Abstract
The clinical reality of cell therapy for heart disease dates back to the 1990s, when autologous skeletal myoblasts were first transplanted into failing hearts during open-chest surgery. Since then, the focus has shifted to bone marrow-derived cells and, more recently, cells extracted from the heart itself. While progress has been nonlinear and often disheartening, the field has nevertheless made remarkable progress. Six major breakthroughs are notable: 1) The establishment of safety with intracoronary delivery; 2) The demonstration that therapeutic regeneration is possible; 3) The rise of allogeneic cell therapy; 4) The impact of increasing mechanistic insights; 5) Glimmers of clinical efficacy; and 6) The progression to phase 2&3 studies. Here I review these landmark developments individually in some detail. Collectively, I conclude that the field has reached a new phase of maturity where the prospect of clinical impact is increasingly imminent.
Introduction
Each year, ~1M Americans suffer myocardial infarction (MI)1. While acute mortality has declined in recent decades due to the universal adoption of reperfusion therapy2, up to 36% of MI survivors will develop heart failure (HF)3, and will consequently be at increased risk for premature death3. Whether due to MI or to another etiology, HF affects ~5M Americans1. Patients are unable to exercise normally (in the extreme, they become bed bound), and suffer from shortness of breath. Current therapy relies on drugs that block various maladaptive signaling pathways, such as beta-adrenergic blockers and angiotensin inhibitors. Additional benefit can sometimes be gained from pacemakers that attempt to normalize the pattern of cardiac contraction. While such drugs and devices can attenuate the progression of HF, no treatment modality currently available addresses the root cause, which is a loss of functional heart muscle4. Cell therapy for heart disease aims to regenerate viable myocardial tissue which has been lost to disease. The main targets to date have been MI and HF. In the case of MI, the goal is to avert the progression to HF; in already-established HF, cell therapy seeks to halt further deterioration or even to reverse the disease. Clinical trials have resulted in inconsistent partial restoration of cardiac structure and function5, giving cause for optimism but leaving much room for improvement.
In reflecting upon the field, I have identified six major developments which have the potential to shape future progress. Time will tell just how durable these developments may be, and whether they will ultimately be hailed as genuine breakthroughs, but here I list and discuss these one at a time. The perspective is personal, as will be evident from the fact that the work highlighted in 3 of the 6 bullets is my own. Nevertheless, I attempt to temper what may be seen as self-congratulatory enthusiasm with a number of caveats and concerns regarding the vast remaining gaps in our knowledge.
Breakthrough #1: Establishment of safety with intracoronary delivery
Skeletal myoblasts were the first cells to be applied to heart disease, on the logical premise that autologous satellite cells might develop into mature contractile units when implanted ectopically into the diseased heart6. The paradigm involved harvesting skeletal muscle biopsies from patients with HF who were to undergo elective cardiac surgery; myoblasts would be grown ex vivo, then reimplanted by direct intramyocardial injection at the time of surgery. Despite early enthusiasm, skeletal myoblasts eventually proved to be risky (ventricular arrhythmias were frequent), and without much functional benefit: the 300-patient phase 2 MAGIC trial was halted after an interim analysis of the first 97 randomized patients revealed no robust trend to efficacy7.
Since then, the focus has shifted to other cell types and to percutaneous catheter-based delivery methods. In 20018, the first acute MI patient was treated with bone marrow-derived mononuclear cells (BMMCs). The paradigm has been oft-repeated and, collectively, forms the basis for the most substantive clinical experience to date with cell therapy for heart disease. After conventional intervention to restore patency of the occluded coronary artery, patients undergo bone marrow aspiration for derivation of BMMCs. The cells are rather finicky: details of manufacturing importantly influence potency, likely contributing to heterogeneous results among trials9,10. Typically 1–14 days post-MI, BMMCs are re-introduced into the patient via the intracoronary route using a balloon catheter inflated at the site of the initial blockage.
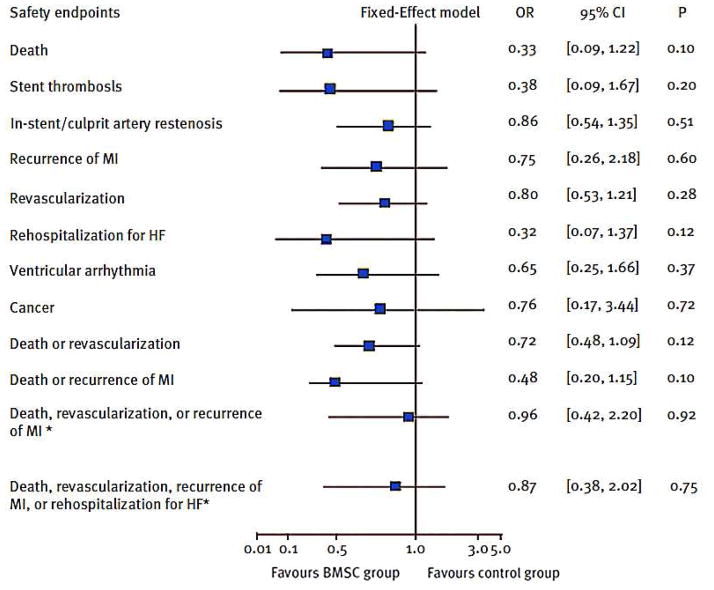
The salient finding has been the superior safety record of intracoronary BMMCs. Fig. 1 shows the results of a meta-analysis of 7 trials involving 660 patients11. Compared to baseline, BMMC transfer performed 4–7 days post-MI significantly decreased revascularization, cumulative clinical events of death or recurrent MI, culprit artery restenosis and ventricular arrhythmia. The lack of excess arrhythmias in BMMC-treated patients is particularly notable. While BMMCs are the only cell type for which large numbers of patients are available, the general pattern of safety with intracoronary delivery has held up so far with cardiac-derived cells as well12–14. One feature that BMMCs and cardiac-derived cells share is a predominantly indirect mechanism of action: long-term engraftment is not required for durable benefit15–17. The problem of arrhythmia is related to conduction block and to inhomogeneity of repolarization; these factors are likely to be much more severe with skeletal myoblasts (that do not integrate electrically in the myocardium) or with pluripotent-cell-derived products. Indeed, Cingolani and I18 have speculated that indirectly-acting cells will be less arrhythmogenic than those which engraft, differentiate and proliferate in vivo. The idea is that endogenous regeneration is likely to cause less electrical instability than transplantation of highly-proliferative cells; the latter may colonize the heart, producing barriers to conduction and/or aberrant repolarization.
Figure 1.
Meta-analysis of safety data from five major BMMC trials, as performed by Zhang et al11. Odds ratios of BMMC infusion therapy with respect to individual and cumulative clinical endpoints. Adapted from Clin Cardiol11 with permission. Abbreviations: CI = confidence interval; HF = heart failure; MI = myocardial infarction; OR = odds ratio; BMSC = bone marrow stem cell.
The finding that intracoronary delivery of non-engrafting cells is safe, particularly with regard to arrhythmia, represents a major breakthrough for the field of cell therapy.
Breakthrough #2: Demonstration of therapeutic regeneration
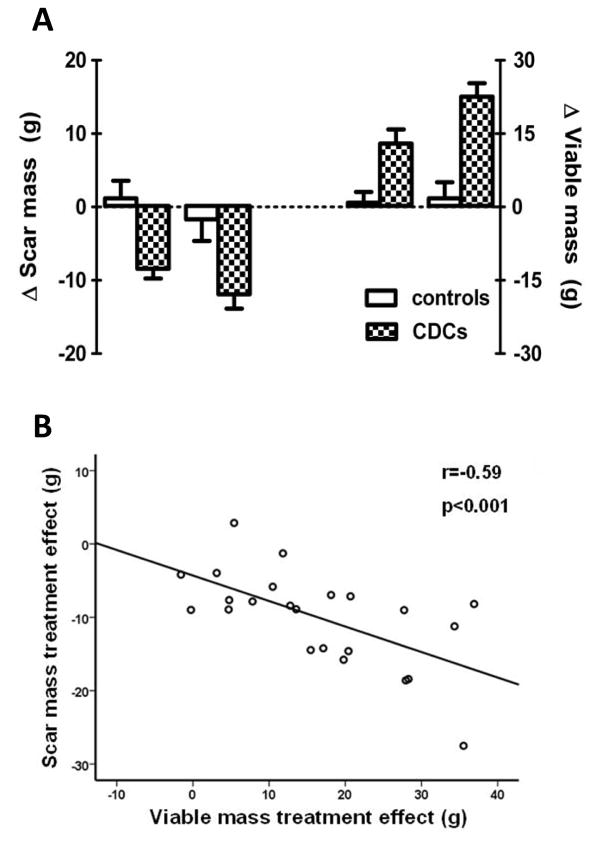
Regeneration is defined as “regrowth of lost or destroyed parts or organs.”19 While human BMMC studies have reported reductions in scar size (e.g.,20), the effect is solely on scar mass with no reciprocal increase in viable myocardium. Thus, such changes report a decrease in the extent of injury, but not regrowth of destroyed parts. Over the past 10 years, my lab has developed cardiosphere-derived cells (CDCs) as a candidate cell type for regenerative therapy post-MI21. These heart-derived cells are stem cells in that they exhibit multilineage potential and clonogenicity22, but they work primarily through indirect mechanisms15. At least six independent labs worldwide have reproduced the published methodology and verified CDCs’ identity and utility17,23–27. CDCs were first used clinically in the CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) trial13,14, conducted by the author and colleagues. CADUCEUS tested the safety and efficacy of intracoronary autologous CDCs in 17 patients with LV dysfunction and a recent MI (1.5–3 months prior), compared to 8 routine-care controls. In >12 months of follow-up, safety endpoints, including arrhythmia, were not significantly different in control and CDC-treated groups. Contrast-enhanced magnetic resonance imaging (MRI) revealed reductions of scar mass at 6 and 12 months in CDC-treated patients (but not in control subjects; left-hand panel, Fig 2 A). Scar reduction is notable, but tissue regeneration was manifested by an unprecedented increase in viable tissue (Fig. 2A, right-hand panel). The reductions in scar mass correlated with the increases in viable mass (Fig. 2B), consistent with (but not proving) the idea that scar is being converted to viable tissue as a consequence of treatment with CDCs. Histological analysis in animal models reveals that CDCs do not induce myocyte hypertrophy28,13; in contrast, cell size tends to be smaller, consistent with an increase in cell number. Moreover, porcine studies comparing contrast-enhanced MRI images with histological sections confirm that MRI accurately reports scarred and viable myocardium after CDC therapy28.
Figure 2.
Changes in scar mass and viable mass in CADUCEUS. A: Differences in scar mass from baseline to 6 or 12 months (left panel), and differences in viable LV mass from baseline to 6 or 12 months (right panel). B: Correlation between the changes in scar mass and the changes in viable mass in CDC-treated subjects at 6 and 12 months. Adapted from Lancet13 and JACC14 with permission.
Neither scar mass nor viable heart mass changed over time in the CADUCEUS control subjects13,14, in line with the traditional belief that chronic MI injury is irreversible: once formed, scar does not resolve on its own, and, once lost, living heart muscle does not spontaneously regrow. CADUCEUS was the first controlled clinical trial to demonstrate an increase in viable tissue as a result of cell therapy13,14. Limited MRI data from the SCIPIO trial of c-kit+ cells also showed increases in viable myocardium in cell-treated patients, but no control subjects were imaged29.
The finding that iatrogenic cardiac regeneration is indeed possible represents a major breakthrough for the field of cell therapy.
Breakthrough #3: The rise of allogeneic cell therapy
During the first decade of cell therapy for heart disease, the vast majority of clinical trials were conducted using autologous cells. This approach has the advantage that it avoids immunologic rejection, but autologous therapy requires patient-specific tissue harvesting, cell processing and quality control, imposing significant risk, expense and inflexibility with regard to the timing of treatment. In addition, cell efficacy may vary with donor age and comorbidities. The use of allogeneic cells, if safe and effective, would obviate such limitations, enabling the generation of highly-standardized “off the shelf” cell products. The obvious disadvantage is the risk of immune rejection, which may limit effectiveness whether or not it poses safety hazards. Nevertheless, since the vast majority of the observed functional benefit is attributable to indirect pathways even with heart-derived cells15–17, rejection of allogeneic cells may not be an issue if it occurs after the cells have exerted their beneficial paracrine effects and if the resulting benefits are durable.
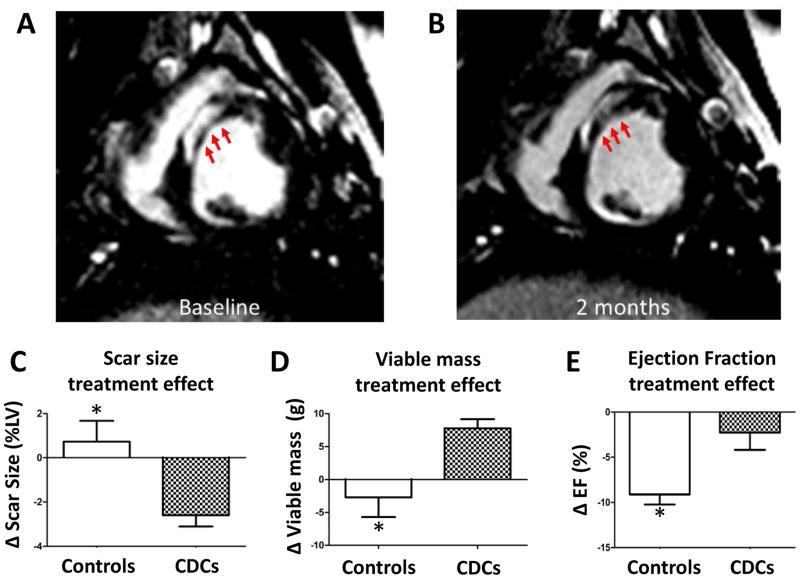
For some time, mesenchymal stem cells (MSCs) have been developed for potential therapeutic application. Allogeneic MSCs or their precursors have been used in various early-phase human trials of MI and HF, with no safety concerns reported to date30. The ALLSTAR trial of allogeneic CDCs post-MI, currently in progress (http://clinicaltrials.gov/show/NCT01458405), is based upon my lab’s discoveries that allogeneic CDC transplantation without immunosuppression is safe, promotes cardiac regeneration and improves heart function in rats17 and pigs28 with MI. Fig. 3 shows results from a porcine study of allogeneic CDCs post-MI28, with reduction of scar, increase of viable tissue, and preserved global function in cell-treated animals relative to vehicle-only controls. The indirect mechanism of action rationalizes the persistence of benefit despite the evanescent survival of transplanted cells. Once activated, endogenous regenerative pathways have their own momentum, not requiring the continued presence of CDCs.
Figure 3.
Allogeneic CDCs attenuate adverse remodeling and improve global function in a porcine preclinical post-MI model. Matched cine short-axis images at baseline (3 weeks post-MI, A) and 2 months after CDC treatment (B). C,D,E: Changes in scar mass, viable mass and ejection fraction respectively between placebo-treated controls and CDC-treated animals 2 months post therapy, versus baseline values in each animal.
The increasing recognition of the safety and efficacy of allogeneic cells, specifically MSCs and CDCs, represents a major breakthrough for the field of cell therapy.
Breakthrough #4: Increasing mechanistic insights
The guiding principle underlying the use of stem cells to achieve regeneration is the idea that injected cells will engraft, proliferate, and differentiate, thereby repopulating the injured heart. However, in many published studies, cell transplantation produces beneficial effects despite poor retention and minimal long-term survival of transplanted cells31. How can transient, paltry short-term cell survival suffice to produce lasting benefits? Multiple lines of evidence now indicate that most of the beneficial effects of transplanted CDCs are indirect (e.g., Fig. 4 shows that, after human CDCs are injected in SCID mice, most of the “new” heart and vascular cells are of mouse origin15); in the extreme, allogeneic CDCs are cleared completely within several weeks, but their functional and structural benefits persist at least 6 mos17. Thus, long-term transplanted cell survival is not required for sustained benefit. This appears to be true for many other nonpluripotent cells5,16.
Figure 4.
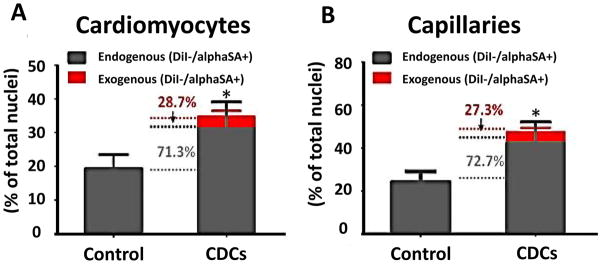
Indirect regeneration from CDCs. 3 weeks after MI, human CDC-treated mice had more cardiomyocytes (A) and capillaries (B) in the infarct area, as compared to controls (injected only with vehicle). More than 70% of the additional cardiomyocytes and capillaries are of endogenous (murine) origin (green).
CDCs are rich biological factories, secreting diffusible factors that promote angiogenesis, recruit endogenous progenitor cells, and coax surviving heart cells to proliferate15,32,33; on the other hand, injected CDCs suppress maladaptive LV remodeling32, apoptosis34,35, tissue fibrosis36, and inflammation after MI. While it is possible that CDCs secrete a complex medley of individual growth factors that collectively produce distinctive benefits, the involvement of master regulators such as microRNAs (miRs) would help tie together the various effects without postulating complex mixtures of many secreted factors. miRs are short noncoding (nc) RNAs that regulate gene expression by targeting families of transcripts for degradation, and thereby play pivotal roles in development, homeostasis and disease37. The roles of several miRs in the heart have been elucidated by recent studies; cardiovascular effects include modulation of susceptibility to oxidative stress, and induction of cardiomyocyte proliferation38,39. Moreover, miRs are known to confer long-lasting benefits and fundamental alterations of the injured microenvironment37.
How might miRs be transferred from CDCs to surrounding myocardium? Exosomes are lipid-bilayer vesicles secreted by a variety of cells that play important roles in paracrine and autocrine signaling40; they have, for example, been implicated as mediators of the angiogenic effects of CD34+ endothelial progenitor cells41. Exosomes are particularly rich in ncRNA including miRs42. Exosomes can cross biological membranes, and their lipid bilayer structure protects the cargo from degradation, enabling the natural delivery of miRs to targets. We are presently testing the hypothesis that CDC-exosomes mimic, and mediate, the beneficial effects of CDCs, and that these exosomes are replete with miRs43.
Regardless of the precise mediators that may turn out to be operative, there has been a major conceptual shift from canonical stem cell-based mechanisms to the notion that most clinically-applied cells work indirectly. The practical implications are multifarious: we have already alluded to the implications for arrhythmogenicity and for allogeneic therapy. The recognition of dominant indirect mechanisms also stimulates the search for next-generation therapeutic candidates that may be able to harness the benefits of cell therapy without the vagaries of cell harvesting, processing and delivery.
By rationalizing allogeneic therapy and opening up new prospects for cell-free products, our increasing mechanistic understanding represents a major breakthrough for the field of cell therapy.
Breakthrough #5: Glimmers of clinical efficacy
The BMMC experience has been notable for little evidence of benefit in surrogate endpoints, namely an inconsistency of improvements in ejection fraction and in scar size, and the absence of rigorous evidence of genuine myocardial regeneration. Nevertheless, it is intriguing that significant benefits on clinical endpoints have been reported. The REPAIR-AMI study demonstrated favorable clinical outcomes associated with cell-therapy, sustained at 5 years of follow up44, despite being underpowered to detect such differences. At 5 years, the composite endpoint of death, MI or revascularization exhibited an odds ratio of 0.62 in favor of the BMMC-treated group relative to placebo (p=0.03). In addition, trends in favor of BMMNC therapy with regard to hard clinical endpoints have also emerged from meta-analyses11,45.
In an attempt to reconcile equivocal functional benefits with extraordinary clinical outcomes, the following considerations are relevant: 1) Ejection fraction (which is load-dependent) may not be the best-suited surrogate marker for assessing the effects of cell therapy. In patients with MI, scar size measured by MRI is a better predictor of mortality compared to ejection fraction46. 2) Patients enrolled in the first generation of BMMC clinical trials had well-preserved ventricular function on average, leaving little room for improvement. The principal patient population has been a first-MI population, receiving prompt reperfusion and state-of-the-art medical therapy, so that the extent of injury is limited. Meanwhile, the greatest benefits of stem cell therapy occur in patients with the greatest MI-induced myocardial damage (e.g., in the REPAIR-AMI47, FINCELL48 and REGENT49 studies, the major determinant of functional recovery after cell therapy was low baseline ejection fraction). This finding has major implications for the design of future clinical studies: cell therapy may maximize its potential for successful myocardial repair and regeneration by targeting a sicker patient population. 3) The effects of BMMC therapy on surrogate endpoints, although seemingly modest, are comparable to what is achieved by established therapeutic strategies including primary PCI, thrombolysis, angiotensin converting enzyme inhibition, or β-blocker therapy, which are used routinely in clinical practice and confer a survival benefit50. However, this conclusion is largely based on meta-analyses and these should be taken with a grain of salt.
The increasing recognition that BMMCs have clinical benefits, despite little signal in terms of surrogate endpoints, represents a major breakthrough for the field of cell therapy. The results give reason to hope that emerging cell types, with greater effects on scar size or ejection fraction, will have superior clinical benefits.
Breakthrough #6: Progression to phase 2&3 studies
All too often in cell therapy, as in many other fields, promising early-phase trial results fail to be confirmed in larger studies51. Well-powered and rigorously designed (randomized, placebo-controlled, double-blind) large-scale clinical trials with long-term follow-up, focusing on hard clinical endpoints, are mandatory to determine whether the changes in surrogate endpoints (e.g., scar size, ventricular volumes and ejection fraction) are consistent, and if they translate into increased survival and reduced morbidity52. Fortunately, several such trials are in progress. Table 1 shows the major ongoing trials, their phase (2 or 3), corporate sponsors (BAMI, exceptionally, is paid by public funds from the European Union) and the cell type under study. Notable among these are the phase 2 ALLSTAR trial of allogeneic CDCs (http://clinicaltrials.gov/show/NCT01458405) and the phase 3 BAMI trial of BMMCs (http://clinicaltrials.gov/show/NCT01569178). The progress from small-scale observational studies to larger studies focusing on clinical endpoints reflects the increasing interest in specific therapeutic candidates by commercial entities. Without such commercial sponsorship, the potential of the field will never fully materialize, and the wide dissemination of reliable products will be impossible.
Table 1.
Selected ongoing phase 2 and 3 clinical trials targeted at heart disease. See www.clinicaltrials.gov for details of each study.
| Indication | Cell Type | Sponsor | Phase |
|---|---|---|---|
| HFa | Autologous Adipose Derived Cells | Cytori | 2 |
| HF | Autologous expanded BMb fractions | Aastrom | 2 |
| HF | Autologous BM cardiopoietic MSCsc | Cardio3Biosciences | 2 |
| HF | SDF-1 plasmid | Juventas | 2 |
| HF | Allogeneic MPCs d | Teva | 3 |
| Post-MIe | Autologous CD34 | Amorcyte | 2 |
| Post-MI | Allogeneic CDCsf | Capricor | 2 |
| Post-MI | Autologous BMMNCsg | EUh | 3 |
| Post-MI | Autologous BM CD133 | Asklepios proresearch | 2 |
Abbreviations:
HF = heart failure;
BM = bone marrow;
MSC = mesenchymal stem cell;
MPC = mesenchymal precursor cells;
MI = myocardial infarction;
CDCs = Cardiosphere derived cells;
BMMNC = bone marrow mononuclear cell;
EU = European Union
The progression of selected cell products into advanced-phase clinical trials represents a major advance for the field of cell therapy.
Concluding Remarks
Over the last several years, we have progressed from a profusion of hype to the point of having a solid basis for moving forward. With the good fortune of prevalent safety to date, we have managed to avoid the sort of debacle that derailed gene therapy for more than a decade53. The demonstration that therapeutic regeneration is possible, in a setting where conventional wisdom teaches that scar is irreversible, catapults the field onto a new plane yet to be achieved by any other treatment approach. The increasing evidence that allogeneic cells can be safe and effective takes cell preparation and manufacturing into the commercial mainstream, and away from the cottage industry paradigm where it has been stalled for so long. Our increasing insights into the mechanism of action of transplanted cells helps us to decide what is rational, and what is not, as we set priorities for future work. The glimmers of clinical efficacy in trials to date, coupled with the increasing number of advanced-phase clinical studies currently in progress, give new reasons for excitement. Other laudable developments not reviewed here include efforts to increase efficacy by conditioning the myocardial environment (e.g., CELLWAVE trial54) or enhancing cardiogenesis of non-resident stem cells (C-CURE trial55); such efforts can only enhance progress. In summary, the field has reached an unprecedented phase of maturity in which the prospect of clinical impact is increasingly plausible, if not likely. Exciting times lie ahead.
Acknowledgments
Work in my laboratory is funded by the NIH (R01 HL083109), the Department of Defense (CSR205330/221349) and the California Institute for Regenerative Medicine (RB4-06215).
Abbreviations
- MI
myocardial infarction
- HF
heart failure
- BMMCs
bone marrow mononuclear cells
- CDCs
cardiosphere derived cells
- CADUCEUS
CArdiosphere-Derived a Utologous stem CElls to reverse ventricUlar dySfunction
- MSCs
mesenchymal stem cells
- MRI
magnetic resonance imaging
Footnotes
Financial Support and Disclosure:
Work in the author’s laboratory is supported by NIH, the Department of Defense, and the California Institute for Regenerative Medicine. Eduardo Marbán is founder of, unpaid advisor to, and owns equity in Capricor Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Nat Rev Cardiol. 2012;9(11):620–633. doi: 10.1038/nrcardio.2012.122. [DOI] [PubMed] [Google Scholar]
- 3.Jhund PS, McMurray JJV. Heart Failure After Acute Myocardial Infarction: A Lost Battle in the War on Heart Failure? Circulation. 2008;118(20):2019–2021. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 4.Koudstaal S, OFJ, Lorkeers SJ, et al. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med. 2013;2(6):434–443. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malliaras K, Kreke M, Marban E. The Stuttering Progress of Cell Therapy for Heart Disease. Clin Pharmacol Ther. 2011;90(4):532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 6.Menasche P, Hagege AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 7.Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 8.Strauer BE, Brehm M, Zeus T, et al. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch Med Wochenschr. 2001;126(34–35):932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- 9.Marban E, Malliaras K. Mixed results for bone marrow–derived cell therapy for ischemic heart disease. JAMA. 2012;308(22):2405–6. doi: 10.1001/jama.2012.64751. [DOI] [PubMed] [Google Scholar]
- 10.Seeger FH, Rasper T, Fischer A, et al. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circulation Research. 2012;111(7):854–62. doi: 10.1161/CIRCRESAHA.112.265678. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Sun A, Xu D, et al. Impact of timing on efficacy and safety of intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009;32(8):458–466. doi: 10.1002/clc.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence for therapeutic regeneration in the final 1-year results of the CADUCEUS trial. Journal of the American College of Cardiology. 2014;63(2):110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chimenti I, Smith RR, Li T-S, et al. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. Circulation Research. 2010;106(5):971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KU, Li QH, Guo Y, et al. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108(3):346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malliaras K, Li TS, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marban E, Cingolani E. Heart to heart: cardiospheres for myocardial regeneration. Heart Rhythm. 2012;9(10):1727–1731. doi: 10.1016/j.hrthm.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Harcourt HM. [Accessed 12/05/2013];The American Heritage Dictionary of the English Language. Available at: http://ahdictionary.com.
- 20.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 21.Smith RR, Barile L, Cho HC, et al. Regenerative Potential of Cardiosphere-Derived Cells Expanded From Percutaneous Endomyocardial Biopsy Specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 22.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4(9):7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghila Rani KG, Kartha CC. Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth factors (Chur, Switzerland) 2010;28(3):157–165. doi: 10.3109/08977190903512628. [DOI] [PubMed] [Google Scholar]
- 24.Gaetani R, Ledda M, Barile L, et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovascular Research. 2009;82(3):411–420. doi: 10.1093/cvr/cvp067. [DOI] [PubMed] [Google Scholar]
- 25.Mishra R, Vijayan K, Colletti EJ, et al. Characterization and Functionality of Cardiac Progenitor Cells in Congenital Heart Patients. Circulation. 2011;123(4):364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled Delivery of Basic Fibroblast Growth Factor Promotes Human Cardiosphere-Derived Cell Engraftment to Enhance Cardiac Repair for Chronic Myocardial Infarction. Journal of the American College of Cardiology. 2008;52(23):1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 27.Tang YL, Zhu W, Cheng M, et al. Hypoxic Preconditioning Enhances the Benefit of Cardiac Progenitor Cell Therapy for Treatment of Myocardial Infarction by Inducing CXCR4 Expression. Circulation Research. 2009;104(10):1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malliaras K, Smith RR, Kanazawa H, et al. Validation of Contrast-Enhanced MRI to Monitor Regenerative Efficacy after Cell Therapy in a Porcine Model of Convalescent Myocardial Infarction. Circulation. 2013;128(25):2764–75. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle AJ, McNiece IK, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- 31.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circulation Research. 2010;106(3):479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. Journal of the American College of Cardiology. 2011;57(4):455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 33.Stastna M, Chimenti I, Marban E, et al. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10(2):245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li TS, Cheng K, Lee ST, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28(11):2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng K, Malliaras K, Li TS, et al. Magnetic enhancement of cell retention, engraftment and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplantation. 2012;21(6):1121–35. doi: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseliou E, Pollan S, Malliaras K, et al. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. Journal of the American College of Cardiology. 2013;61(10):1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 37.Osman A. MicroRNAs in health and disease--basic science and clinical applications. Clin Lab. 2012;58:5:6, 393–402. [PubMed] [Google Scholar]
- 38.Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 39.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11(11):860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 41.Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation Research. 2011;109(7):724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iguchi H, Kosaka N, Ochiya T. Secretory microRNAs as a versatile communication tool. Commun Integr Biol. 2010;3(5):478–481. doi: 10.4161/cib.3.5.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim AG, Cheng K, Marban E. Abstract 19186: Role of exosomes and their microRNA constituents in mediating the therapeutic benefits of human cardiosphere-derived cells in vitro and in mice with myocardial infarction. Abstract presented at Circulation. 2013;128 [Google Scholar]
- 44.Leistner D, Asmuss B, Erbs S, et al. Abstract 13940: Intracoronary Infusion of Bone Marrow-Derived Mononuclear Cells in Acute Myocardial Infarction: 5 Year Clinical Outcome and MRI Data of the Randomized, Double-Blind, Placebo-Controlled REPAIR-AMI Trial. Circulation. 2011;124 [Google Scholar]
- 45.Zhang SN, Sun AJ, Ge JB, et al. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int J Cardiol. 2009;136(2):178–185. doi: 10.1016/j.ijcard.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 46.Roes SD, Kelle S, Kaandorp TA, et al. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100(6):930–936. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 48.Huikuri HV, Kervinen K, Niemela M, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 49.Tendera M, Wojakowski W, Ruzyllo W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 50.Reffelmann T, Konemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. Journal of the American College of Cardiology. 2009;53(4):305–308. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Lara PN, Redman MW. The hazards of randomized phase II trials. Ann Oncol. 2012;23(1):7–9. doi: 10.1093/annonc/mdr567. [DOI] [PubMed] [Google Scholar]
- 52.Malliaras K, Marban E. Moving beyond surrogate endpoints in cell therapy trials for heart disease. Stem Cells Transl Med. 2014;3(1):2–6. doi: 10.5966/sctm.2013-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson JM. Medicine. A history lesson for stem cells. Science. 2009;324(5928):727–728. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]
- 54.Assmus B, Walter DH, Seeger FH, et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309(15):1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 55.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology. 2013;61(23):2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]