Abstract
Objectives
1. Determine whether stress in preterm infants, measured with salivary cortisol, decreases after five days of Kangaroo Care (KC) compared to five days of Standard Care (SC). 2. To determine whether kangaroo care provides sustainable pain relief beyond the period of skin-to-skin holding.
Study Design
Preterm infants (n=38) born at 27-30 weeks gestational age were randomized to either the KC or the SC group and received the allocated intervention starting on day of life (DOL) five and continuing for five days. Salivary cortisol was collected on DOL five and again on DOL ten. Differences were analyzed using repeated measures ANOVA and t tests. Pain during nasal suctioning over five days was assessed using the Premature Infant Pain Profile (PIPP).
Result
1. Adequate saliva samples for salivary cortisol were collected for 13 KC infants and 11 SC infants. There was no main effect of group (p=0.49), but there was a significant main effect of age (DOL five versus DOL ten), with salivary cortisol levels decreasing in both groups (p=0.02). 2. Pain scores for both groups (n=38) indicted mild to moderate pain during suctioning, with no significant difference in pain scores between groups.
Conclusion
1. KC did not affect salivary cortisol levels in preterm neonates, but levels in both the KC and SC groups decreased over time from DOL five to ten. Salivary cortisol may vary with age of infant. 2. Infants experience pain during routine suctioning and may require pain management.
Keywords: kangaroo care, preterm infants, salivary cortisol, pain
1. Introduction
Each year approximately 64,000 preterm infants in the United States are born weighing <1500 grams [1], resulting in major public health problems [2] because of the increased risk for medical and developmental sequelae. The multiple stressors experienced in the neonatal intensive care unit (NICU) along with repeated painful procedures may compound the risk for sequelae by causing immediate physiologic distress [3] or increasing the likelihood of neurodevelopmental disabilities later in life [4-6].
Kangaroo care (KC) or skin-to-skin holding on the parenty's chest in an upright position, has been identified as one method that can reduce pain responses during painful procedures in newborn infants [7-12]. KC also facilitates bonding and may have benefits for infant neurodevelopment [13] and reduced morbidity and mortality [14]. However, it is not known whether daily KC reduces the general stress of being a preterm infant in a NICU and whether stress reduction is sustainable beyond the immediate holding period.
Salivary cortisol levels are often used as surrogates for plasma cortisol in neonates because they are noninvasive but still accurate [15]. Several studies on pain and stress in infants have measured salivary cortisol to evaluate the effectiveness of pain or stress relief measures [9,12,16-20] or to analyze adverse effects of pain and stress [5,6,21].
2. Objectives
The first objective of this study was to determine whether salivary cortisol levels in preterm infants decrease after five days of KC compared to five days of standard care (SC), therefore reflecting a reduction in stress for infants receiving KC. The second objective of this study was to determine whether the analgesic effects of KC extend beyond the immediate period of skin-to-skin holding. Previous studies have shown that kangaroo care reduces pain responses in preterm infants while the infant is being held [9,22,23], but it is not known whether this effect is sustainable and provides analgesia or calming between periods of holding. Specifically, the study analyzed the effects of KC versus SC group on pain response during tracheal or nasal suctioning. The International Evidence-Based Group for Neonatal Pain emphasizes the importance of providing pain relief during routine therapeutic procedures such as tracheal suctioning [22].
3. Study Design
This study was part of a larger randomized, controlled trial that evaluated the effects of KC on preterm infants hospitalized in a NICU at an academic medical center. The local Institutional Review Board granted approval for the study. Infants were randomized using a computer generated randomization table to receive either KC or SC for five consecutive days from day of life (DOL) five through DOL nine. Parents gave consent to participate in the study before randomization to group assignment. Infants assigned to the KC group were held skin-to-skin on the mother's or father's chest for a minimum of two hours daily from DOL five through nine. Infants assigned to the SC group remained in the incubator but at the parent's request could be held 15 minutes daily. The NICU used for this study provides private rooms for each infant and rooming-in capability for parents. Therefore, families were assured privacy and a quiet environment for skin-to-skin holding. Saliva samples were collected and analyzed for salivary cortisol on days five and ten of the study. Pain scores were assessed during routine suctioning via tracheal or nasal routes as these procedures were considered painful for the neonates [22].
3a. Study Population
Infants were recruited over a period of 20 months. Eligible neonates were less than 5 days old and were estimated to be 27-30 weeks gestational age as assessed by best obstetrical criteria at birth. All neonates weighed at least 1000 grams at birth, and were receiving mechanical ventilation, nasal continuous positive airway pressure (CPAP), or nasal cannula flow. Parents enrolled in the study were willing and able to hold the infant for a minimum of two hours daily for five consecutive days.
Infants with the following conditions were excluded from the study: documented maternal opiate use prior to delivery; clinical instability, severe congenital defects, or major surgery; Apgar score of three or less at five minutes of age, a cord blood pH of <7.0 or base deficit <−15. A total of 38 infants received the allocated intervention for the study, with19 assigned to the KC group and 19 to the SC group.
Data Collection and Analysis
3b. Salivary Cortisol
A saliva sample for measurement of cortisol was collected on the morning of the fifth DOL using an eye sponge BD VIsispear ™ (sorbette with plastic handle) from Salimetrics LLC (State College, PA). The sorbette was held alongside the buccal mucosa or under the tongue for 20 -25 minutes until it was moist and evenly puffed up. Saliva samples were collected at approximately 9 AM each morning as described previously [24].
On the morning of the tenth DOL, a repeat salivary cortisol was obtained as described above. The study was completed when the salivary cortisol sample was collected on DOL ten. Saliva was analyzed according to the methods detailed in our previous publication [24].
Data were collected on demographics and other variables that might affect stress levels or cortisol production. These included number of days on the ventilator, number of daily painful procedures encountered by infants [25], birth weight, and timing of maternal prenatal steroids. The Welch two sample t test was used to determine whether the KC and SC groups differed significantly in these characteristics.
Differences in means of salivary cortisol levels between the two groups and the two collection times (DOL five and ten) were analyzed using repeated measures analysis of variance (RMANOVA) followed by Tukey post-hoc testing to adjust for multiple comparisons. Linear regression was used to analyze relationships between infant characteristics and salivary cortisol levels.
3c. Pain during routine suctioning
Pain scores immediately following all routine tracheal or nasal suctioning procedures were assessed using the Premature Infant Pain Profile (PIPP). The PIPP is used to assess pain in preterm and term infants, and content and construct validity, reliability, sensitivity, and specificity have been established for this tool [26,27]. The PIPP gives a composite pain score based on behavioral (facial expressions), physiologic (heart rate and oxygen saturation) and contextual (gestational age and sleep/wake state) indicators. PIPP scores are based on a 30 second observation, and were assessed by staff nurses who were assigned to the infants for routine care. Staff nurses who suctioned the infants also gave the PIPP scores, and nurses were not blinded to infants’ group assignment. Suctioning was performed only as needed, and PIPP scores were therefore given at random times once or twice a day or sometimes every other day depending on the infants’ needs for suctioning. All staff nurses who participated were given individual and daily ongoing training on the correct use of the PIPP. Random inter-rater reliability checks were performed on 13% of infants with one investigator assessing PIPP scores along with staff nurses. The Pearson correlation for the two sets of PIPP scores was 0.98.
A PIPP score of 6 or less indicates mild pain, while a score of 12 or greater indicates moderate to severe pain. The maximum score for preterm infants is 21. Pain responses were evaluated using t-tests, to compare neonates who received KC vs. SC and to determine if there was a difference in PIPP scores over time.
4. Results
Fifty-four infants were recruited and randomized to interventions, 28 to the KC group and 26 to the SC group. Of these infants, nine in the KC group and seven in the SC group became ineligible after randomization. Figure 1 presents the enrollment and flow of infants through the study. Although original plans and power analysis indicated a sample size of 100, recruitment of infants was discontinued after 54 infants were enrolled because the culture of the NICU had changed. When the study began, KC was not a routine protocol for 27-30 week infants in this NICU. However, as the study progressed, KC was becoming routine and more widely accepted. Staff nurses were all educated in providing KC to infants in this age group and parents were provided with brochures encouraging them to participate. Randomization to the SC group was no longer congruous with the environment.
Figure 1.
Enrollment Flow Diagram for Infants in Pain and Stress Arm of Kangaroo Care Study
Although 38 infants received the interventions (19 per group), only 13 infants in the KC group and 11 in the SC group had adequate saliva volumes on both DOL five and DOLten to analyze changes in salivary cortisol levels after five days in the study. For the first ten samples of the study, the sorbette was left in the mouth for only three minutes, resulting in only 30% having sufficient volume of saliva for analysis. For the subsequent samples, the sorbette was left in the mouth for 20 minutes with a resulting 83% success rate [24].
Demographic data are presented in Table 1. A summary of variables that were analyzed for possible effects on salivary cortisol production is presented in Table 2. These variables included number of days on the ventilator, number of daily painful procedures encountered by infants [25], birth weight, and timing of maternal prenatal steroids. The Welch two-sample t test found no significant differences in these characteristics between groups, indicating that the KC and SC groups were similar. Linear regression showed no relationship between these variables and salivary cortisol levels (p=0.2) or percentage change in salivary cortisol levels (p=0.27).
Table 1.
Demographic Data (n=38)
| Group | Gender | Race | Ethnicity | Gestational age (weeks) |
|---|---|---|---|---|
| KC group n=19 | Males=8; Females=11 | Caucasian=17 African American=2 | Non-hispanic=17 Hispanic=2 | 29 |
| SC group n=19 | Males=11; Females=8 | Caucasian=16; African American=3 | Non-hispanic=19 Hispanic=0 | 28.5 |
Table 2.
Characteristics of Infants in Kangaroo Care (KC) and Standard Care (SC) Groups
| KC (n=13] |
SC (n=11) |
||
|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | p value |
| Days on ventilator | 1.85 (1.75) | 2.54 (1.56) | 0.15 |
| # painful procedures (daily) | 2.7 | 3.5 | 0.11 |
| Birth weight (grams) | 1311. 5 (216.3) | 1213.2 (186.4) | 0.16 |
| # days between maternal steroids and delivery | 12.4 (6.4) | 8.5 (8.4) | 0.18 |
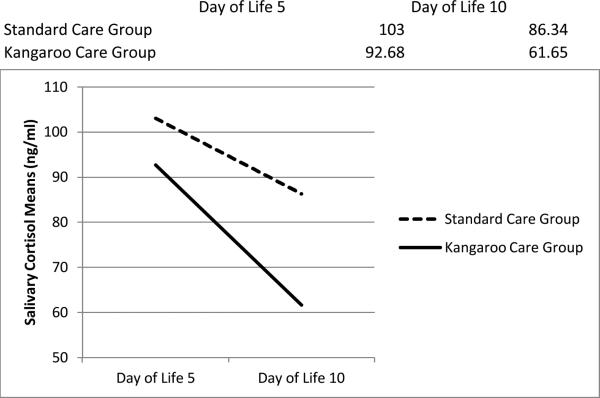
Analysis of salivary cortisol levels was based on raw values (ng/ml) that were normally distributed. No interaction of day (DOL five or ten) and group (KC versus SC) was found for salivary cortisol levels (p=0.475). Additionally, there was no main effect of group (p=0.49, Table 3), while there was a significant main effect for DOL five versus DOL ten (p=0.02), with salivary cortisol levels decreasing in both groups. Figure 2 illustrates the reduction of salivary cortisol levels in both groups from DOL five to DOL ten. Post hoc analysis of the main effect of treatment groups and day of life on salivary cortisol means is presented in Table 3.
Table 3.
Main Effect of Treatment Group and Day of Life on Salivary Cortisol (ng/ml)
| Mean | SE | p value | |
|---|---|---|---|
| Treatment Group | |||
| Kangaroo Care | 77.17 | 15.29 | 0.49 |
| Standard Care | 94.70 | 20.66 | |
| Day of Life | |||
| 5 | 97.89 | 14.95 | *0.02 |
| 10 | 73.99 | 12.46 | |
Figure 2.
Changes in Salivary Cortisol Means (ng/ml) from Day of Life Five to Day of Life Ten
PIPP scores were elevated after suctioning indicating mild to moderate pain, compared to baseline PIPP scores before suctioning that showed an absence of pain. After suctioning, average PIPP scores were 7.64 ± 0.40 SE (KC group) and 7.89 ± 0.21 SE (SC group, p=0.59). There were no significant changes in scores over the 5 days of data collection (Table 4, p=0.17). All PIPP scores were based on nasal suctioning except for two infants who underwent tracheal suctioning for the first two days of the study only. PIPP scores during tracheal suctioning ranged from 5-12, while PIPP scores during nasal suctioning ranged from 4-13.
Table 4.
Premature Infant Pain Profile (PIPP) Scores for Kangaroo Care (KC) and Standard Care (SC) Groups over Five Days
| KC (n=19] |
SC (n=19) |
|
|---|---|---|
| Day | Mean PIPP (SE) | Mean PIPP (SE) |
| 1 | 8.04 (0.54) | 8.4 (0.72) |
| 2 | 7.75 (0.62) | 7.0 (0.33) |
| 3 | 9.03 (0.73) | 8.15 (0.51) |
| 4 | 6.81 (0.80) | 8.14 (0.54) |
| 5 | 6.58 (0.69) | 7.74 (0.73) |
| Overall | 7.64 (0.40) | 7.89 (0.21) p=0.59 |
5. Discussion
Our work agrees with Cignacco et al. [28] who found a decrease in salivary cortisol levels in preterm infants over 14 days from DOL 2-14. The neonates in that study were exposed to an average of 12.9 painful procedures daily and their gestational age was higher (average 29 5/7 weeks). Although Cignacco found that salivary cortisol levels decreased over time, there were no significant changes in salivary cortisol levels immediately before and after heel sticks (p=0.55). In contrast, Cong [12] found that 30 minutes of KC immediately before, during and after a heelstick procedure significantly decreased cortisol levels in infants during KC compared to neonates who were exposed to incubator care only. However, the study only assessed the reactivity of the hypothalamic pituitary adrenal (HPA) response to a painful procedure (heelstick), and made no attempt to compare overall stress response over time.
Continued research is needed on the impact of KC (skin-to-skin care) on stress levels in preterm infants and on the rationale behind any calming effects. It is not known whether direct skin contact is the factor that provides calming, or whether the multisensorial stimulation of being held and rocked combined with the mother's fragrance, heart-beat, voice and breathing provide stress relief for the infant [7]. Morelius [18] studied the effects of skin-to-skin care on stress levels in 17 pairs of mothers and preterm infants and found that salivary cortisol levels increased in some infants (38%) and decreased in others (38%). Morelius [29] also found no significant difference in salivary cortisol levels between preterm infants who were randomized to a family-centered care group (n=152) where parents could stay 24 hours a day and preterm infants randomized to a standard care group (n=137).
The failure in the current study to find a significant difference between salivary cortisol levels between the KC group and the SC group (p=0.49, Table 3) may be related to several factors. First, KC was only administered for two hours daily from DOL five to ten, which may be inadequate for long-term stress relief and long-term down regulation of the HPA axis. However, more time spent in KC would have been difficult given the time constraints of both parents and health care providers. Many parents were recovering from operative deliveries, and increased time in KC would have caused physical stress for some parents. Second, our neonates were only exposed to an average of 2.7 (KC group) and 3.5 (SC group) painful procedures per day. Many studies of preterm neonates have reported a greater number of painful events in neonates of this age and gestation [21,25,28]. There could possibly be a more significant difference in the benefits of KC compared to SC when infants are undergoing more frequent painful procedures. Third, there was no attempt to initiate KC before and during painful procedures (suctioning). Human [21] and animal [30,31] data have demonstrated the importance of stressors such as pain and maternal separation on the responsiveness of the neuroendocrine system. Our design did not attempt to study the responsiveness of the HPA axis, but only measured baseline levels of salivary cortisol with and without KC. Since KC decreases pain responsiveness in preterm neonates [23,32,33], it is possible that cortisol responsiveness would have also decreased if infants had been held during suctioning [12]. Finally, the responsiveness of the HPA axis is known to decrease during hospitalization in the NICU [20]. The immaturity of the HPA axis during the neonatal period is well documented, and may result from non-responsiveness of the adrenal gland to adrenocorticotropin hormone [34]. Our finding of decreasing baseline cortisol levels from DOL five to ten may be related to cumulative stress during the NICU stay that could overwhelm the neuroendocrine self-regulation system of preterm neonates and dampen the response to stress. Alternatively, as preterm neonates mature and become more stable, they may be exposed to less pain and stress, unless clinical deterioration occurs, as was demonstrated by one of our subjects who developed sepsis with a resultant marked increase in salivary cortisol. The responsiveness of the HPA axis, cortisol regulation, and normal cortisol levels in preterm infants are very complex, and continued research is needed [35].
It is important to continue to study baseline cortisol measurements in preterm infants because elevated levels may be associated with adverse long-term consequences. Elevated fasting cortisol levels are found in lower birth weight neonates, and these higher levels are in turn associated with glucose intolerance and elevated blood pressure [36,37]. Thus, fetal programming with up-regulation of the HPA axis and change in glucose metabolism and cardiovascular status likely plays a significant role in the increased incidence of metabolic syndrome found in ex-preterm adults. In addition, baseline cortisol levels have been associated with a variety of anxiety producing disorders such as depression in children [38,39], while others have suggested that cortisol levels are not as important as HPA reactivity [40]. Continued study is needed on possible long-term consequences of elevated cortisol measurements in preterm infants.
Measurement of pain responses in preterm infants during routine procedures is important because of the frequency of painful procedures in this population. The pain that infants experience on a daily basis may be underestimated or ignored, with acute and long-term consequences [6]. Results showed that infants in both groups experienced mild to moderate pain during suctioning. Our study agrees with the work of Anand et al. [22] in the Consensus Statement for the Prevention and Management of Pain in the Newborn that routine procedures such as suctioning are painful and that infants may need non-pharmacological measures to relieve pain on a daily basis. No other studies have assessed pain scores during routine suctioning, and this study shows that nasal as well as tracheal suctioning procedures are painful. There was no significant difference in pain scores between the KC and SC groups, indicating that the analgesic or calming effects of KC are not sustainable or do not extend beyond the immediate period of skin-to-skin holding.
Our study had some limitations. SC subjects were allowed to receive 15 minutes of KC daily and many took advantage of the opportunity. KC was so well accepted by parents and providers that it would not have been possible to have a pure SC group in that environment. Second, many salivary cortisol samples were inadequate at the beginning of the study. As the research group became more experienced at collecting these samples, the success rate increased dramatically [24]. Third, the study was limited to 5 days of data collection, and a longer duration of kangaroo care may have decreased salivary cortisol levels to a greater extent (Figure 1). Fourth, suctioning was carried out 24 hours around the clock at random times according to infant need, and therefore several staff nurses were required to do the suctioning and give PIPP scores. Additionally, nurses were not blinded to infant assignment. Finally, the sample size was small, limiting the power of the study and indicating the need to replicate the study with a larger group.
Our study had several strengths. First, subjects were randomized to either KC or SC prior to enrollment, and parents did not know when they consented whether their infant had been randomized to the KC or the SC group. This assured a more uniform distribution of patients, because parents did not self-select their treatment group. Self-selection could have resulted in a biased distribution of the population base that could have affected results. Second, cortisol samples were collected at approximately the same time each day. Although cortisol levels may not be related to Circadian rhythms until 6-8 weeks of age even in preterm neonates [41], collecting samples at the same time daily eliminated that variable.
Study findings are generalizable only to stable preterm infants from 27 – 30 weeks who weigh at least 1000 grams. Infants who were clinically unstable or who had undergone birth asphyxia were excluded from the study. Similarly, the study is generalizable only to infants whose parents are able to stay with them and to hold them on a daily basis.
6. Conclusion
KC did not affect baseline salivary cortisol levels in preterm neonates compared with a SC group, but levels decreased in both groups over time from DOL five to ten. The DOL should be considered when assessing salivary cortisol in preterm neonates. KC did not change PIPP scores after suctioning in preterm neonates if they were not being held at the time of the painful stimulus. PIPP scores after suctioning were high enough to indicate that infants may need pain relief during suctioning.
Acknowledgments
7c. Other Disclosures
This project was supported by grants from the National Center for Research Resources (5P20RR020146-09) and the National Institute of General Medical Sciences (8 P20 GM103425-09) from the National Institutes of Health.
Footnotes
7. Disclosure Statements
7a. Financial Disclosure Statement
The authors declare no conflict of interest.
7b. Human Research Statement
This research was conducted in accordance with the ethical standards of the Institutional Review Board and the World Medical Association's Helsinki Declaration. Parental consent was obtained before randomizing infants to a treatment group.
Contributor Information
Anita J. Mitchell, University of Arkansas for Medical Sciences (UAMS), College of Nursing 4301 West Markham, Slot 529, Little Rock, AR 72205 Phone: 501-266-1551; Fax: 501-686-8350 AMitchell@uams.edu.
Charlotte C. Yates, University of Central Arkansas Department of Physical Therapy University of Arkansas for Medical Sciences, Center for Translational Neuroscience cyates@uca.edu.
D. Keith Williams, University of Arkansas for Medical Sciences, College of Medicine, Department of Biostatistics WilliamsDavidK@uams.edu.
Jason Y. Chang, University of Arkansas for Medical Sciences, College of Medicine, Department of Neurobiology and Developmental Sciences JYChang@uams.edu.
Richard Whit Hall, University of Arkansas for Medical Sciences, College of Medicine, Department of Pediatrics and Neonatology; Center for Translational Neuroscience HallRichardW@uams.edu.
References
- 1.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 2.Myers E, Ment LR. Long-term outcome of preterm infants and the role of neuroimaging. Clin Perinatol. 2009;36:773. doi: 10.1016/j.clp.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Als H. Toward a syntactive theory of development: Promise for the assessment and support of infant individuality. Infant Ment Health J. 1982;3:229–43. [Google Scholar]
- 4.Anand KJ. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–29. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- 5.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–6. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunau RE, Holsti L, Peters JWB. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006 Aug;11:268–75. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Campbell-Yeo M, Fernandes A, Johnston C. Procedural pain management for neonates using nonpharmacological strategies: Part 2: Mother-driven interventions. Adv Neonatal Care. 2011;11:312–20. doi: 10.1097/ANC.0b013e318229aa76. [DOI] [PubMed] [Google Scholar]
- 8.Chermont AG, Falcao LF, EH, Balda R, X, Guinsburg R. Skin-to-skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics. 2009;124:e1101–e1107. doi: 10.1542/peds.2009-0993. [DOI] [PubMed] [Google Scholar]
- 9.Johnston CC, Filion F, Campbell-Yeo M, et al. Kangaroo Mother Care diminishes pain from heel lance in very preterm neonates: A crossover trial. BMC Pediatr. 2008;8:13. doi: 10.1186/1471-2431-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–87. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnock FF, Castral TC, Brant R, et al. Brief report: Maternal Kangaroo Care for neonatal pain relief: A systematic narrative review. J Pediatric Psychol. 2010;35:975–84. doi: 10.1093/jpepsy/jsp123. [DOI] [PubMed] [Google Scholar]
- 12.Xiaomei C, Ludington-Hoe S, Walsh S. Randomized crossover trial of Kangaroo Care to reduce biobehavioral pain responses in preterm infants: A pilot study. Biol Res Nurs. 2011;13:204–16. doi: 10.1177/1099800410385839. [DOI] [PubMed] [Google Scholar]
- 13.Nyqvist KH, Anderson GC, Bergman N, et al. State of the art and recommendations. Kangaroo mother care: Application in a high-tech environment. Acta Paediatr. 2010;99:812–9. doi: 10.1111/j.1651-2227.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. ‘Kangaroo Mother Care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39(Suppl 1):i144–i154. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE., Jr Correlation between plasma and salivary cortisol levels in preterm infants. J Pediatr. 2002;140:116–8. doi: 10.1067/mpd.2002.120765. [DOI] [PubMed] [Google Scholar]
- 16.Gibbins S, Stevens B, Beyene J, Chan PC, Bagg M, Asztalos E. Pain behaviours in extremely low gestational age infants. Early Hum Dev. 2008;84:451–8. doi: 10.1016/j.earlhumdev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Kleberg A, Warren I, Norman E, et al. Lower stress responses after newborn individualized developmental care and assessment program care during eye screening examinations for retinopathy of prematurity: A randomized study. Pediatrics. 2008;121:e1267–e1278. doi: 10.1542/peds.2006-2510. [DOI] [PubMed] [Google Scholar]
- 18.Morelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116:1105–13. doi: 10.1542/peds.2004-2440. [DOI] [PubMed] [Google Scholar]
- 19.South MMT, Strauss RA, South AP, Boggess JF, Thorp JM. The use of non-nutritive sucking to decrease the physiologic pain response during neonatal circumcision: A randomized controlled trial. Am J Obstet Gynecol. 2005;193:537–43. doi: 10.1016/j.ajog.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 20.White-Traut RC, Schwertz D, McFarlin B, Kogan J. Salivary cortisol and behavioral state responses of healthy newborn infants to tactile-only and multisensory interventions. J Obstet Gynecol Neonatal Nurs. 2009;38:22–34. doi: 10.1111/j.1552-6909.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Grunau R, Holsti L, Haley D, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–80. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 23.Johnston CC, Stevens B, Pinelli J, et al. Kangaroo Care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003;157:1084–8. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell A, Chang J, Yates C, Hall RW. Challenges, guidelines, and systemic review of salivary cortisol research in preterm infants. e-Journal Neonatol Res. 2012:2. [Google Scholar]
- 25.Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 26.Ballantyne M, Stevens B, McAllister M, Dionne K, Jack A. Validation of the Premature Infant Pain Profile in the clinical setting. Clin J Pain. 1999;15:297–303. doi: 10.1097/00002508-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: Development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Cignacco E, Denhaerynck K, Nelle M, Buhrer C, Engberg S. Variability in pain response to a non-pharmacological intervention across repeated routine pain exposure in preterm infants: A feasibility study. Acta Paediatr. 2009;98:842–6. doi: 10.1111/j.1651-2227.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 29.Morelius E, Brostrom EB, Westrup B, Sarman I, Ortenstrand A. The Stockholm Neonatal Family-Centered Care Study: Effects on salivary cortisol in infants and their mothers. Early Hum Dev. 2012 Jul;88:575–81. doi: 10.1016/j.earlhumdev.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Anisman H, Lacosta S, Kent P, McIntyre DC, Merali Z. Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress. 1998;2:209–20. doi: 10.3109/10253899809167284. [DOI] [PubMed] [Google Scholar]
- 31.Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 32.Kostandy RR, Ludington-Hoe SM, Cong X, et al. Kangaroo Care (skin contact) reduces crying response to pain in preterm neonates: Pilot results. Pain Manag Nurs. 2008;9:55–65. doi: 10.1016/j.pmn.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinall J, Riddell RP, Greenberg S. The influence of culture on maternal soothing behaviours and infant pain expression in the immunization context. Pain Res Manag. 2011;16:234–8. doi: 10.1155/2011/707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol. 2008;28:797–802. doi: 10.1038/jp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng PC. Is there a “normal” range of serum cortisol concentration for preterm infants? Pediatrics. 2008 Oct;:873–875. doi: 10.1542/peds.2008-0516. [DOI] [PubMed] [Google Scholar]
- 36.Clark PM. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. Eur J Pediatr. 1998;157:S7–S10. doi: 10.1007/pl00014289. [DOI] [PubMed] [Google Scholar]
- 37.Walker BR. Cortisol--cause and cure for metabolic syndrome? Diabet Med. 2006;23:1281–8. doi: 10.1111/j.1464-5491.2006.01998.x. [DOI] [PubMed] [Google Scholar]
- 38.Greaves-Lord K, Huizink AC, Oldehinkel AJ, Ormel J, Verhulst FC, Ferdinand RF. Baseline cortisol measures and developmental pathways of anxiety in early adolescence. Acta Psychiatr Scand. 2009;120:178–86. doi: 10.1111/j.1600-0447.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009 Oct;34:1272–83. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothenberger SE, Moehler E, Reck C, Resch F. Prenatal stress: Course and interrelation of emotional and physiological stress measures. Psychopathology. 2011;44:60–7. doi: 10.1159/000319309. [DOI] [PubMed] [Google Scholar]
- 41.Castro M, Elias PC, Martinelli CE, Jr., Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000 Oct;33:1171–5. doi: 10.1590/s0100-879x2000001000006. [DOI] [PubMed] [Google Scholar]