Abstract
The treatment of tuberculosis by chemotherapy is complicated due to multiple drug prescriptions, long treatment duration, and adverse side effects. We report here for the first time an in vitro therapeutic effect of nanocomposites based on para-aminosalicylic acid with zinc layered hydroxide (PAS-ZLH) and zinc-aluminum layered double hydroxides (PAS-Zn/Al LDH), against mycobacteria, Gram-positive bacteria, and Gram-negative bacteria. The nanocomposites demonstrated good antimycobacterial activity and were found to be effective in killing Gram-positive and Gram-negative bacteria. A biocompatibility study revealed good biocompatibility of the PAS-ZLH nanocomposites against normal human MRC-5 lung cells. The para-aminosalicylic acid loading was quantified with high-performance liquid chromatography analysis. In summary, the present preliminary in vitro studies are highly encouraging for further in vivo studies of PAS-ZLH and PAS-Zn/Al LDH nanocomposites to treat tuberculosis.
Keywords: Zn/Al-layered double hydroxides, zinc layered hydroxides, tuberculosis, para-aminosalicylic acid (PAS), antimicrobial agents
Introduction
Tuberculosis (TB) has been lethal to humans for centuries and despite significant technological advances, it still claims millions of precious human lives. According to a recent global TB report by the World Health Organization, about 8.6 million new cases of TB were reported with about 1.3 million deaths in 2013.1
TB is a bacterial infectious disease caused by Mycobacterium tuberculosis, which generally targets the lungs (pulmonary TB) but can also infect other body organs like the liver, spleen, kidneys, tonsils, brains, intestine, etc, and is called extrapulmonary TB.2 The aforementioned classification is based on the target site of the infection. However, TB can also be classified according to the treatment prescription, namely: 1) drug susceptible TB (DSTB) (the most common form of TB, which can be cured by the four first-line anti-TB drugs: isoniazid, rifampicin, pyrazinamide, and ethambutol), 2) multidrug-resistant TB (MDR-TB) (the form of TB when bacteria become resistant to multiple anti-TB drugs, especially against isoniazid and rifampicin; MDR-TB is treated with second-line anti-TB drugs, namely para-aminosalicylic acid [PAS], cycloserine, aminoglycosides, fluoroquinolones, thioamides, and cyclopeptides),3 and 3) extensively resistant TB, where the bacteria become resistant to first-line anti-TB drugs, namely rifampicin and isoniazid, as well as against second-line anti-TB drugs, namely amikacin, kanamycin, capreomycin, and any of the fluoroquinolones.3 Clearly, one the most significant problems with treating TB today is that chemotherapy is complicated by the long treatment duration (ie, 6–24 months depending on the type of TB discussed above) and the adverse effects of anti-TB drugs.3,4
PAS was first used in clinical trials in 1948 and was found effective in eradicating TB bacteria.5 Initially, it was prescribed for the treatment of DSTB, but due to its adverse effects, such as nausea, vomiting, abdominal cramps, anorexia, and epigastria distress, etc, it is no longer used for DSTB. However, it is still used for the treatment of MDR-TB.6,7 Sustained-release formulations of PAS could possibly minimize its side effects.7 Different formulations of PAS have been tried by physicians to minimize its side effects, such as PAS-buffered tablets, in the form of granules, PAS transformed in its salt form, etc. The intrinsic half-life of PAS is between 45–60 minutes.8 Sustained-release formulations of PAS would not only reduce side effects, but also improve its intrinsic half-life.
Different drug delivery approaches have been attempted for anti-TB drugs such as polymers like poly-L-lactic acid and poly (D-L lactic acid). Composite formations with chitosan, montmorillonite and hydrogel formulations have also been applied, etc.9–12 There are some adverse effects related to these drug delivery systems like decreased cytocompatibility, insolubility, and accumulation in the body, etc.3 Saifullah et al wrote a comprehensive review on the different drug delivery systems applied for anti-TB drugs.3
In an effort to develop new drug delivery systems for treating TB, we have developed two-dimensional layered double hydroxides (LDHs) as a versatile material with numerous applications. LDHs are being applied as catalysts, magnetization devices, polymerization tools, flame retardant materials, and for the removal of toxic materials from the environment.13–16 LDHs have brucite-like structures but carry positive charges due to the addition of trivalent metal cations while the positive charge is balanced by a variety of anions.17 One variation of LDHs is metal layered hydroxides, where only a divalent metal is used.18
The general formula for LDHs is:
| (1) |
and the general formula for metal layered hydroxides is:
| (2) |
where MII is a divalent metal ion, MIII are trivalent cations, x represents the molar fraction, and An− is the counter anion.17,19
In recent years, LDHs have emerged as an ideal material for biomedical applications, especially for drug delivery purposes. The fabulous features of LDHs (such as biocompatibility; easy excretion from the body; the tendency to accommodate different kinds of organic and inorganic anions, and biomolecules like DNA and RNA; ease of preparation; and the tendency to release the intercalated molecule in a sustained manner) make them excellent drug delivery systems.15,17,20–24
We have successfully intercalated PAS into zinc/aluminum LDHs (Zn/Al LDH) by two methods; namely, coprecipitation (nanocomposite A) and ion exchange (nanocomposite B) as previously described.25 We have also intercalated PAS into zinc layered hydroxides (PAS-ZLH) using a ZnO suspension method (nanocomposite C).26
Here, we report for the first time, the antimycobacterial activities and antimicrobial activities of the nanocomposites A, B, and C against Gram-positive and Gram-negative bacteria. We also describe the low cytotoxic effect of nanocomposite C against normal human MRC-5 lung cells. We already reported concerning the high biocompatibility of nanocomposite A and nanocomposite B on normal human lung cells and 3T3 mouse fibroblast cells.25 In this manner, this study continues to support the study of such nanocomposites as a new effective method to treat TB.
Material and methods
Materials
Analytical grade chemicals were used without any further purification. The drug, PAS (99% pure), zinc nitrate hexahydrated, aluminum hydrate nonahydrated, and zinc oxide were bought from Sigma-Aldrich Co, (St Louis, MO, USA). The solvent, dimethyl sulfoxide, was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Preparation of PAS with Zn/Al LDH (PAS-Zn/Al LDH) and PAS-ZLH
PAS-Zn/Al LDH nanocomposites were prepared by two different methods, namely coprecipitation (nanocomposite A) and an ion exchange method (nanocomposite B) as described previously.25 PAS-ZLH nanocomposites were prepared by the direct addition of PAS into zinc oxide suspensions and the pH of the solution was raised to 7.9 by the addition of an aqueous (0.5 mol/L NaOH) solution as described previously.26
Nanocomposite characterization
X-ray diffraction (XRD) analysis was carried out on a Shimadzu XRD-6000 diffractometer (Shimadzu Corporation, Kyoto, Japan). XRD patterns were recorded in the range of 2θ=2–60° with cuKα radiation at 30 kV and 30 mA. The PAS loading was determined using a Sykam high-performance liquid chromatography (HPLC) system with an autoinjector Sykam 5300, Sykam S3250 ultraviolet visible (UV/Vis) detector, and a Sykam quaternary pump system 5300 (Sykam GmbH, Eresing, Germany), with a Zorbax Rx-Sil 4.6 × 150 mm column with 5 μm particle sizes (Agilent Technologies, Santa Clara, CA, USA). For the quantification of the metallic elements zinc and aluminum, an inductively coupled plasma optical emission spectrometer (Optima 2000 DV; PerkinElmer Inc., Waltham, MA, USA) was used. Details of the characterization instruments were provided in our previously reported research articles.25,26
HPLC analysis
The loading of PAS in the nanocomposites was determined using an HPLC method. The previously reported method was used with slight modification for PAS quantification.27 The mobile phase used in this work was a combination of methanol and 17.5 mM potassium phosphate buffer (equal molar concentrations of both monobasic and dibasic potassium salts at a pH of 3.5 adjusted by phosphoric acid) at a ratio of 85:15.27 The standard solutions of different PAS concentrations of 0 mg/L, 20 mg/L, 40 mg/L, 60 mg/L, and 80 mg/L were prepared in 50 mL solutions (45 mL mobile phase plus 5 mL HCl). Ten milligrams of each nanocomposite was dissolved in 50 mL of solution (5 mL HCl and 45 mL of the mobile phase). The samples, standards, and mobile phase were filtered with microfilters with a pore size of 0.20 μm. The flow rate was kept at 1 mL/minute, the oven temperature was kept at 30°C, and a wavelength at 235 nm was selected for PAS detection.
Antimycobacterial antimicrobial susceptibility test
The drug susceptibility testing (DST) of PAS and its nanocomposites was performed using the non-radiometric fluorescence-based method of the BACTEC™ MGIT™ 960 Mycobacterial Detection System (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) against M. tuberculosis (ATCC® 25618™; American Type Culture Collection, Manassas, VA, USA); the minimum inhibitory concentrations (MICs) of the nanocomposites were determined. The Mycobacteria Growth Indicator Tube (MGIT) with BD BACTEC™ MGIT™ 960 growth supplement for DST was used in the MGIT™ 960 instrument (Becton, Dickinson and Company) as described previously.28,29 The standard protocol for DST in MGIT™ 960 was strictly followed as recommended for primary drugs. Culture suspensions for inoculation were well dispersed with no large clumps to avoid false-resistant results. After thorough mixing and homogenization of the culture suspensions, the tubes were allowed to rest for at least 15 minutes, and the supernatant was used to inoculate the drug-containing media and the control according to the manufacturer’s instructions for DST of first-line drugs. All inoculated drug-containing MGIT™ 960 tubes were placed in the DST set carrier and entered into the MGIT™ 960 instrument and labelled as “unknown drugs” using the DST entry feature. For the DST set containing “unknown drugs,” the instrument flagged the DST set “complete” when the growth control reached a growth unit (GU) value of 400. At that point, the GU values of drug-containing tubes were retrieved from the instrument by printing out a DST set report, and the results were interpreted manually. If the GU of the drug-containing tube was more than 100 when the GU of the growth control was 400, the results were defined as resistant. If the GU values of the drug-containing tubes were equal to or less than 100, the results were considered susceptible. Experiments were repeated at various concentrations of PAS nanocomposite suspensions until the MICs were determined.
Non-mycobacterium antimicrobial susceptibility test
The synthesized PAS nanocomposites were tested for their antimicrobial activity against different microorganisms, including Gram-positive (Staphylococcus aureus) bacteria, Gram-negative (Pseudomonas aeruginosa and Escherichia coli) bacteria, and Candida albicans using the plate colony counting method.30 The microorganisms Staphylococcus aureus (ATCC 43300), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), and Candida albicans (ATCC 20408) were purchased from the American Type Culture Collection (ATCC), Manassas, VA, USA.
In vitro assay for cell viability
Cell culture
Human lung fibroblast cells, MRC-5 (ATCC® CCL-171™), were purchased from the American Type Culture Collection. The cells were cultured in Dulbecco’s modified Eagle medium (DMEM) and Roswell Park Memorial Institute (RPMI) 1640 media containing 10% fetal bovine serum (Hyclone; Thermo Fisher Scientific). Growth media contained 100 units/mL penicillin and 50 μg/mL streptomycin, respectively. The cells were maintained at 37°C in a humidified atmosphere in the presence of 5% CO2. To determine and compare the cytotoxicity of the synthesized nanocomposites, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assays were performed according to a previously optimized method.25,31 Briefly, human lung fibroblast MRC-5 cells were cultured in DMEM and RPMI 1640 medium containing 10% fetal bovine serum. Growth media contained 100 units/mL penicillin and 50 μg/mL streptomycin, respectively, and these cells were maintained at 37°C in a humidified atmosphere of 5% CO2. The cells were seeded into 96-well culture plates at 1 × 104 cells per well. The cells were incubated with the above cell culture medium (100 μL) containing dispersed nanocomposites at various concentrations from 0.781 μg/mL to 50 μg/mL for 24, 48, and 72 hours. Plates treated with the medium but without the dispersed nanocomposites were run in parallel and were used as controls. Following treatment, the amount of formazan crystals formed was measured after 4 hours of exposure to a MTT solution in phosphate-buffered saline and absorbance values were measured at 570 nm by an enzyme-linked immunosorbent assay plate reader. Cytotoxicity experiments were performed in triplicate, and cytotoxicity results were calculated according to a previously described method and the results are presented as mean ± standard deviation.32 All experiments were completed in triplicate and repeated at least three different times.
Statistical analysis
The unpaired t-test was used to compare between the MICs of PAS and nanocomposites A, B, and C against M. tuberculosis. Statistical analysis was used to compare the percentage inhibition of PAS and nanocomposites A, B, and C against different microorganisms using a two-way analysis of variance (ANOVA) test. The Prism V6.01 statistical software (GraphPad Software, Inc., La Jolla, CA, USA) was used for data management and statistical analysis. ANOVA followed by Student’s t-tests were used to determine the differences between means of cell viability (%). All data are shown as mean ± standard deviation unless indicated differently.
Results and discussion
Powder XRD
Figure 1 shows the XRD patterns for the nanocomposite PAS-Zn/Al LDH prepared by the coprecipitation method (nanocomposite A), PAS-Zn/Al LDH prepared by the ion exchange method (nanocomposite B), and PAS-ZLH from ZnO (nanocomposite C), respectively. The interlayer distance (basal spacing) of LDH having the nitrate ion as a counter anion was 8.9 Å as reported previously.17,19,33 The basal spacing increased to 15.9 Å, 16.8 Å, and 25.2 Å, from 8.9 Å, for nanocomposite A, B, and C, respectively, as shown in Figure 1. The increased basal spacing for the samples as observed from XRD patterns is the strongest evidence of the successful intercalation of the PAS in ZLH and Zn/Al-LDH. The second and third reflections were also present in all three spectra, which indicates a higher crystallinity of the samples. For a more detailed discussion of the characterization of the present materials and the molecular orientation of PAS between the interlayer galleries, please refer to our previously published manuscripts.25,26
Figure 1.
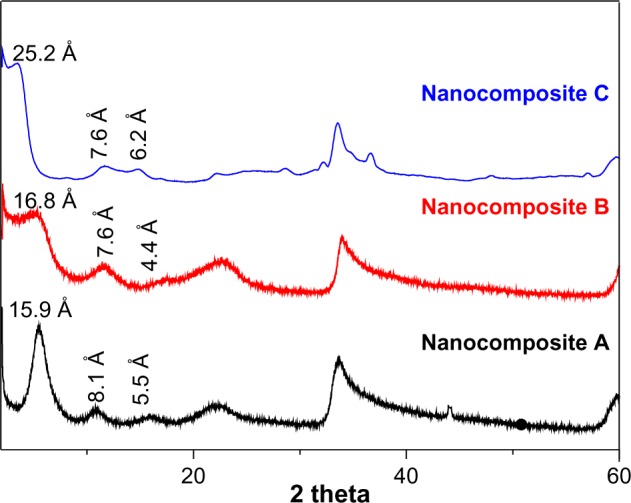
X-ray diffraction patterns of nanocomposite A, nanocomposite B, and nanocomposite C.
HPLC analysis
Figure 2 shows the calibration curve standard for the PAS solutions. The correlation coefficient, R2, for the standard solutions was 0.970. The percentage loading of PAS in the nanocomposites determined using HPLC is given in Table 1. In our previous report, we determined the PAS loading using an UV/Vis spectrophotometer for the nanocomposites A and B and the percent loading for the nanocomposites was determined by carbon, hydrogen, nitrogen and sulfur (CHNS) elemental analysis (N percentage).25,26 The percentage loading of PAS quantified by HPLC analysis was almost equivalent to the loading determined by UV/Vis spectrophotometry and by N percentage; the difference between PAS loading by the two techniques was found to be around 2%.
Figure 2.
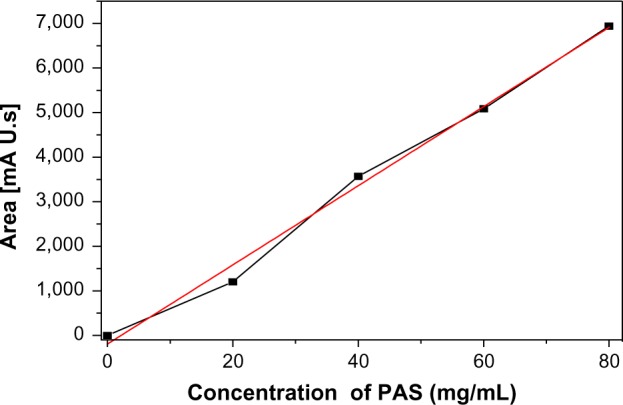
Calibration curve of PAS determined using HPLC with standard concentrations of 0 mg/mL, 20 mg/mL, 40 mg/mL, 60 mg/mL, and 80 mg/mL.
Notes: The red line shows linear fitting of calibration curve. The black line shows the experimental data.
Abbreviations: HPLC, high-performance liquid chromatography; PAS, para-aminosalicylic acid.
Table 1.
Percentage loading of PAS in nanocomposites A, B, and C as determined by HPLC, UV/Vis spectrophotometry, and nitrogen elemental analysis
| Number | Sample name | Percentage loading PAS by HPLC analysis | Percentage loading by UV/Vis or by N percentage |
|---|---|---|---|
| 1 | Nanocomposite A | 25.00 | 22.80 (UV/Vis) |
| 2 | Nanocomposite B | 16.00 | 16.60 (UV/Vis) |
| 3 | Nanocomposite C | 15.00 | 16.86 (Nitrogen) |
Notes: Nanocomposite A is PAS-Zn/Al LDH prepared by the coprecipitation method. Nanocomposite B is PAS-Zn/Al LDH prepared by the ion exchange method. Nanocomposite C is PAS-ZLH prepared using ZnO as the precursor.
Abbreviations: HPLC, high-performance liquid chromatography; PAS, paraaminosalicylic acid; PAS-ZLH, PAS with zinc layered hydroxide; PAS-Zn/Al LDH, PAS with Zn/Al layered double hydroxides; UV/Vis, ultraviolet visible.
Anti-TB and antimicrobial activity
The MICs (mean ± standard error of the mean) of the as-synthesized PAS nanocomposites A, B, and C against M. tuberculosis were found to be 7.9, 12.4, and 5.5 μg/mL, respectively, as compared to that of the free drug (PAS), which was 5.0 μg/mL (Figure 3). The percentage of drug loading for the nanocomposites A, B, and C was found to be 25%, 16%, and 15%, respectively. Furthermore, the back calculation of PAS loading revealed that the amount of PAS present in the nanocomposites was much lower than the total amount of nanocomposites shown in the MICs. The amount of PAS present in 7.9 μg/mL of nanocomposite A was actually 1.97 μg/mL. Similarly, the amount of PAS in 12.4 μg/mL of nanocomposite B was 1.98 μg/mL and the amount of PAS present in 5.5 μg/mL of nanocomposite C was 0.83 μg/mL.
Figure 3.
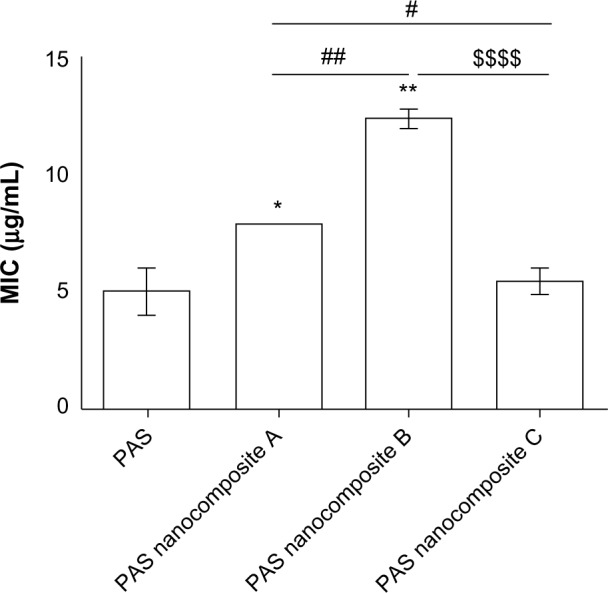
MICs (μg/mL) of PAS nanocomposites as compared to PAS alone against Mycobacterium tuberculosis as determined using the MGIT with BD BACTEC™ MGIT™ 960 growth supplement for DST and measured by the MGIT™ 960 instrument.
Notes: Results were analyzed using unpaired t-tests with Welch’s correction of PAS-nanocomposite. $$$$: Nanocomposite C versus B. ##: Nanocomposite B versus A. #: Nanocomposite C versus A. *P<0.05; nanocomposite A versus PAS. **P<0.05; nanocomposite B versus PAS.
Abbreviations: DST, drug susceptibility testing; MICs, minimum inhibitory concentrations; MGIT, Mycobacteria Growth Indicator Tube; PAS, para-aminosalicylic acid.
The amount of free drug (PAS) required for MIC was determined to be 5.0 μg/mL. On the other hand, the actual MIC amount of PAS present in nanocomposites A, B, and C was 1.97 μg/mL, 1.98 μg/mL, and 0.83 μg/mL, respectively. As a consequence, a very small amount of MIC intake was made possible because of the nanocomposite. Based on these results, it can be inferred that nanocomposites A and B enhanced the efficacy of PAS by 2.5 times and nanocomposite C improved the efficacy of PAS by five times compared to the free drug PAS. Improved efficacy of PAS in the nanocomposite formulation can be attributed to the nanoscaled size and sustained-release characteristics of the nanocomposites A, B, and C.
Based on these results, we can conclude that the nanocomposite formulations A, B, and C would decrease adverse side effects associated with PAS by reducing the amount of PAS required for efficacy.
Vesenbeckh et al adopted an analogically similar approach for PAS by encapsulating it in gelatin, and the side effects (like rash, acral cyanosis, and even shivering with fever) were mostly eliminated.34
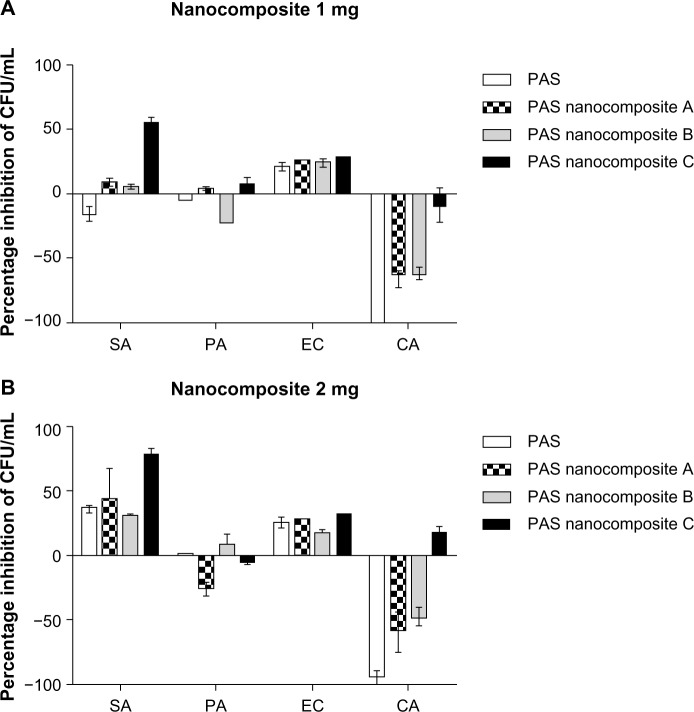
The unpaired t-test with Welch’s correction was used to compare between the MICs of PAS and nanocomposites A, B, and C against M. tuberculosis. The results of the antimicrobial testing showed that the nanocomposites had significant antibacterial activity against Gram-positive bacteria, Gram-negative bacteria, and C. albicans, as shown in Figure 4 from the percentage inhibition of each compound against the different organisms. It was found that the nanocomposites were more potent against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria than P. aeruginosa and C. albicans.
Figure 4.
Effect of PAS nanocomposites on the inhibition of microbial growth using the plate colony counting method at two concentrations: 1 mg (A) and 2 mg (B).
Notes: All experiments were completed in triplicate and repeated at least three different times. Results are presented as mean ± standard deviation.
Abbreviations: CA, Candida albicans; CFU, colony-forming units; EC, Escherichia coli; PA, Pseudomonas aeruginosa; SA, Staphylococcus aureus; PAS, para-aminosalicylic acid.
Cytotoxicity study of nanocomposite C (PAS-ZLH) against normal human lung cells
The most commonly used method for determining in vitro cytotoxicity is by the colorimetric MTT assay. The MTT assay protocol is based on the color change from yellow (due to tetrazolium salt MTT) to purple, due to the formation of formazan crystals.35 The color change takes place due to the reduction of the MTT compound to formazan and the reduction by the mitochondria of living cells; the concentration of formazan was determined with a spectrophotometer which is directly proportional with the viability of the living cells.35
The biocompatibility of nanocomposites A and B was previously reported against normal human lung cells MRC-5 and 3T3 mouse fibroblast cells, by using an MTT assay.25 These nanocomposites were found to be highly biocompatible with these two cell lines even at higher concentrations of 50 μg/mL for 72 hours and cell viability was found to be above 80%.25
The biocompatibility of nanocomposite C (PAS-ZLH) was also previously determined using the MTT assay against 3T3 mouse fibroblast cells.26 Cell viability was found to be about 70% at a concentration of 25 μg/mL for 72 hours. However, at a higher concentration of 50 μg/mL, nanocomposite C was found to be toxic.26 Here, we report for the first time the cytotoxicity of nanocomposite C against normal human lung cells, MRC-5 cells, with various concentrations from 0.7821 μg/mL to 50 μg/mL for 24, 48, and 72 hours, as shown in Figure 5. Nanocomposite C was found to be extremely cytocompatible with normal human lung cells even at the very highest concentration of 50 μg/mL after 72 hours of incubation with a cell viability of about 80%. Thus, we conclude that nanocomposites A, B, and C are biocompatible with normal human lung MRC-5 cells and 3T3 mouse fibroblast cells, and possess anti-TB and antimicrobial properties.
Figure 5.
Cell viability (MTT assay) of human lung fibroblasts, MRC-5, against various gradient concentrations of nanocomposite C after 24, 48, and 72 hours of exposure.
Abbreviation: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Conclusion
Nanocomposites of PAS with Zn/Al LDH and with ZLH were found to possess strong antimycobacterial and antimicrobial properties. The MIC of intercalated PAS in nanocomposites A, B, and C is much lower compared to PAS in its free form, which means better therapeutic efficacy. In addition, these formulations were found to be highly biocompatible, ie, about 80% cell viability against normal human lung cells (which are the cells that most commonly reside in the place of M. tuberculosis) and mouse fibroblast cells (a standard cell line used in cytotoxicity studies). With such a high biocompatibility and good antimycobacterial and antimicrobial properties (with a sustained release of drugs), the present nanocomposites should be further studied in vivo as novel anti-TB materials.
Acknowledgments
The authors would like to thank the Ministry of Education (MOE), Malaysia, Fundamental Research Grant Scheme, the Malaysian Higher Education Commission, and Northeastern University, Boston, Massachusetts, USA.
Funding for this research was provided by: the Higher Education Commission of Malaysia under the Commonwealth Scholarship and Fellowship Plan (Ref: KPT.B.600-6/3, Vol 68), for Bullo Saifullah; by the Fundamental Research Grant Scheme (FRGS) (FRGS/2/2013/SG06/UPM/01/1 with vote #5524467), for Mohd Zobir B Hussein; and by the Northeastern University in Boston, Massachusetts, USA, for funding the anti-TB experiments, for Thomas J Webster.
Footnotes
Disclosure
The authors report no conflicts of interest in work.
References
- 1.World Health Organization . Global Tuberculosis Report 2013. Geneva: World Health Organization; 2013. [Accessed May 26, 2014]. Available from: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1. [Google Scholar]
- 2.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120(4):316–353. [PubMed] [Google Scholar]
- 3.Saifullah B, Hussein MZ, Hussein Al Ali SH. Controlled-release approaches towards the chemotherapy of tuberculosis. Int J Nanomedicine. 2012;7:5451–5463. doi: 10.2147/IJN.S34996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21(6):587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- 5.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4(9):796–806. [PubMed] [Google Scholar]
- 6.Boman G. Serum concentration and half-life of rifampicin after simultaneous oral administration of aminosalicylic acid or isoniazid. Eur J Clin Pharmacol. 1974;7(3):217–225. doi: 10.1007/BF00560384. [DOI] [PubMed] [Google Scholar]
- 7.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348(2):119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 8.Peloquin CA, Berning SE, Huitt GA, Childs JM, Singleton MD, James GT. Once-daily and twice-daily dosing of p-aminosalicylic acid granules. Am J Respir Crit Care Med. 1999;159(3):932–934. doi: 10.1164/ajrccm.159.3.9807131. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima Y, Serigano T, Hino T, Yamamoto H, Takeuchi H. Surface-modified antiasthmatic dry powder aerosols inhaled intratracheally reduce the pharmacologically effective dose. Pharm Res. 1998;15(11):1753–1759. doi: 10.1023/a:1011968914726. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R, Saxena D, Dwivedi AK, Misra A. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm Res. 2001;18(10):1405–1410. doi: 10.1023/a:1012296604685. [DOI] [PubMed] [Google Scholar]
- 11.Hua S, Yang H, Wang W, Wang A. Controlled release of ofloxacin from chitosan–montmorillonite hydrogel. Appl Clay Sci. 2010;50(1):12–117. [Google Scholar]
- 12.Soto E, Kim YS, Lee J, Kornfeld H, Ostroff G. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers. 2010;2(4):681–689. [Google Scholar]
- 13.Wang Q, O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112(7):4124–4155. doi: 10.1021/cr200434v. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Li D, Wang J, Li S, Evans DG, Duan X. Synthesis, flame-retardant and smoke-suppressant properties of a borate-intercalated layered double hydroxide. Clays Clay Miner. 2005;53(3):294–300. [Google Scholar]
- 15.Newman SP, Jones W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J Chem. 1998;22(2):105–115. [Google Scholar]
- 16.Cavani F, Trifirò F, Vaccari A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal Today. 1991;11(2):173–301. [Google Scholar]
- 17.Duan XE, Evans DG, editors. Layered Double Hydroxides; Series: Structure and Bonding. Volume 119. Berlin: Springer; 2006. [Google Scholar]
- 18.Hussein Al Ali SH, Al-Qubaisi M, Hussein MZ, Ismail M, Bullo S. Hippuric acid nanocomposite enhances doxorubicin and oxaliplatin-induced cytotoxicity in MDA-MB231, MCF-7 and Caco2 cell lines. Drug Des Devel Ther. 2013;7:25–31. doi: 10.2147/DDDT.S37070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccari A. Layered double hydroxides: present and future: Rives V, editor. Nova Science Publishers, Inc., New York, 2001, IX+439 pp, ISBN 1-59033-060-9. Appl Clay Sci. 2002;22(1):75–76. [Google Scholar]
- 20.Mohsin SM, Hussein MZ, Sarijo SH, Fakurazi S, Arulselvan P, Hin TY. Synthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen application. Chem Cent J. 2013;7(1):26. doi: 10.1186/1752-153X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kura AU, Hussein Al Ali SH, Hussein MZ, Fakurazi S, Arulselvan P. Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int J Nanomedicine. 2013;8:1103–1110. doi: 10.2147/IJN.S39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Chung H-E, Choi SJ. Acute oral toxicity and kinetic behaviors of inorganic layered nanoparticles. Journal of Nanomaterials. 2013 [Google Scholar]
- 23.Del Hoyo C. Layered double hydroxides and human health: An overview. Appl Clay Sci. 2007;36(1–3):103–121. [Google Scholar]
- 24.Hussein-Al-Ali SH, Al-Qubaisi M, Hussein MZ, Ismail M, Zainal Z, Hakim MN. In vitro inhibition of histamine release behavior of cetirizine intercalated into zn/al- and mg/al-layered double hydroxides. Int J Mol Sci. 2012;13(5):5899–5916. doi: 10.3390/ijms13055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saifullah B, Hussein MZ, Hussein-Al-Ali SH, Arulselvan P, Fakurazi S. Antituberculosis nanodelivery system with controlled-release properties based on para-amino salicylate-zinc aluminum-layered double-hydroxide nanocomposites. Drug Des Devel Ther. 2013;7:1365–1375. doi: 10.2147/DDDT.S50665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saifullah B, Hussein MZ, Hussein-Al-Ali SH, Arulselvan P, Fakurazi S. Sustained release formulation of an anti-tuberculosis drug based on para-amino salicylic acid-zinc layered hydroxide nanocomposite. Chem Cent J. 2013;7(1):72. doi: 10.1186/1752-153X-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasbinder E, Van Der Weken G, Vander Heyden Y, et al. Quantitative determination of p-aminosalicylic acid and its degradation product m-aminophenol in pellets by ion-pair high-performance liquid chromatography applying the monolithic Chromolith Speedrod RP-18e column. Biomed Chromatogr. 2004;18(1):55–63. doi: 10.1002/bmc.292. [DOI] [PubMed] [Google Scholar]
- 28.Walters SB, Hanna BA. Testing of susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by mycobacterium growth indicator tube method. J Clin Microbiol. 1996;34(6):1565–1567. doi: 10.1128/jcm.34.6.1565-1567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palaci M, Ueki SY, Sato DN, Da Silva Telles MA, Curcio M, Silva EA. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol. 1996;34(3):762–764. doi: 10.1128/jcm.34.3.762-764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine. 2013;8(1):4467–4479. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen CC1, Chen HM, Chen SS, et al. Specific microtubule-depolymerizing agents augment efficacy of dendritic cell-based cancer vaccines. J Biomed Sci. 2011;18:44. doi: 10.1186/1423-0127-18-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhammad AA, Pauzi NAS, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from moringa oleifera lam. Biomed Res Int. 2013 doi: 10.1155/2013/974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rives V, editor. Layered Double Hydroxides: Present and Future. Hauppauge: Nova Science Publishers; 2001. [Google Scholar]
- 34.Vesenbeckh SM, Becker J, Huhnt C, et al. Successful oral desensitization to iv para-aminosalicylic acid (PAS) using encapsulated PAS dry substance. Infection. 2012;40(2):199–202. doi: 10.1007/s15010-011-0172-y. [DOI] [PubMed] [Google Scholar]
- 35.Hussain SM, Frazier JM. Cellular toxicity of hydrazine in primary rat hepatocytes. Toxicol Sci. 2002;69(2):424–432. doi: 10.1093/toxsci/69.2.424. [DOI] [PubMed] [Google Scholar]