Abstract
Immunotherapy is an attractive option for patients with high risk neuroblastoma due to their poor long-term survival rates after conventional treatment. Neuroblastoma cells are derived from the embryonic neural crest and therefore express tumor antigens not widely seen in normal cells, making them potential targets for immunologic attack. There is already considerable experience with monoclonal antibodies that target these tumor associated antigens, and in this review we focus on more exploratory approaches, using tumor vaccines and adoptive transfer of tumor-directed T cells.
Keywords: Neuroblastoma, immunotherapy, adoptive T cell transfer, chimeric antigen receptors, vaccine trials
INTRODUCTION
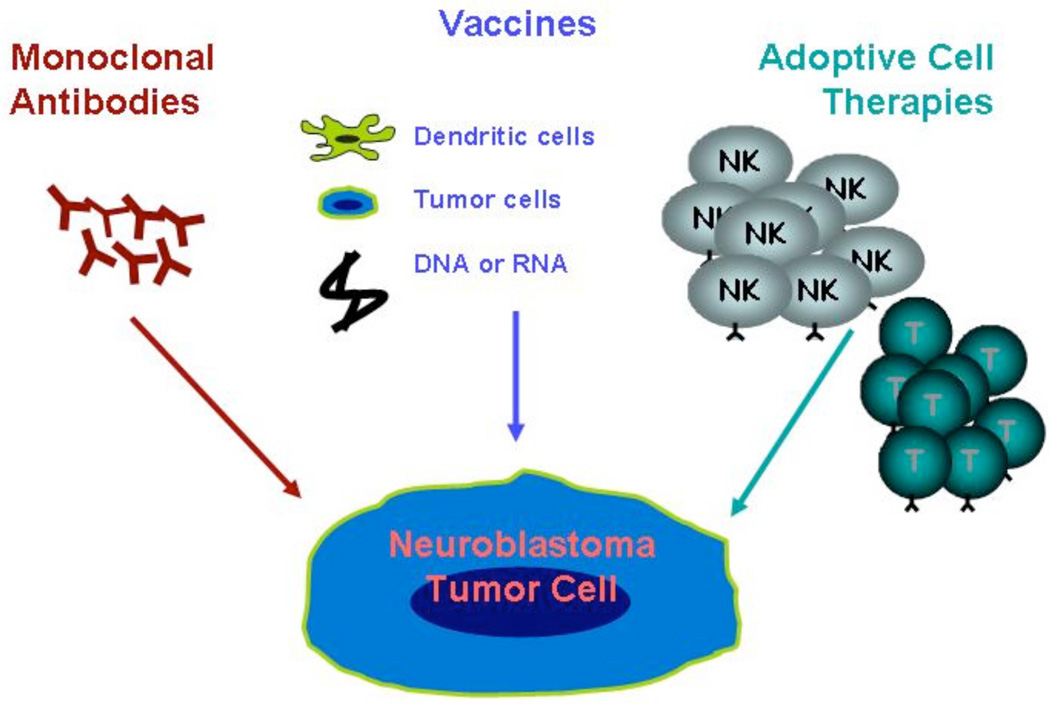
Over the past 20 years, efforts to improve the outcome for patients with high-risk neuroblastoma have focused on dose-intensifying chemotherapy, to levels that induce myeloablation and require autologous stem cell rescue. Biologic therapies with 13-cis-retinoic acid have also been added [1–5]. Unfortunately, despite improved remission rates, long-term survival in this population remains less than 40% [5, 6]. The limitations of current therapies have increased interest in experimental treatment options such as tumor-directed immunotherapies. Three types of immunotherapy have been used for patients with neuroblastoma: monoclonal antibodies, vaccination, and adoptive cellular therapy (Fig. 1).
Fig. (1). Immunotherapy for neuroblastoma.
Currently there are 3 forms of immunotherapy that have been used to treat patients with neuroblastoma: (a) monoclonal antibody therapy, (b) vaccine therapy, and (c) adoptive cell transfer.
MONOCLONAL ANTIBODY THERAPY
Monoclonal antibodies (MAbs) recognize tumor specific antigens and recruit effector mechanisms that kill tumor cells, usually by antibody dependent cell mediated cytotoxicity or complement mediated lysis. These effector mechanisms can be supplemented by coupling MAbs to toxins or radionucleides [7], or to "linker" molecules to bind them to effector T cells [8]. Antigens recognized by monoclonal antibodies must be present on the cell surface and may be almost any type of molecule including proteins, carbohydrates, lipids, or a combination thereof. Antigens currently used as targets for monoclonal antibodies are not expressed exclusively on tumor cells [7, 9]; "on-target but off-target-organ" adverse-effects of MAb may therefore be significant, depending on the degree and distribution of antigen expression on normal cells. In addition, many MAb contain peptide sequences or entire protein chains remaining from their rodent origin, and may induce an immune response leading to serious hypersensitivity reactions [10].
Monoclonal antibodies targeting neuroblastoma tumor associated antigens (TAA) have been used since the mid 1980’s [11] and were extensively reviewed by Johnson et al. in 2007 [7]. Tumor associated antigens targeted by MAb include the gangliosides GD2, (see Adoptive T Cell Therapy below) GD3, and GM3 and the glycoproteins CD56, L1-CAM, and GP95 [7, 12]. Clinical responses, including complete remissions, have been seen when monoclonal antibodies have been used alone [13–15]. In addition to single center studies, the Children’s Oncology Group has sponsored Phase I, II, and III studies using MAb to treat relapsed/refractory disease, as well as incorporating these agents after front-line myeloablative chemotherapy and stem cell rescue. Hence, as in adult malignancies, it appears likely that MAb will come to have a defined and significant role in the standard treatment of high-risk neuroblastoma.
VACCINE THERAPY
Developing an effective vaccine for neuroblastoma is a considerable challenge. While vaccines have been extremely successful at preventing infectious diseases, they are much less beneficial once illness is established. With only a few exceptions, cancer vaccines will be used after patients have developed their malignancies, and not for cancer prevention. For those with established disease, the generation of sustained and effective anti-tumor immune responses to TAA may be difficult [16]. In patients with neuroblastoma, development of a safe and effective vaccine for generalized use may be further hampered by the heterogeneity of tumor pathobiology, since some malignancies undergo spontaneous regression, while others are highly metastatic and minimally responsive to most intensive therapies. Moreover, downregulation of major histocompatibility complex (MHC) and co-stimulatory molecules by neuroblastoma cells may limit the effectiveness of any tumor-specific T cell immune response induced by the vaccine [17–20].
Despite obstacles to developing a successful vaccine therapy for neuroblastoma, a number of studies have been reported, and tumor responses, including complete remissions, have been observed. In part because of the heterogeneity of the disease, neuroblastoma tumor vaccines have been composed of cellular extracts or whole cell products. These complex products have the advantage of allowing multiple tumor antigens to be presented and investigators have used autologous or allogeneic neuroblastoma tumor cells, dendritic cells (DCs) loaded with peptides, mRNA, or tumor cell lysates (Table 1) [21–24]. Whole cell vaccines are amenable to genetic-modification as a way to enhance induction of an anti-tumor immune response [16, 18], but unlike peptide, protein or other "sub-unit" vaccines they are more difficult to standardize, store and distribute. The advantages and disadvantages of each type of vaccine product are listed in Table 2.
Table 1.
Vaccine Trials in Patients with Neuroblastoma
| Vaccine Product Authors | [Reference] |
|---|---|
| DCs pulsed with tumor lysate | Geiger et al. [22] |
| DCs pulsed with tumor RNA | Caruso et al. [21] |
| Autologous tumor cells genetically modified with IL-2 | Bowman et al. [23] |
| Allogeneic tumor cells genetically modified with IL-2 | Bowman et al. [24]; Haight et al. [54] |
| Autologous tumor cells genetically modified with IL-2 and lymphotactin | Russell et al. [55] |
| Allogeneic tumor cells genetically modified with IL-2 and lymphotactin | Rousseau et al. [28] |
Abbreviations: Dendritic cell (DC); Interleukin (IL).
Table 2.
Tumor Vaccine Products: Advantages and Disadvantages
| Product | Advantages | Disadvantages |
|---|---|---|
| Single peptide | Easy to produce and standardize Immune monitoring of a single antigen |
Prior identification of the single TAA Antigen-loss tumor variants |
| DCs pulsed with peptide, mRNA or apoptotic bodies | Antigen presentation using both MHC class I and II molecules | Complex production Antigen-loss variants Peptide use requires prior identification of the single TAA |
| Autologous whole cell tumor vaccine | No need to identify TAAs All tumor antigens likely represented and correct MHC restriction insured |
Requires patient tumor sample Complex production |
| Allogeneic whole cell tumor vaccine | No need to identify TAAs Easier to produce and standardize vs. autologous product MHC mismatch may be overcome by cross priming |
Inappropriate tumor antigen presentation Correct antigens may be missing |
Modified from Rousseau et al. 2005 [16].
Abbreviations: Dendritic cell (DC); Major histocompatibility complex (MHC); Tumor associated antigen (TAA).
One of the earliest vaccine trials for patients with neuroblastoma used tumor cells combined with bacilli Calmette-Guérin. Subcutaneous injection of this product caused local inflammation and induced transient tumor responses, although the results were never formally published [16]. Subsequently, a second group evaluated DCs loaded with tumor cell lysates. Although an increased number of in vivo tumor-specific T cells were detected, no sustained clinical responses were observed [22].
Bowman et al. [23] tested the hypothesis that modifying autologous neuroblastoma cells to secrete IL-2 could generate an immunogenic whole-cell vaccine. Local IL-2 secretion recruits T cells and natural killer (NK) cells, and also induces interferon gamma (IFN-γ) release by NK cells and some T lymphocytes [16, 25]. Release of IFN-γ may have significant consequences, since it increases MHC molecule expression by neuroblasts [16] and thereby augments both their immunogenicity and vulnerability to MHC restricted T cell killing. The group found that administration of IL-2 gene-modified autologous tumor cells to patients with high-risk disease was safe and led to a local infiltration of CD3+CD4+ T cells at the injection site. In the peripheral blood, there were increased numbers of activated T cells, and to a lesser extent NK cells, eosinophils and monocytes. Furthermore, IgG anti-tumor antibodies were identified in 4 of 9 patients. Of the 10 patients treated, 3 had clinical responses after vaccination (1 complete response, 1 partial response, and 1 patient with stable disease) [23]. Additionally, 3 of the remaining 7 patients developed responses (1 complete, 2 partial responses) after low dose oral etoposide [16].
While autologous tumor vaccine products should express all the tumor antigens necessary to generate effective immune responses in a given patient, they are difficult and slow to prepare, and require access to tumor samples from each subject. An allogeneic “off-the-shelf” product is easier to make and standardize, and would broaden the eligible pool of patients in clinical trials. The same group of investigators therefore compared the efficacy of an allogeneic tumor product transduced to express IL-2. Of 12 subjects, 3 had transient increases in the frequency of cytotoxic T lymphocyte precursor cells that reacted to the tumor cell line, but no patients developed cytotoxic immune function against their own tumor cells. Clinically, 1 patient had a >90% partial response after receiving this vaccine [24].
Pre-clinical studies then showed that the T-lymphocyte recruiting chemokine lymphotactin (LTN) [26] could substantially increase the immunogenicity of IL-2 gene modified neuroblastoma cells [27]. This led Rousseau et al. [28] to modify the previously used allogeneic cell line to secrete both IL-2 and LTN. Subcutaneous administration to subjects with neuroblastoma led to increased local infiltration of CD4+ and CD8+ T cells, eosinophils, and Langerhan's cells. Systemically, increased NK cells and IgG antibodies to the vaccine cell line were detected in the peripheral blood. Amongst 28 patients, there were 4 complete responses (2 sustained > 4 years after vaccination), 1 very good partial response, 1 partial response, and 5 patients with stable disease [16, 28] (and unpublished data).
Hence, cell based vaccine therapies for patients with neuroblastoma can be given safely and are able to induce both immune and clinical responses. We and others are now evaluating the safety, immune response, and efficacy of neuroblastoma vaccines given to patients whose disease burden has recently been minimized by high-dose chemotherapy and stem cell rescue. The hope is that vaccination at this time will ensure the best possible ratio of effector cells to tumor cells, and minimize the influence of regulatory T lymphocytes. One of these studies uses an IL-2 and LTN secreting allogeneic cell line injected with a second, unmodified, cell line expressing a distinct set of TAA. This combination of lines should increase the breadth of the resulting immune response, and thereby increase the probability of a response targeting antigens expressed by the patients own tumor.
ADOPTIVE T CELL THERAPY
Adoptive cellular immunotherapies for neuroblastoma are clinically less developed than monoclonal antibodies or vaccines. The technical and regulatory demands of ex vivo manufacture and subsequent administration of cellular products are substantial. Nonetheless, there are clear potential advantages in preparing an engineered effector T cell product in an ex vivo environment, since it will be free of the immunosuppressive influences of an established tumor. These hypothetical benefits may make adoptive T cell transfer more effective than vaccination, which relies on activation and recruitment of an immune system already damaged both by the tumor and its treatment.
The native T cell receptor recognizes processed protein antigens presented in association with MHC molecules on the cell surface. Thus T cells, unlike MAbs, may recognize processed internal and external antigens, but may be thwarted if the cancer cells have defects in antigen processing/presentation, or fail to express MHC molecules. Despite this limitation, adoptively transferred T cells have several advantages over MAbs. They can expand in vivo following transfer and produce long-term re-population of the host with effector cells. They can actively migrate through multiple tissue planes. Lastly, they use direct and indirect cytotoxic effector mechanisms, reducing the risk that tumor cells will be invulnerable to killing.
Prerequisites for generating antigen-specific cytotoxic T lymphocytes (CTL) include the identification of appropriate TAA and the availability of suitable antigen-presenting cells (APCs) [10]. Once identified, antigen-specific CTL lines can be generated by co-culturing T cells with APCs that express the TAA, and expanded by antigen re-stimulation and exposure to cytokines such as IL-2, IL4 and IL7.
Neuroblastoma expresses several antigens of the oncofetal or cancer testis (CTA) group that could be targets for the native receptor of T cells. These include melanoma antigen encoding gene (MAGE)-A1, MAGE-A3/A6, NY-ESO-1, B melanoma antigen (BAGE) and G antigen (GAGE) [29–33]. Although primary neuroblastoma cells express few if any MHC molecules [29, 34], these structures become markedly upregulated following chemotherapy, radiation, or any inflammatory response. Once MHC molecules are expressed, tumor cells may become susceptible to killing by TAA specific T cells. With the increasing successes of adoptive immunotherapy of T cells directed to oncofetal/CTA, interest has been renewed in attacking neuroblastoma by the same means, but no clinical studies have yet been reported.
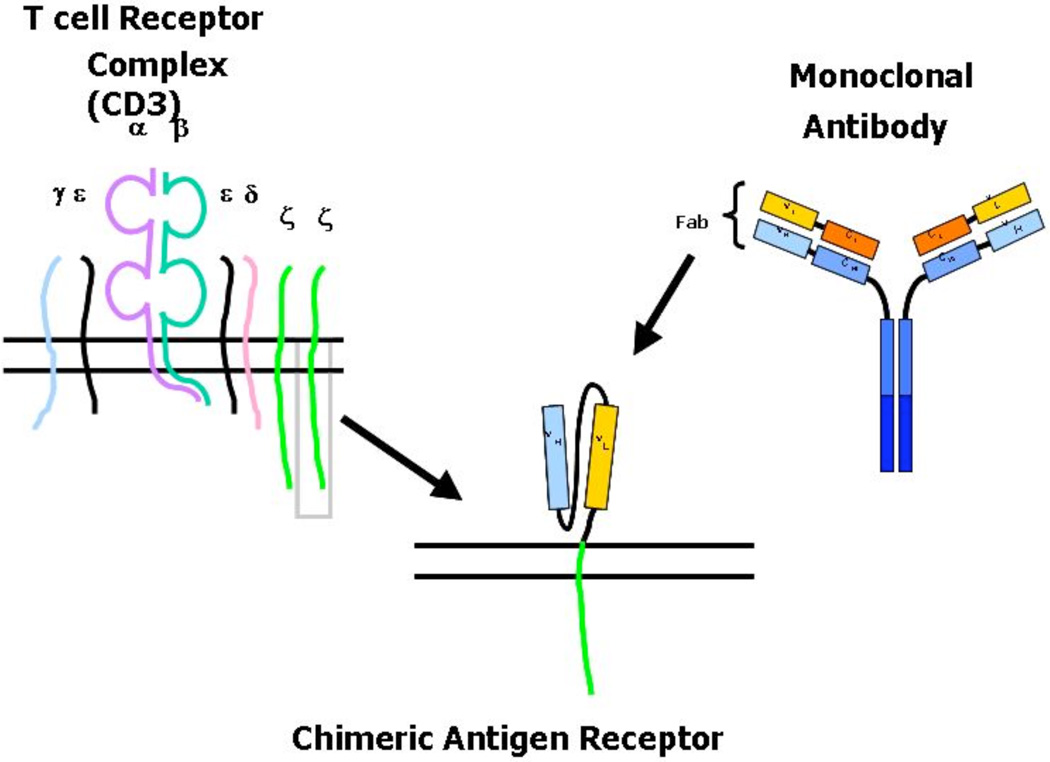
As neuroblastoma, like other cancer cells, can downregulate MHC molecule expression [35], there is also interest in the adoptive transfer of T cells expressing an MHC-independent antigen receptor. Such chimeric antigen receptors (CARs) can be generated by linking the heavy and light chain variable regions of a TAA-specific monoclonal antibody. The single chain variable fragment (scFv) is then coupled to the transmembrane and intracellular portion of the ζ–chain signaling moiety from native T cell receptors (Fig. 2) [10, 36, 37]. This construct combines the specificity of monoclonal antibodies with the trafficking and cytotoxic effects of a T cell.
Fig. (2). Generation of a chimeric antigen receptor.
Chimeric antigen receptors (CARs) consist of an extracellular antigen-specific region and an intracellular chain with kinase activity (endodomain). Upon binding of the target antigen, the endodomain delivers a downstream signal initiating T cell activation.
CAR-specific T cells have been used in two clinical trials in patients with neuroblastoma. Gonzalez et al. described the generation of CE7R CAR-expressing CD8+ clones targeting CD171 [38], the L1 adhesion molecule that is overexpressed by neuroblastoma cells [17, 39, 40]. The CAR-T cells were used in a Phase I trial for patients with recurrent/refractory disease, and of the 6 patients treated, no severe toxicities were observed and one had a partial, but un-sustained, response after CE7R T cell infusion [41].
In the second trial, patients with a history of high-risk neuroblastoma were infused simultaneously with two different T cell populations, activated T cells (ATC), and EBV specific CTL [42]. Earlier adoptive transfer studies had shown that EBV-CTLs are long lived in the peripheral circulation [43–45]. This characteristic is likely attributable to the stimulation and co-stimulation received following native antigen receptor engagement by the EBV antigens chronically expressed by B-lymphocytes in EBV seropositive individuals. The purpose of the study was to discover if long-term survival of CTL continued when the cells also expressed a CAR targeting a neuroblastoma TAA. Prolonged survival of effector cells would be desirable since this would likely increase their anti-tumor activity compared to cells only present transiently.
The subjects in this study received ATC and CTL that both expressed a CAR specific for GD2 (see Monoclonal Antibodies above). However, the CAR used in each cell population contained a unique oligonucleotide that allowed them to be molecularly distinguished [42]. The results showed that GD2-CAR CTL were present longer and at higher levels than CAR ATC, and demonstrated the in vivo survival advantage of CTL even when genetically modified to express a CAR. Clinically, no dose limiting toxicities were identified, and in particular no patient suffered neurological pain - a common adverse effect with GD2 MAb and attributable to reaction with GD2 expressed at low levels on peripheral pain fibers. Of 12 patients treated, 3 had no evidence of disease at the time of infusion. One remains disease free at >17 months post infusion, and the other 2 are alive with disease 13 and 34 months post infusion, respectively. Of the 9 patients with relapsed/resistant disease at the time of infusion, 3 have had stable disease (from 10 to 16 months), 2 developed areas of tumor inflammation and necrosis, 1 had clearance of marrow disease at 6 weeks, and 1 has a complete response sustained for more than 24 months [42]. Of note, although patients with neuroblastoma have elevated serum levels of GD2 [11, 46], CAR-CTL function is not impaired in the presence of their soluble ligand, presumably because of greater avidity of the CAR for antigen on the surface of the tumor cells [10, 47].
While the above approach is encouraging, it has several limitations. Not all patients are EBV seropositive, and in those who are, the manufacture of EBV-CTLs adds to the time, cost and complexity to adoptive cellular immunotherapy. One way to by-pass CTL generation is by modifying CARs to provide additional signals to the T cell following chimeric receptor engagement. T cells require multiple co-stimulatory signals for an optimal response to binding at their antigen specific receptor. These co-stimulatory signals are rarely provided by tumor cells [10], and primary neuroblastoma tumor cells lack most co-stimulatory molecules, including CD40, CD80, CD86, OX40L and 4IBB-L [48, 49]. It is possible to modify CARs to express endodomains from these co-stimulatory molecules. CAR binding to the TAA then leads to an array of stimulatory and co-stimulatory signals, and enhanced cell activation, survival and proliferation. Although this approach has worked well in vitro [50], it has yet to be assessed in vivo.
In the future, other forms of CAR T cell engineering will be evaluated for use in adoptive T cell transfer. Two areas currently being investigated are increasing the tumor-homing capacity of adoptively transferred T cells by incorporating receptors targeting chemokines secreted by tumor cells or cells in the tumor stromal environment [51–53], and modifying the CAR exodomains to reduce the immune response caused by the rodent sequences they contain [10, 38].
CONCLUSION
Immunotherapy using monoclonal antibodies, active immunization or adoptive transfer of antigen specific T cells may provide less-toxic, therapeutic options for patients with neuroblastoma and can produce additional sustained clinical responses. Over the next decade the challenge will be to more firmly establish the effectiveness of these immune approaches and to discover how they may best be integrated with current multimodality treatments.
REFERENCES
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Pole JG, Casper J, Elfenbein G, Gee A, Gross S, Janssen W, et al. High-dose chemoradiotherapy supported by marrow infusions for advanced neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991;9:152–158. doi: 10.1200/JCO.1991.9.1.152. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JR, Coccia PF. Is more better? Dose intensity in neuroblastoma. J Clin Oncol. 1991;9:902–904. doi: 10.1200/JCO.1991.9.6.902. [DOI] [PubMed] [Google Scholar]
- 4.Shuster JJ, Cantor AB, McWilliams N, Pole JG, Castleberry RP, Marcus R, et al. The prognostic significance of autologous bone marrow transplant in advanced neuroblastoma. J Clin Oncol. 1991;9:1045–1049. doi: 10.1200/JCO.1991.9.6.1045. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 6.De BB, Nicolas B, Boni L, Indolfi P, Carli M, Cordero Di ML, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–1601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 7.Johnson E, Dean SM, Sondel PM. Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev Mol Med. 2007;9:1–21. doi: 10.1017/S1462399407000518. [DOI] [PubMed] [Google Scholar]
- 8.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson J. Reengineered monoclonal antibodies step up to the plate in cancer studies. JAMA. 1995;274:1821–1822. [PubMed] [Google Scholar]
- 10.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 11.Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44:5914–5920. [PubMed] [Google Scholar]
- 12.Cheung NK, Sondel PM. Neuroblastoma immunology and immunotherapy. In: Cohn S, Cheung NK, editors. Neuroblastoma. Springer Press; 2005. pp. 223–242. [Google Scholar]
- 13.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 14.Handgretinger R, Baader P, Dopfer R, Klingebiel T, Reuland P, Treuner J, et al. A phase I study of neuroblastoma with the antiganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau RF, Brenner MK. Vaccine therapies for pediatric malignancies. Cancer J. 2005;11:331–339. doi: 10.1097/00130404-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Verneris MR, Wagner JE. Recent developments in cell-based immune therapy for neuroblastoma. J Neuroimmune Pharmacol. 2007;2:134–139. doi: 10.1007/s11481-007-9065-3. [DOI] [PubMed] [Google Scholar]
- 18.Shilyansky J, Jacobs P, Doffek K, Sugg SL. Induction of cytolytic T lymphocytes against pediatric solid tumors in vitro using autologous dendritic cells pulsed with necrotic primary tumor. J Pediatr Surg. 2007;42:54–61. doi: 10.1016/j.jpedsurg.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262–274. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso DA, Orme LM, Amor GM, Neale AM, Radcliff FJ, Downie P, et al. Results of a Phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer. 2005;103:1280–1291. doi: 10.1002/cncr.20911. [DOI] [PubMed] [Google Scholar]
- 22.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 23.Bowman L, Grossmann M, Rill D, Brown M, Zhong WY, Alexander B, et al. IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998;92:1941–1949. [PubMed] [Google Scholar]
- 24.Bowman LC, Grossmann M, Rill D, Brown M, Zhong WY, Alexander B, et al. Interleukin-2 gene-modified allogeneic tumor cells for treatment of relapsed neuroblastoma. Hum Gene Ther. 1998;9:1303–1311. doi: 10.1089/hum.1998.9.9-1303. [DOI] [PubMed] [Google Scholar]
- 25.Main EK, Lampson LA, Hart MK, Kornbluth J, Wilson DB. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–246. [PubMed] [Google Scholar]
- 26.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 27.Dilloo D, Bacon K, Holden W, Zhong W, Burdach S, Zlotnik A, et al. Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nat Med. 1996;2:1090–1095. doi: 10.1038/nm1096-1090. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau RF, Haight AE, Hirschmann-Jax C, Yvon ES, Rill DR, Mei Z, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 29.Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, et al. Expression of MHC class I, MHC class II, cancer germline antigens in neuroblastoma. Cancer Immunol Immunother. 2005;54:400–406. doi: 10.1007/s00262-004-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soling A, Schurr P, Berthold F. Expression and clinical relevance of NY-ESO-1, MAGE-1 and MAGE-3 in neuroblastoma. Anticancer Res. 1999;19:2205–2209. [PubMed] [Google Scholar]
- 31.Prigione I, Corrias MV, Airoldi I, Raffaghello L, Morandi F, Bocca P, et al. Immunogenicity of human neuroblastoma. Ann N Y Acad Sci. 2004;1028:69–80. doi: 10.1196/annals.1322.008. [DOI] [PubMed] [Google Scholar]
- 32.Ishida H, Matsumura T, Salgaller ML, Ohmizono Y, Kadono Y, Sawada T. MAGE-1 and MAGE-3 or-6 expression in neuroblastoma-related pediatric solid tumors. Int J Cancer. 1996;69:375–380. doi: 10.1002/(SICI)1097-0215(19961021)69:5<375::AID-IJC4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Cheung IY, Barber D, Cheung NK. Detection of microscopic neuroblastoma in marrow by histology, immunocytology, and reverse transcription-PCR of multiple molecular markers. Clin Cancer Res. 1998;4:2801–2805. [PubMed] [Google Scholar]
- 34.Whelan JP, Chatten J, Lampson LA. HLA class I and beta 2-microglobulin expression in frozen and formaldehyde-fixed paraffin sections of neuroblastoma tumors. Cancer Res. 1985;45:5976–5983. [PubMed] [Google Scholar]
- 35.Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 36.Eshhar Z. Tumor-specific T-bodies: towards clinical application. Cancer Immunol Immunother. 1997;45:131–136. doi: 10.1007/s002620050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez S, Naranjo A, Serrano LM, Chang WC, Wright CL, Jensen MC. Genetic engineering of cytolytic T lymphocytes for adoptive T-cell therapy of neuroblastoma. J Gene Med. 2004;6:704–711. doi: 10.1002/jgm.489. [DOI] [PubMed] [Google Scholar]
- 39.d'Uscio C, Blaser K. Establishment of anti-human neuroblastoma-selective isotype-switch variants. J Immunol Methods. 1992;146:63–70. doi: 10.1016/0022-1759(92)90049-y. [DOI] [PubMed] [Google Scholar]
- 40.d'Uscio CH, Jungi TW, Blaser K. Cellular cytotoxicity mediated by isotype-switch variants of a monoclonal antibody to human neuroblastoma. Br J Cancer. 1991;64:445–450. doi: 10.1038/bjc.1991.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 42.Pule M, Savoldo B, Myers G, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in neuroblastoma patients. Nat Med. 2008 doi: 10.1038/nm.1882. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 44.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Savoldo B, Huls H, Lopez T, Gee A, Wilson J, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res. 2002;159:123–133. doi: 10.1007/978-3-642-56352-2_15. [DOI] [PubMed] [Google Scholar]
- 46.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–1567. [PubMed] [Google Scholar]
- 47.Hombach A, Koch D, Sircar R, Heuser C, Diehl V, Kruis W, et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 1999;6:300–304. doi: 10.1038/sj.gt.3300813. [DOI] [PubMed] [Google Scholar]
- 48.Airoldi I, Lualdi S, Bruno S, Raffaghello L, Occhino M, Gambini C, et al. Expression of costimulatory molecules in human neuroblastoma. Evidence that CD40+ neuroblastoma cells undergo apoptosis following interaction with CD40L. Br J Cancer. 2003;88:1527–1536. doi: 10.1038/sj.bjc.6600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Morandi F, Bocca P, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–161. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 50.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bracci L, Moschella F, Sestili P, La SV, Valentini M, Canini I, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 53.Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 54.Haight AE, Bowman LC, Ng CY, Vanin EF, Davidoff AM. Humoral response to vaccination with interleukin-2-expressing allogeneic neuroblastoma cells after primary therapy. Med Pediatr Oncol. 2000;35:712–715. doi: 10.1002/1096-911x(20001201)35:6<712::aid-mpo50>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother. 2007;30:227–233. doi: 10.1097/01.cji.0000211335.14385.57. [DOI] [PubMed] [Google Scholar]