver tumor ablation. In addition, the site concordance rate decreased as the margin size increased (p < 0.001) in the current study, which supports the hypothesis that when the ablation margin is adequate, progression is probably the result of de novo growth of another lesion rather than proliferation of residual viable cancer cells that remained in the ablation zone.
As previously shown, we found that tumor size was associated with LTP [1, 4, 6, 7, 10, 11]. The influence of a nearby vessel on the ablation margin and the effectiveness of RFA due to the “heat-sink effect” has been described [1, 6, 7, 10, 19, 43]. This study did not detect any significant difference of LTP for tumor adjacent to a 3–6-mm vessel. This disparity may be in part attributed to the overall small tumor size in this cohort and our exclusion of tumors incompletely treated with residual disease at the first post ablation CT.
An irregular ablation defect eccentrically located around the tumor resulting in a small minimal margin was identified as a risk factor in univariate analysis, highlighting the importance of accurate electrode positioning and necessity of overlapping ablations to create an adequate margin all around the tumor.
This study showed that a close margin (<5 mm) can result from a relatively small ablation zone or the eccentric location of the ablation zone in relation to the tumor center. Description of the ablation zone using maximum/effective diameter, defect/tumor diameter ratio volume, defect/tumor volume ratio, or ablation margin calculated as the difference between the maximum defect and tumor diameter divided by 2 does not take into account the nonellipsoid tumor geometry or eccentric ablation zone [11, 12, 44]. This study demonstrated that the creation of a uniform margin >5 mm all around the tumor in every direction is essential to achieve local tumor control. Another point of interest in our work is the fact that despite technical efforts to achieve a margin >5 mm homogeneously around the tumor, this was frequently not evident on the first postablation CT, 4–8 weeks later. Although it is conceivable that the margin immediately after ablation was larger than the one we calculated 4–8 weeks later, it is clear that an accurate estimation of the margin on CT landmarks is time-consuming and may not be possible to accurately estimate visually during the ablation procedure. To that effect, the development and application of image registration and fusion software that can superimpose the ablation zone on the target tumor and calculate the margin immediately after ablation is necessary and may dramatically improve outcomes of tumor ablation [45]. We also recommend that ablation margins evaluated on the day of ablation exceed the 5-mm threshold and be at least 10 mm all around the target tumor, whenever this is safe.
Our methodology for the assessment of minimal margins relies on the choice of anatomic landmarks that can be used as fiducials in the pre- and postablation imaging. Based on our experience, the bifurcation of intrahepatic vessel was the most reliable landmark, because the vessel bifurcation is a 3D fixed point in the liver. In contrast, a smooth vessel segment is a 2D fixed segment and more points on the same vessel can be chosen potentially as landmarks, increasing variability between observers and diminishing reproducibility. Surgical clips serve as a less reliable landmark than other radiodense or radiolucent lesions in the liver (such as tiny calcifications and cysts). This is due to possible shift of staples or clips over time as a result of scarring and healing after resection. The smooth portion of the liver capsule or the ligament are the least reliable landmarks, because they are extremely variable and shift with respiration and positioning.
There are several limitations of this study. Our study used a CT examination 4–8 weeks after the ablation, because these were available for all patients enrolled in the study and because published series and reporting standards guidelines recommend this first postprocedure imaging as a universally accepted time point for the evaluation of ablation effectiveness [19]. This evaluation however has its limitations, because the margin calculated at 4–8 weeks postablation does not represent the margin on the day of the ablation, which is probably larger. Our methodology has limitations similar to other techniques, such as the changes in measurements that can be attributed to different patient positions and variations on the respiratory phases during pre- and postablation imaging. Another limitation of our methodology is the semiquantitative evaluation of the margin due to the relatively limited z-axis CT spatial resolution, the tissue structural changes after ablation and the inconspicuous nature of tumor and/or ablation defect periphery in certain cases. Also, all of our work is based on the evaluation of the ablation margin by CT without any pathological assessment for confirmation. Another limitation is that this cohort included only tumors that were considered completely treated by CT at 4–8 weeks postablation. As such, it is possible that lesions not completely ablated, with residual tumor at the site of ablation due to the heat-sink phenomenon, were excluded from this cohort. A final limitation is that ablation margin measurement is a time-consuming process, which requires a comparative and meticulous review of the images between prior and posts CT on several “same levels” and calculations in several directions based on the optimal and carefully chosen landmarks for each ablated tumor. This last limitation is however at the same time an advantage of the presented methodology, because it demonstrated how to evaluate the ablation margin only by landmarks identified in CT. This allows the application of this methodology anywhere in the world where CT is used. This is particularly important in less fortunate places in the world where dedicated fusion and registration software or even PACS are not available.
In conclusion, the minimal margin size estimated using anatomic landmarks on contrast-enhanced CT is an independent imaging surrogate biomarker of LPFS after RFA of CLM. A minimal margin >5 mm in all directions around the target CLM on the 4–8 weeks post-RFA CT is associated with improved local tumor control and prolonged LPFS. Accounting for the possible decrease of the ablation zone within the 4–8-week period since RFA and based on the surgical data, a margin of 10 mm all around the tumor is recommended whenever safe and feasible.
Acknowledgments
This work was supported in part by NIH Grant No. 5R21CA131763.
Footnotes
Conflict of interest Authors Xiaodong Wang, Constantinos T. Sofocleous, Joseph P. Erinjeri, Elena N. Petre, Mithat Gonen, Kinh G. Do, Karen T. Brown, Anne M. Covey, Lynn A. Brody, William Alago, Raymond H. Thornton, and Kemeny NE have no conflict of interest to declare. Stephen B. Solomon—consultant for Covidien (Mansfield, MA) and Johnson and Johnson (Piscataway, NJ) and has a research contract with GE Healthcare (Milwaukee, WI).
Abstract was presented to CIRSE Meeting 2011.
Contributor Information
Xiaodong Wang, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA; Department of Interventional Radiology, Peking University Cancer Hospital and Institute, Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education, Beijing 100142, China.
Constantinos T. Sofocleous, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Joseph P. Erinjeri, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Elena N. Petre, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Mithat Gonen, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Kinh G. Do, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY, USA
Karen T. Brown, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Anne M. Covey, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Lynn A. Brody, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
William Alago, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA.
Raymond H. Thornton, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
Nancy E. Kemeny, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY, USA
Stephen B. Solomon, Section of Interventional Radiology, Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., Room H118, New York, NY 10065, USA
References
- 1.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008;15(10):2757–2764. doi: 10.1245/s10434-008-0043-7. [DOI] [PubMed] [Google Scholar]
- 2.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25(6):445–460. doi: 10.1159/000184736. [DOI] [PubMed] [Google Scholar]
- 3.Leen E, Horgan PG. Radiofrequency ablation of colorectal liver metastases. Surg Oncol. 2007;16(1):47–51. doi: 10.1016/j.suronc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Ayav A, Germain A, Marchal F, et al. Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg. 2010;200(4):435–439. doi: 10.1016/j.amjsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Sofocleous C, Nascimento R, Petrovic L, et al. Histopathology of tissue extracted on the probe after radiofrequency ablation of liver malignancy can predict local progression: initial results. Radiology. 2008;249(1(Oct)):364–374. doi: 10.1148/radiol.2491071752. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59(3):432–441. doi: 10.1016/j.ejrad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg Oncol. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepato-cellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195(3):758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 9.Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43(1):71–78. doi: 10.1007/s00535-007-2123-z. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188(2):480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 11.Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol. 2010;20(4):877–885. doi: 10.1007/s00330-009-1610-4. [DOI] [PubMed] [Google Scholar]
- 12.Frich L, Hagen G, Brabrand K, et al. Local tumor progression after radiofrequency ablation of colorectal liver metastases: evaluation of ablative margin and three-dimensional volumetric analysis. J Vasc Interv Radiol. 2007;18(9):1134–1140. doi: 10.1016/j.jvir.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008;190(6):1544–1551. doi: 10.2214/AJR.07.2798. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka C, Horiguchi J, Ishifuro M, et al. A feasibility study: evaluation of radiofrequency ablation therapy to hepato-cellular carcinoma using image registration of preoperative and postoperative CT. Acad Radiol. 2006;13(8):986–994. doi: 10.1016/j.acra.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Schraml C, Clasen S, Schwenzer NF, et al. Diagnostic performance of contrast-enhanced computed tomography in the immediate assessment of radiofrequency ablation success in colorectal liver metastases. Abdom Imaging. 2008;33(6):643–651. doi: 10.1007/s00261-007-9351-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim KW, Lee JM, Klotz E, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011;196(5):W565–W572. doi: 10.2214/AJR.10.5122. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235(3):728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H, Loyer EM, DuBrow RA, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics. 2001;21(Spec No):S41–S54. doi: 10.1148/radiographics.21.suppl_1.g01oc08s41. [DOI] [PubMed] [Google Scholar]
- 21.Pua BB, Sofocleous CT. Imaging to optimize liver tumor ablation. Imaging Med. 2010;2(4):433–443. doi: 10.2217/IIM.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics 2003. CA Cancer J Clin. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Kulaylat MN, Gibbs JF. Thermoablation of colorectal liver metastasis. J Surg Oncol. 2010;101(8):699–705. doi: 10.1002/jso.21504. [DOI] [PubMed] [Google Scholar]
- 25.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132(4):605–611. doi: 10.1067/msy.2002.127545. discussion 611-612. [DOI] [PubMed] [Google Scholar]
- 26.Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10(9):1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761. doi: 10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90(10):1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 29.Machi J, Oishi AJ, Sumida K, et al. Long-term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first- and second-line management. Cancer J. 2006;12(4):318–326. doi: 10.1097/00130404-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Elias D, De Baere T, Smayra T, Ouellet JF, Roche A, Lasser P. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89(6):752–756. doi: 10.1046/j.1365-2168.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 31.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19(5):1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 32.Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197(6):728–736. doi: 10.1016/j.amjsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol (Stockholm, Sweden: 1987) 2007;48(3):253–258. doi: 10.1080/02841850601161539. [DOI] [PubMed] [Google Scholar]
- 34.Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy–local tumor control rate and survival data. Radiology. 2004;230(2):450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi M, Inoue Y, Komeda K, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248(4):626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 37.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 38.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246(2):295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sofocleous CT, Klein KM, Hubbi B, et al. Histopathologic evaluation of tissue extracted on the radiofrequency probe after ablation of liver tumors: preliminary findings. AJR Am J Roentgenol. 2004;183(1):209–213. doi: 10.2214/ajr.183.1.1830209. [DOI] [PubMed] [Google Scholar]
- 40.Snoeren N, Jansen MC, Rijken AM, et al. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg. 2009;26(1):56–62. doi: 10.1159/000194946. [DOI] [PubMed] [Google Scholar]
- 41.Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18(13):3702–3710. doi: 10.1245/s10434-011-1762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a Prognostic Biomarker of Survival after Radiofrequency Ablation of Liver Malignancies. 2012. (In Press) [DOI] [PMC free article] [PubMed]
- 43.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14(10):1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 44.Keil S, Bruners P, Schiffl K, et al. Radiofrequency ablation of liver metastases-software-assisted evaluation of the ablation zone in MDCT: tumor-free follow-up versus local recurrent disease. Cardiovasc Intervent Radiol. 2010;33(2):297–306. doi: 10.1007/s00270-009-9681-9. [DOI] [PubMed] [Google Scholar]
- 45.Wood BJ, Locklin JK, Viswanathan A, et al. Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future. J Vasc Interv Radiol. 2007;18(1 Pt 1):9–24. doi: 10.1016/j.jvir.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]