Abstract
Objective
Massive perivillous fibrin deposition (MPFD) and maternal floor infarction (MFI) are related placentallesions often associated with fetal death and fetal growth restriction. A tendency to recur in subsequent pregnancies has been reported. This study was conducted to determine whether this complication of pregnancy could reflect maternal anti-fetal rejection.
Methods
Pregnancies with MPFD were identified (n=10). Controls consisted of women with uncomplicated pregnancies who delivered at term without MPFD (n=175). Second-trimester maternal plasma was analyzed for panel-reactive anti-HLA class I and class II antibodies. The prevalence of chronic chorioamnionitis, villitis of unknown etiology, and plasma cell deciduitis was compared between cases and controls. Immunohistochemistry was performed on available umbilical vein segments from MPFD cases (n=4) to determine whether there was evidence of complement activation (C4d deposition). Specific maternal HLA-antibody and fetal HLA-antigen status were also determined in paired specimens (n=6). Plasma CXCL-10/IP-10 concentrations were measured in longitudinal samples of cases (n=28 specimens) and controls (n=749 specimens) by ELISA. Linear mixed models were used to test for differences in plasma CXCL-10 concentration.
Results
1) The prevalence of plasma cell deciduitis in the placenta was significantly higher in cases with MPFD than in those with uncomplicated term deliveries (40% vs. 8.6%, p=0.01); 2) patients with MPFD had a significantly higher frequency of maternal anti-HLA class I seropositivity during the second trimester than those in uncomplicated term deliveries (80% vs. 36%, p=0.01); 3) strongly positive C4d deposition was observed on umbilical vein endothelium in cases of MPFD; 4) specific maternal antibody against fetal HLA antigen class I or II was identified in all cases of MPFD; and 5) the mean maternal plasma concentration of CXCL-10 was higher in patients with evidence of MPFD than in those without evidence of MFPD (p <0.001).
Conclusions
Collectively, the data presented herein suggest that a subset of patients with MPFD has a signature of maternal anti-fetal rejection as a mechanism of disease.
Keywords: PRA, MPFD, fibrinoid deposition, stillbirth, HLA, plasma cell deciduitis, villitis
Introduction
Massive perivillous fibrin deposition (MPFD) and maternal floor infarction (MFI) are related placental lesions characterized by extensive deposition of fibrinoid material in the intervillous space, and associated with hypoplastic and sclerosis of the engulfed villi.1–3 Fibrin and/or fibrinoid material deposition interferes with perfusion and gas/nutrient exchange in the intervillous space, resulting in “chronic placental insufficiency”.4–8 Pregnancies with MPFD are associated with serious obstetrical complications, such as spontaneous abortion,3, 9, 10 fetal growth restriction,3, 4, 6, 7, 11–15 and fetal death.3, 4, 6, 7, 10, 12, 14–22 The mechanisms responsible for MPFD are unknown.3, 23
The fetus is the most successful semi-allograft. Therefore, maternal immune tolerance of the fetus is essential for successful pregnancy.24–47 Failure of maternal tolerance to the fetus has been proposed to be a mechanism of disease in recurrent pregnancy loss,48–52 preterm delivery,18, 44, 53, 54 fetal growth restriction (FGR),4, 12, 55 fetal death4, 12, 56 and preeclampsia (PE).44, 50, 52, 55, 57–60 Allograft rejection involves both the innate and adaptive limb of the immune response.61, 62 The most important alloantigens are within the major histocompatibility complex (MHC) class I and class II, and are part of the human leukocyte antigen (HLA) system.61, 63
An important feature of humoral antibody-mediated rejection after allograft transplantation is the generation of donor-specific HLA. To screen for the presence of these antibodies, HLA panel reactive antibodies (PRA) can be used.64–66 HLA sensitization is a risk factor for graft rejection.67, 68 HLA panel-reactive antibodies (PRA) are used to determine the HLA sensitization status of recipients69, 70 and to assess the likelihood of graft rejection in patients who undergo transplantation.71–74 The presence of HLA-antibodies in early pregnancy is associated with a reduced chance of live birth.75 Moreover, the presence of C4d deposition (a degradation product of complement factor C4) is considered to be an evidence of antibody-mediated rejection of the allografts.76, 77 For example, in a renal transplant, immunostaining for C4d in glomerular endothelial cells and peritubular capillaries in renal allograft biopsies has been shown to be an important indication of graft pathology.78, 79
Recently, we and other investigators proposed that chronic chorioamnionitis (infiltration of maternal T cells in the chorioamniotic membranes)54, 56, 80, 81, villitis of unknown etiology (VUE)19, 20, 82, 83 and chronic deciduitis with plasma cell74 are the placental lesions associated with maternal anti-fetal rejection. Prior reports showed that maternal HLA PRA positivity before 16 weeks74 and at the time of diagnosis54 are associated with the presence of chronic chorioamnionitis. Furthermore, maternal, fetal plasma and amniotic fluid concentration of CXCL-10, an anti-angiogenic T-cell chemokine, is higher in patients with evidence of placental lesions associated with maternal anti-fetal rejection than in those without these lesions.19, 20
We postulated that if maternal anti-fetal rejection is involved in the mechanism of disease of MPFD, evidence in support of graft rejection may be present. Consequently, the objective of this study was to examine whether in MPFD there is: 1) a difference in the frequency of placenta lesions associated with maternal anti-fetal rejection; 2) an increased frequency of maternal anti-HLA seropositivity; 3) evidence of complement activation in the fetus (C4d deposition on umbilical cord vessels); and 4) a change in the maternal plasma concentration of CXCL-10.
Material and Methods
Placental pathologic specimens from the Bank of Biological Materials of the Wayne State University/Detroit Medical Center/Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, from 2006 to 2011 were reviewed. Cases meeting the criteria of MPFD were identified. The criterion for the diagnosis was the identification of perivillous fibrinoid material encasing at least 50% of the villi on a minimum of one slide3. Either cases with fibrinoid material only on the maternal floor side of the placenta or cases with transplacental fibrinoid deposition were eligible (n=10). Controls (n=175) were women without MPFD in the placenta who had uncomplicated pregnancies, delivered a neonate whose birth weight was appropriate for gestational age (10th – 90th percentiles)84 and had plasma samples available for at least five of the following gestational age intervals: 6–15, 16–20, 20–24, 25–28, 28–32, 32–36 and ≥37 weeks. These patients had been enrolled in a longitudinal protocol to identify biological markers for the prediction of PE, SGA, and stillbirth. Venous samples were collected every 4 weeks until 24 weeks and every 2 weeks thereafter until delivery. Exclusion criteria were 1) multiple gestations; and 2) congenital fetal anomaly.
All women provided written informed consent before participating in the study, and the use of clinical data and the collection and utilization of biological samples for research purposes were approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver Nation al Institute of Child Health and Human Development, National Institutes of Health.
Placental Pathology
Histopathologic changes of the placenta were identified using the diagnostic criteria of the Perinatal Section of the Society for Pediatric Pathology.85 Plasma cell deciduitis was defined as a lymphoplasmacytic infiltration of the decidua of the basal plate.86 The diagnosis of VUE and chronic chorioamnionitis were based on previously published descriptions.18, 20
Briefly, the diagnosis of VUE is made when lymphohistiocytic infiltration is present in more than five villi on multiple slides. Chronic chorioamnionitis is confirmed when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue is observed.
Flow Cytometry of Panel-Reactive Maternal Anti-HLA Antibodies
HLA PRA seropositivity was determined in plasma of both cases and controls. Flow cytometry was performed using the FlowPRA-I and FlowPRA-II Screening Tests (One Lambda Inc., Canoga Park, CA). The test represents a pool of 30 HLA antigens of each class I and class II and is designed to provide a non-specific assessment of the presence or absence of anti-HLA-I or anti-HLA-II antibodies. We followed the manufacturer recommendations for the assay; 20 µl of plasma were incubated with FlowPRA beads for 30 min at room temperature. The manufacturer’s wash buffer was added and vortexed, and the supernatant was discarded a total of three times. The beads were then stained with 100 µl of 1X FITC labeled anti-human IgG antibody for 30 min. After washing the beads twice with wash buffer and adding fixing solution (PBS with 0.5% formaldehyde), the FL1 fluorescence of 5,000 events was analyzed using BD LSRII flow cytometry (BD Biosciences, San Jose, CA). Plasma that was anti-HLA IgG positive showed a fluorescent channel shift as compared with the negative serum; the percentage of PRA was represented by the percentage of beads that reacted positively with the serum, and a figure of >10% was considered a positive result.
The frequency of HLA PRA seropositivity in MPFD was compared to the control group. For this comparison, only the specimens collected closest to the time of delivery of MPFD cases were included. Samples from the control group were matched for gestational age at delivery (n=143) of the majority of cases with MPFD.
Flow Cytometry of Specific Maternal and Fetal Anti-HLA Antibodies
Specific antibody reactivity in maternal blood was then determined in the same cases using maternal blood collected just prior to delivery and fetal umbilical cord DNA from specimens obtained at the time of delivery (sample available in 6/10 cases). First, to assess the maternal HLA antibody, the LABScreen Single Antigen test (One Lambda Inc., Canoga Park, CA) was used. Five µL of each bead group were incubated in 20 µL of test plasma with gentle shaking. After washing, 1 µL of diluted FITC-conjugated anti-human IgG was added and incubated for another 30 min at room temperature. The sample was washed twice and then analyzed by flow cytometry. A negative control was used to generate flow cytometer settings prior to running prepared serum, according to the manufacturer’s specifications. Data analysis was conducted with HLA Fusion 2.0 software (One Lambda Inc., Canoga Park, CA).
To assess fetal specificity of maternal HLA, the PRA LABType SSO typing kit (One Lambda Inc., Canoga Park, CA) was used to obtain the fetal HLA genotype. Genomic DNA was obtained from cord blood. To collect cord blood, a small piece of snap-frozen umbilical cord was ground with liquid nitrogen in a mortar. Genomic DNA was isolated from the ground tissue using the DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA). For fetal HLA genotyping, locus-specific polymerase chain reaction (PCR) amplification was performed with 2 µl of genomic DNA from the umbilical cord (20 to 50 ng/µl), D-mix (One Lambda Inc., Canoga Park, CA), locus-specific amplification primers (One Lambda Inc., Canoga Park, CA) and Taq DNA Polymerase (Applied Biosystems, Carlsbad, CA). PCR was conducted during the following cycles: 1 cycle at 96 °C for 3 min; 5 cycles at 96 °C for 20 s, 60 °C for 20 s, and 72 °C for 20 s; 30 cycles at 96 °C for 10 s, 60 °C for 15 s, and 72 °C for 20 s; 1 cycle at 72 °C for 10 min. The amplified PCR products were then denatured and neutralized, followed by hybridization with LABType SSO beads at 60°C for 15 min. After three washes with wash buffer, the beads were labeled with R-PE Conjugated Streptavidin and washed twice. Data acquisition was performed with Luminex 100 (Luminex Corporation, Austin, TX), and data analysis was conducted with HLA Fusion 2.0 software (One Lambda Inc., Canoga Park, CA)
C4d Deposition Assessment
Immunohistochemistry was used to assess for C4d deposition on umbilical vein segments as specimens became available (n=4). Cases with a fetal death were excluded due to autolysis of tissue. Five-µm segments of formalin-fixed and paraffin-embedded umbilical cord were stained using a Ventana Discovery automatic staining system (Ventana Medical Systems, Tucson, AZ). A mouse monoclonal anti-human C4d antibody (1:100, ALPCO Diagnostics, Salem, NH) was used for immunostaining, and the Discovery DAB Map Kit (Ventana Medical Systems, Tucson, AZ) was used to detect the chromogen reaction of horseradish peroxidase. Positivity for C4d staining was defined by widespread circumferential venous epithelial staining.
CXCL-10/IP-10 Expression Determination
Plasma CXCL-10 concentrations were determined in samples collected longitudinally from cases (n=10 patients; n=28 samples) and the entire control population (n=175 patients; n= 749 samples) by ELISA. Immunoassays for CXCL-10 (R&D Systems, Minneapolis, MN) were used and maternal plasma samples were added to assay diluent RD1-56 (R&D Systems, Minneapolis, MN) in duplicate to plates pre-coated with monoclonal antibody specific for human CXCL-10. After two hours of incubation, each well was aspirated and washed a total of four times with wash buffer. Two-hundred µL of CXCL-10 conjugate was added to each well and incubated for two hours. Aspiration and wash were repeated four times. Two-hundred µL of substrate solution (R&D Systems, Minneapolis, MN) were then added to each well and incubated for 30 min. After the addition of 50 µL of stop solution, the optical density of each well was determined using a microplate reader, according to the manufacturer’s specifications. The inter-assay coefficient of variation (CV) was 3.6% while the intra-assay CV was 3.7%. The sensitivity of the assay for CXCL-10/IP-10 was <4.95 pg/mL.
Statistical Analysis
Medians and interquartile ranges were calculated for continuous variables and frequencies and percentages were calculated for categorical variables. The Fisher’s exact test, Chi-square test, and Mann-Whitney U-test were used to compare groups as appropriate. A p-value <0.05 was considered significant. Statistical analyses were performed using SPSS Version 15.0 software (SPSS, Inc., Chicago, IL).
Linear mixed models were used to test for differences in the log-transformed average plasma CXCL-10 concentration overall and across four gestational length-defined periods (<14 weeks, 14–16 weeks, 17–19 weeks, and 20–30 weeks), adjusting for factors significantly associated with MPFD [maternal age, body mass index (BMI), African-American ethnicity] and using a robust covariance matrix estimator. Analysis was performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC).
Results
Eighty percent of MPFD cases resulted in pregnancy loss (40% had a fetal death and 40% had a second-trimester spontaneous abortion). Patients with MPFD were significantly older, had a lower median gestational age at delivery, and lower median birthweight than those in the control group (p < 0.001 all) (Table I).
Table I.
Demographics and clinical characteristics of patients with uncomplicated pregnancy and massive perivillous fibrin deposition
| Uncomplicated pregnancies (n=175) |
MPFD (n=10) |
p-value | |
|---|---|---|---|
| Maternal Age (years) | 23 (20–26) | 31 (26–35) | <0.001 |
| African American | 151 (86%) | 10 (100%) | 0.4 |
| Nulliparity | 62 (35%) | 0 (0%) | 0.03 |
| Pre-pregnancy BMI (kg/m2) | 27 (23–32) | 29 (28–35) | 0.04 |
| Gestational Age at Delivery (weeks) | 39 (39–40) | 23 (17–29) | <0.001 |
| Birth weight (grams) | 3330 (3150–3555) | 277 (175–605) | <0.001 |
| Stillbirth (> 20 weeks) | 0 | 4 (40%) | --- |
| Miscarriage in the Second Trimester (<20 weeks) | 0 | 4 (40%) | --- |
| Fetal Growth Restriction | 0 | 4 (40%) | --- |
| Placental Abruption | 0 | 2 (20%) | --- |
Data are expressed as median (interquartile range) or number (percent).
MPFD: Massive perivillous fibrin deposition
BMI: Body mass index
Figure 1 and 2 demonstrated gross and microscopic section of placenta from MPFD case, respectively. Pathologic findings in MPFD cases are summarized in Table II. There was a significantly higher rate of plasma cell deciduitis [40% (4/10) vs. 8.6% (15/175), p=0.011] and fetal vascular thrombo-occlusive disease [30% (3/10) vs. 6% (11/175), p=0.03] in MPFD than in the control group. There was also a trend towards increased frequency of villitis of unknown etiology in pregnancies complicated by MPFD, although the difference did not reach statistical significance [10% (1/10), vs. 0.6% (1/175), p=0.1]. The frequency of chronic chorioamnionitis in MPFD, however, was not significantly different from that in the control group [20% (2/10) vs. 17% (30/175); p=0.7].
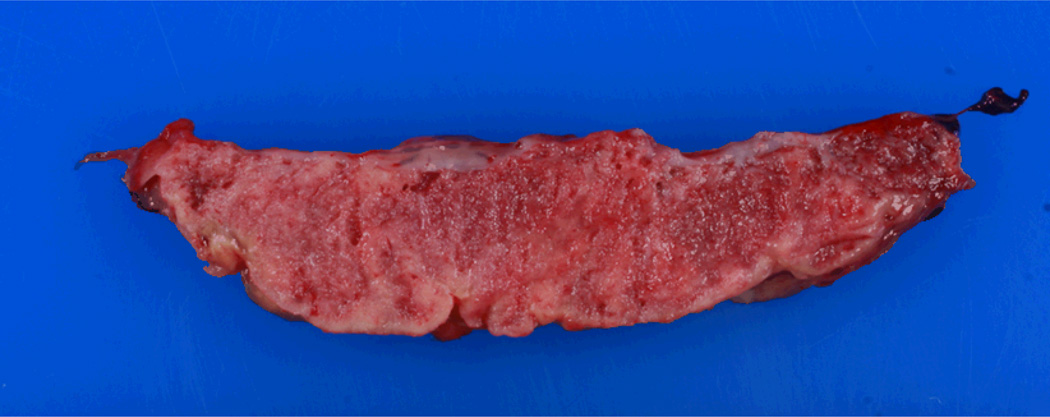
Figure 1.
Gross placental specimen from MPFD case #1. Note thickening and rubber-like texture. Fibrin deposition visualized grossly as yellow/avascular appearing tissue.
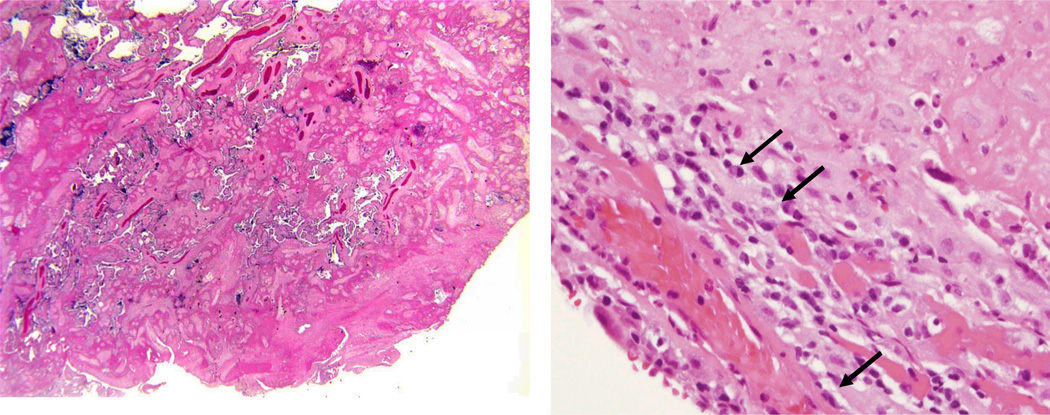
Figure 2.
Microscopic section of placenta from MPFD case #1. Note loss of normal villous architecture and encasement of remaining villi in pale-pink fibrin material (left). Area of chronic deciduitis with plasma cells (denoted by arrows) within large amount of fibrin deposition.This lesion is suggestive of maternal anti-fetal rejection (right).
Table II.
Frequency of pathologic diagnosis in massive perivillous fibrin deposition group and uncomplicated pregnancy group.
| Uncomplicated pregnancies (n=175) |
MPFD (n=10) |
p-value | |
|---|---|---|---|
| Deciduitis with plasma cells | 15 (8.6%) | 4 (40%) | 0.011 |
| Villitis of Unknown Etiology | 1 (0.6%) | 1 (10%) | 0.1 |
| Chronic chorioamnionitis | 30 (17%) | 2 (20%) | 0.7 |
| Acute chorioamnionitis | 42 (24%) | 5 (50%) | 0.1 |
| Fetal vascular thrombo-occlusive disease | 11 (6%) | 3 (30%) | 0.03 |
| Chronic villitis | 18 (10%) | 3 (30%) | 0.1 |
| Evidence of fetal rejection | 45 (26%) | 4 (40%) | 0.5 |
Data are expressed as number (percent).
Evidence for fetal rejection was defined as positive for either chronic chorioamnionitis, villitis, or deciduitis.
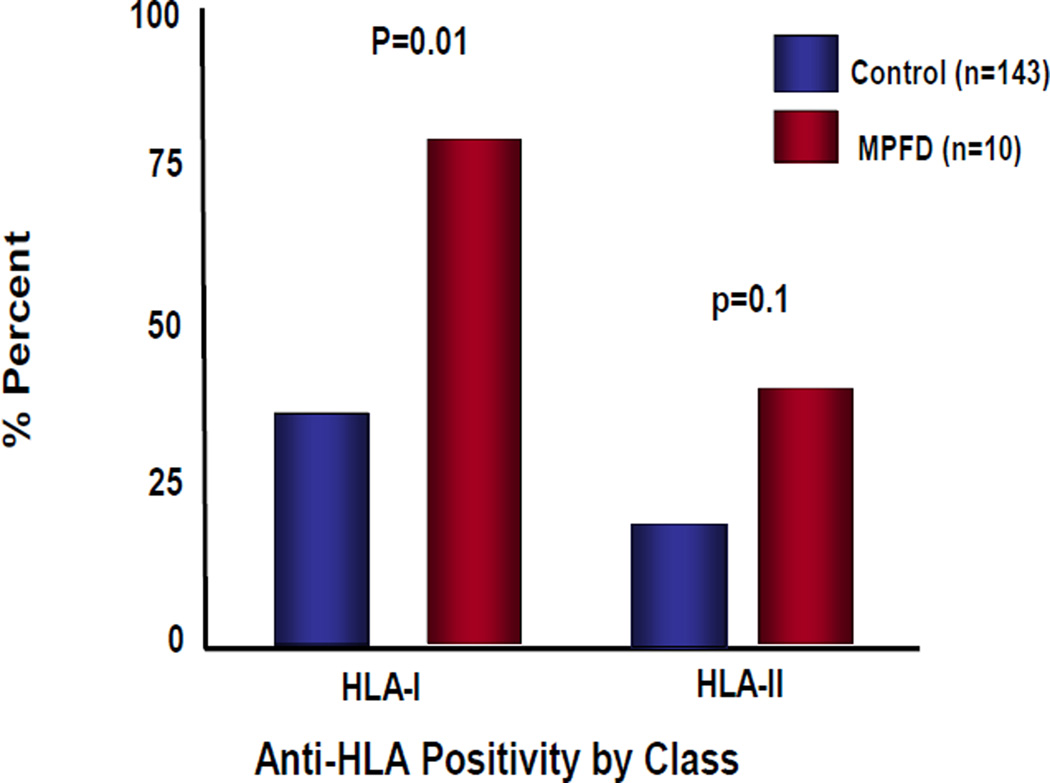
Figure 3 demonstrates the frequency of HLA Class I and HLA Class II seropositivity in patients with MPFD compared to women in the control group. Patients with MPFD had a significantly higher frequency of HLA Class I seropositivity than women with uncomplicated pregnancy [(80% (8/10) vs. 36.4% (52/143); p=0.01)]. There was no significant difference in the frequency of HLA class II seropositivity among 2 groups [40% (4/10) vs. 18.2% (26/143); p=0.1]. Furthermore, fetal specific HLA genotyping and identification of specific maternal HLA antibodies demonstrated the presence of maternal antibodies against fetal specific HLA antigen in all cases for which fetal tissues (umbilical cord and umbilical cord blood) were available (n=6). Table III and Table IV show the results for each MPFD case for fetal HLA specific HLA antigens and identification of maternal specific HLA antibodies.
Figure 3.
Rates of anti-HLA Class I and Class II positivity in maternal blood from MPFD cases and control, uncomplicated term-delivery patients.
Table III.
Fetal HLA-class I type at A, B, and C loci as determined from umbilical cord DNA compared to specific maternal anti-HLA class I antibodies. Maternal antibodies corresponding to fetal antigen are high-lighted/underlined. In all cases except one, maternal antibodies show specificity against fetal HLA antigens.
| Patient Number | HLA A | HLA B | HLA C | Maternal Serum anti-HLA class I Antibodies |
|---|---|---|---|---|
| 1 | 23,74 | 53,57 | 4,7 |  |
| 3 | 2,30 | 7,81 | 8,15 |  |
| 4 | 1,24 | 8,45 | 6,7 |  |
| 5 | 2,3 | 35,45 | 4,16 | 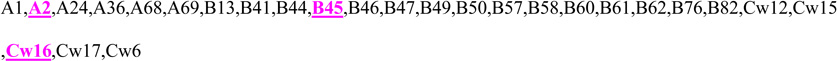 |
| 6 | 2,26 | 8,49 | 3,7 | B13,B27,B47,B48,B57,B60,B61,B7,B81 |
| 7 | 30,34 | 14,49 | 7,8 |
Table IV.
Fetal HLA-class II type at DQ, DR B1, and DR B345 loci as determined from umbilical cord DNA compared to specific maternal anti-HLA class II antibodies. Maternal antibodies corresponding to fetal antigen are high-lighted/underlined. Maternal specificity against fetal class II antigen was shown in 4/6 cases.
| Patient Number |
DQ | DR B1 | DR B345 | Maternal Serum anti-HLA class II Antibodies |
|---|---|---|---|---|
| 1 | 2 | 13,17 | 52 |  |
| 3 | 2,6 | 9,15 | 51,53 | 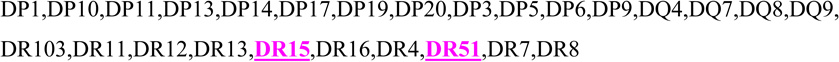 |
| 4 | 2,6 | 15,17 | 51,52 | |
| 5 | 5,6 | 13,15 | 51,52 | Negative |
| 6 | 3,5 | 1,13 | 52 | |
| 7 | 6 | 15 | 51 | Negative |
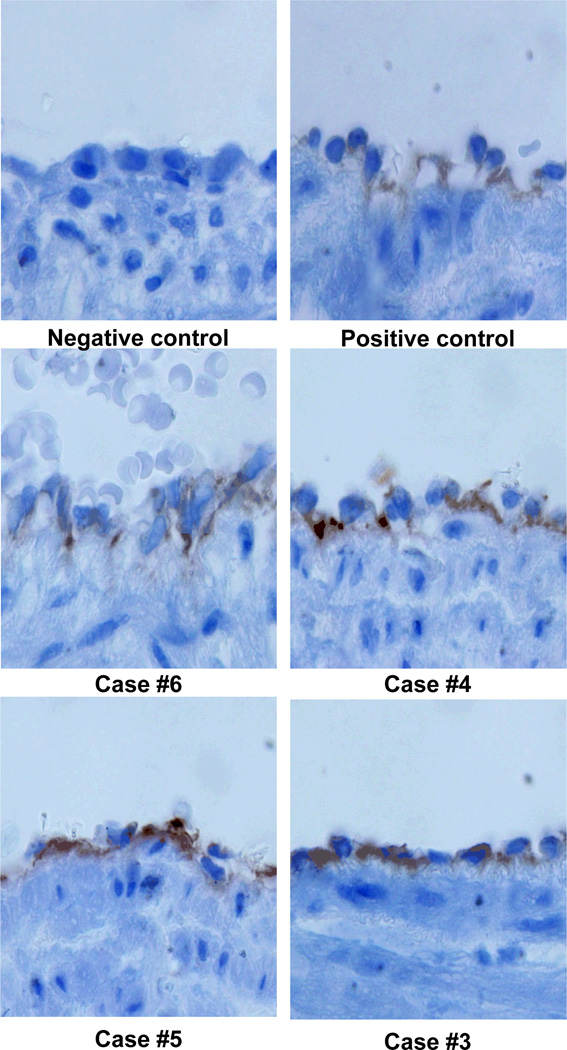
In four cases, the fetus was liveborn and umbilical cord specimens were available for immunostaining–all four cases demonstrated strongly positive C4d deposition in the umbilical vein specimen (Figure 4). Finally, the mean maternal plasma concentration of CXCL-10 was significantly elevated in MPFD cases relative to controls both overall (p<0.001) and further as a function of gestational age (<14 weeks, 14–16 weeks, 17–19 weeks, and 20–30 weeks) (p=0.01); both relationships remain significant after adjustment for maternal age, African-American ethnicity, and pre-pregnancy BMI (p=0.04, p=0.03 respectively).
Figure 4.
C4d deposition on umbilical vein endothelium. All 4 cases demonstrate strong reactivity by immunohistochemistry (brown).
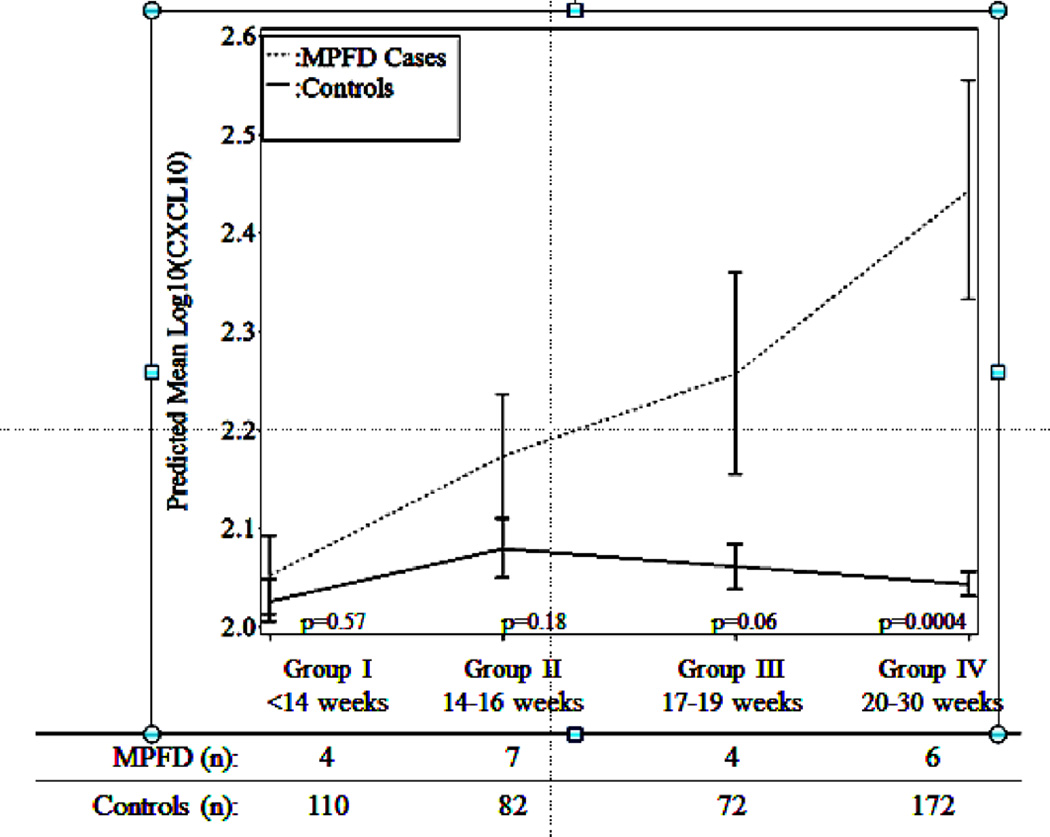
The mean maternal plasma concentration of CXCL-10 remained relatively stable over time and negligibly declined following the 16th week of gestation among controls, while, the mean plasma concentration among cases increased over time starting from 17–19 weeks (p=0.006) and became significantly higher than that in control group at 20–30 weeks of gestation (p=0.004; Figure 5). Thus, evidence of increasing maternal plasma concentration of CXCL-10 was present in patients with MPFD affected pregnancies before the diagnosis of MPFD. Table V summarizes the clinical course, placental pathology, and laboratory assays for cases with MPFD.
Figure 5.
Estimated mean +/− standard error of plasma concentrations (log 10) of CXCL-10 in MPFD and uncomplicated pregnancies by gestational age interval. Estimated mean CXCL-10 concentrations over time are adjusted by maternal age, body mass index and African-American ethnicity; P-values characterize the difference in estimated mean concentrations at each gestational age interval determined by the linear mixed effects model.
Table V.
Clinical description of massive perivillous fibrin deposition cases.
| Case Number |
Age | Gravida, Parity |
GA at Delivery (weeks) |
Clinical Description | Birth Weight (grams, Percentile for GA) |
Placental Pathology | + HLA | Genotype Confirmation |
+C4d Umbilical Vein |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VUE | PD | Other | I | II | I | II | |||||||
| 1 | 35 | G 12 P 8-2-1-8 | 23+1 | The fetus has decreased growth and progressive deterioration of Doppler parameters starting at 20 weeks gestation. Intrauterine fetal death diagnosed at 23 weeks. | 274 (1%) | Yes | Yes | Yes | Yes | Yes | Yes | N/A | |
| 2 | 34 | G 11 P 8-1-1-8 | 28+2 | The fetus has decreased growth and progressive deterioration of Doppler parameters starting at 20 weeks gestation. Fetal death diagnosed and labor was induced at 28 weeks | 454 (1%) | Yes | Yes | Acute Chorioamnionitis | Yes | Yes | N/A | N/A | N/A |
| 3 | 33 | G 10 P 7-1-1-7 | 38+1 | Spontaneous labor at term. | 3285 (51.5%) | Yes | Yes | Chronic Chorioamnionitis | Yes | Yes | Yes | Yes | Yes |
| 4 | 24 | G 4 P 2-0-1-2 | 15+6 | Presented with ruptured membranes and was induced for inevitable abortion. | 150 | No | No | Acute Chorioamnionitis; Chronic Deciduitis without Plasma Cells | Yes | Yes | Yes | Yes | Yes |
| 5 | 27 | G 3 P 0-0-2-0 | 30+0 | Presented with fetal growth restriction, heavy vaginal bleeding and clinical placental abruption. Emergency cesarean delivery was performed. | 755 (1%) | No | No | Yes | No | Yes | No | Yes | |
| 6 | 22 | G 2 P 0-0-1-0 | 22+3 | Short cervix was noted at 20 weeks; membranes ruptured with spontaneous labor at 22 weeks. Intrapartum demise with delivery of a stillborn infant. | 448 (34%) | No | No | Acute Chorioamnionitis | Yes | No | No | Yes | Yes |
| 7 | 28 | G 11 P 0-1-9-1 | 23+6 | The fetus has thickening placenta, multiple placental lacunae, and oligohydramnios at 18 weeks. Fetal death diagnosed and labor was induced at 23+ weeks | 277 (1%) | No | No | Acute Chorioamnionitis Chronic Deciduitis without Plasma Cells | Yes | No | Yes | No | N/A |
| 8 | 43 | G 13 P 3-3-6-4 | 16+4 | Presented with rupture of fetal membranes and fetal demise. | Unknown | No | Yes | Yes | No | N/A | N/A | N/A | |
| 9 | 35 | G 7 P 0-0-6-0 | 17+3 | Cervical length of 0 mm; A rescue cerclage was placed but membranes ruptured shortly afterwards. Induction for inevitable abortion. | 160 | No | No | No | No | N/A | N/A | N/A | |
| 10 | 29 | G 3 P 1-1-0-1 | 17+2 | Presented with abdominal pain and vaginal bleeding. Fetal demise was diagnosed and patient was induced. | 190 | No | No | Acute Chorioamnionitis Chronic Deciduitis without Plasma Cells | No | No | N/A | N/A | N/A |
Cases #1–3 are pregnancies in the same patient, VUE: Villitis of Unknown Etiology, PD: Deciduitis with Plasma Cells
Discussion
Principal findings of the study
1) The frequency of plasma cell deciduitis and fetal vascular thrombo-occlusive disease was significantly higher in the placenta of patients with MPFD than in the control group; 2) patients with MPFD had a significantly higher frequency of maternal anti-HLA class I seropositivity during the second trimester than in those in the control group; 3) maternal plasma antibodies against fetal HLA antigens class I or II were identified in all cases of MPFD; 4) C4d deposition in the umbilical vein was documented in all cases of MPFD; and 5) the mean maternal plasma concentration of CXCL-10 in MPFD cases was significantly higher than in the control group. Taken together, these findings suggest that antibody-mediated maternal anti-fetal rejection operates in cases of maternal floor infarction.
Maternal floor infarction and massive perivillous fibrin deposition
Maternal floor infarction (MFI) was originally described by Benirschke in 1961 as a passing comment accompanied by a figure in an article focused on the examination of the placenta.1 He described a lesion on the maternal surface of the placenta, in which the thin layer of the decidua basalis is covered by small amounts of calcium and fibrin.1 Benirschke suggested that lack of fissuring of the decidua basalis in a term placenta was abnormal, and he coined the term “maternal floor infarction” to emphasize that the deposition of material occurred mainly in the villous tree next to the placental floor.1 Subsequently, Benirschke and Driscoll in 1967 described the lesion in greater detail.2 Fox proposed in 1978 that MFI was a postmortem change of the placenta of stillborns,16 a concept which has been abandoned.
The term “MFI” recognizes that the major feature is deposition of fibrinoid material around the villous tree.1 This material prevents normal gas and nutritive exchange between the maternal and fetal circulations, and consequently can lead to fetal growth restriction3, 4, 6, 7, 11–15 and death.3, 4, 6, 7, 10, 12, 14–18, 20–22 Naeye R et al. reported that some cases develop rapidly in the third trimester because fetal death was not accompanied by fetal growth restriction.6
MPFD and MFI are considered to represent the same process with different severity. Although the term “infarction” has been used repeatedly, several pathologists have emphasized that the lesion does not represent an infarction; yet the term continues to be used.6
Since the original descriptions, the diagnosis of these lesions has been subjective. In 2002, Katzman and Genest proposed semi quantitative histologic criteria based on the review of 80 placentas.3 The frequency of these lesions (MPFD/MFI) has been estimated to be 0.03–0.5% of all deliveries.3
Several mechanisms of disease leading to MPFD have been proposed, including: 1) infection;87, 88 2) cytoxicity due to extravillous trophoblast (formerly referred to as “X cells”);8 3) autoimmune disorders;7 4) coagulation or fibrinolytic disorders;89 and 5) imbalance of angiogenic/antiangiogenic factors.90 The hypothesis that polymorphisms in fibrinolysis or fibrinolysis inhibitor genes might be more frequent in MPFD placentas has not been supported in a study focusing on DNA variants in genes involved in fibrinolysis (PAI-1, thrombin activated fibrinolysis inhibitor, plasminogen activator urokinase, and tissue plasminogen activator).89
Plasma cell deciduitis in MPFD/MFI
We report herein an association between MPFD/MFI and plasma cell deciduitis, a placental lesion associated with maternal anti-fetal rejection.80 Previous reports also demonstrated the association of this placental lesion and preterm birth.53, 80
Antibody-mediated maternal anti-fetal rejection
Antibody-mediated maternal anti-fetal rejection is thought to result from maternal anti-fetal IgG crossing the placenta and inducing a fetal systemic inflammatory response.54 A requirement for antibody-mediated rejection is the presence of antibodies against fetal antigens. We screened for such antibodies using the HLA PRA assay against HLA Class I and HLA Class II antigens. This test has been used to test the recipient for the presence of circulating antibodies before organ transplantation. These antibodies identify patients who are already sensitized against antigens of potential donors and their presence identify patients at risk for rejection and graft failure.91, 92
Sensitization against HLA Class I and Class II antigens may occur through prior transfusions.93, 94 However, pregnancy is a unique state in which there is evidence of bidirectional cell traffic between the mother and fetus.95–98 Indeed, this sensitization against fetal antigens of paternal origin may explain why patients with a prior pregnancy have a higher rate of rejection or graft failure than women who have not been pregnant. We have previously shown that the likelihood of HLA PRA positivity is greater in multiparous than in nulliparous women, and males.99, 100 In the study herein, we demonstrated a higher HLA-class I seropositivity in women with MPFD than in the control group. While HLA-class II seropositivity was higher in patients with MPFD, compared to controls, this finding did not reach statistical significance.
A positive HLA PRA test indicates the presence of circulating antibodies, but does not provide specificity as to the nature of the antibody and the presence of the antigen in the fetus, both of which would be a requirement for maternal anti-fetal rejection to occur. To address this, we determined the fetal HLA status at the A, B, and C loci from umbilical cord DNA and then compared the results to specific HLA antibodies detected in maternal serum using multiplex technology. Among the six patients in whom umbilical cord specimens were available to assess the fetal HLA genotype, all exhibited antibodies specific to the fetal HLA class I or HLA class II antigens.
Even if specific antibodies against fetal antigens are present, activation of complement is required for tissue damage. Indeed, complement activation is an integral part of antibody mediated rejection. We report here C4d immunostaining of the umbilical vein endothelium. C4d deposition is indicative of activation of the classical pathway of complement, and is unique in that it covalently binds to the endothelial basement membranes, leaving immunologic evidence that antibody-mediated complement activation has occurred.101 C4d deposition in peritubular capillaries has been shown to be key in identifying evidence of graft rejection in renal transplants.78, 102 In the current study, all cases with MPFD examined (n=4) showed strong immunoreactivity for C4d in the umbilical vein endothelium. We have previously reported that C4d deposition in umbilical vein endothelium was significantly more frequent in preterm labor (77.1%) than in term labor (11.4%).54
Chemokine associated with rejection is elevated in the maternal circulation in MPFD
CXCL-10 is a chemokine of the CXC family which has both pro-inflammatory and anti-angiogenic properties.103 Overexpression of this chemokine has been demonstrated in serum/plasma,104, 105 urine,106 peripheral white blood cells of patients who have received a transplant.107–112 In addition, this chemokine has also been found within transplanted organs during the course of rejection.103, 107, 108, 110, 113, 114 These findings, coupled with gene deletion experiments have shown that CXCL-10 is an important mediator in allograft rejection.115 During normal pregnancy, the plasma concentration of this chemokine is higher than in non-pregnant women. In the current study, we found that the mean maternal plasma CXCL-10 concentration was higher in MPFD than in the control group.116
Conclusion
Massive perivillous fibrin deposition is associated with: 1) plasma cell deciduitis; 2) the presence of specific anti-HLA antibodies in maternal blood to fetal antigens; 3) evidence of antibody-mediated complement activation on umbilical vein endothelium; and 4) elevation of a maternal plasma concentration of CXCL-10. Collectively, our results support the concept that MPFD is a unique state in which there is maternal anti-fetal rejection. This work has implication for understanding the mechanism of disease, the discovery of biomarkers for patient at risk and potential therapeutic intervention in this serious placental disorder.
Acknowledgement
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
References
- 1.Bernirschke K. Examination of the placenta. Obstet Gynecol. 1961;18:1961. [Google Scholar]
- 2.Benirschke K, Driscoll SG. The Pathology of the Human Placenta. Berlin: Springer-Verlag; 1967. [Google Scholar]
- 3.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol. 2002;5:159–164. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 4.Andres RL, Kuyper W, Resnik R, Piacquadio KM, Benirschke K. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163:935–938. doi: 10.1016/0002-9378(90)91100-q. [DOI] [PubMed] [Google Scholar]
- 5.Katz VL, Bowes WA, Jr, Sierkh AE. Maternal floor infarction of the placenta associated with elevated second trimester serum alpha-fetoprotein. Am J Perinatol. 1987;4:225–228. doi: 10.1055/s-2007-999778. [DOI] [PubMed] [Google Scholar]
- 6.Naeye RL. Maternal floor infarction. Hum Pathol. 1985;16:823–828. doi: 10.1016/s0046-8177(85)80254-9. [DOI] [PubMed] [Google Scholar]
- 7.Bendon RW, Hommel AB. Maternal floor infarction in autoimmune disease: two cases. Pediatr Pathol Lab Med. 1996;16:293–297. [PubMed] [Google Scholar]
- 8.Vernof KK, Benirschke K, Kephart GM, Wasmoen TL, Gleich GJ. Maternal floor infarction: relationship to X cells, major basic protein, and adverse perinatal outcome. Am J Obstet Gynecol. 1992;167:1355–1363. doi: 10.1016/s0002-9378(11)91716-5. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DM, Crouch EC, Curran EM, Farmer DR. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am J Pathol. 1990;136:855–865. [PMC free article] [PubMed] [Google Scholar]
- 10.Bane AL, Gillan JE. Massive perivillous fibrinoid causing recurrent placental failure. BJOG. 2003;110:292–295. [PubMed] [Google Scholar]
- 11.Nickel RE. Maternal floor infarction: an unusual cause of intrauterine growth retardation. Am J Dis Child. 1988;142:1270–1271. doi: 10.1001/archpedi.1988.02150120024020. [DOI] [PubMed] [Google Scholar]
- 12.Mandsager NT, Bendon R, Mostello D, Rosenn B, Miodovnik M, Siddiqi TA. Maternal floor infarction of the placenta: prenatal diagnosis and clinical significance. Obstet Gynecol. 1994;83:750–754. [PubMed] [Google Scholar]
- 13.Redline RW, Jiang JG, Shah D. Discordancy for maternal floor infarction in dizygotic twin placentas. Hum Pathol. 2003;34:822–824. doi: 10.1016/s0046-8177(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 14.Sebire NJ, Backos M, El Gaddal S, Goldin RD, Regan L. Placental pathology, antiphospholipid antibodies, and pregnancy outcome in recurrent miscarriage patients. Obstet Gynecol. 2003;101:258–263. doi: 10.1016/s0029-7844(02)02385-2. [DOI] [PubMed] [Google Scholar]
- 15.Sebire NJ, Backos M, Goldin RD, Regan L. Placental massive perivillous fibrin deposition associated with antiphospholipid antibody syndrome. BJOG. 2002;109:570–573. doi: 10.1111/j.1471-0528.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 16.Fox H, Elston CW. Pathology of the placenta. Major Probl Pathol. 1978;7:1–491. [PubMed] [Google Scholar]
- 17.Clewell WH, Manchester DK. Recurrent maternal floor infarction: a preventable cause of fetal death. Am J Obstet Gynecol. 1983;147:346–347. doi: 10.1016/0002-9378(83)91130-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, Kim CJ. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Adnani M, Kiho L, Scheimberg I. Recurrent placental massive perivillous fibrin deposition associated with polymyositis: a case report and review of the literature. Pediatr Dev Pathol. 2008;11:226–229. doi: 10.2350/07-06-0306.1. [DOI] [PubMed] [Google Scholar]
- 22.Hung NA, Jackson C, Nicholson M, Highton J. Pregnancy-related polymyositis and massive perivillous fibrin deposition in the placenta: are they pathogenetically related? Arthritis Rheum. 2006;55:154–156. doi: 10.1002/art.21710. [DOI] [PubMed] [Google Scholar]
- 23.Becroft DM, Thompson JM, Mitchell EA. Placental infarcts, intervillous fibrin plaques, and intervillous thrombi: incidences, cooccurrences, and epidemiological associations. Pediatr Dev Pathol. 2004;7:26–34. doi: 10.1007/s10024-003-4032-3. [DOI] [PubMed] [Google Scholar]
- 24.Eklund KK. Monovalent cation-induced fusion of acidic phospholipid vesicles. Chem Phys Lipids. 1990;52:199–206. doi: 10.1016/0009-3084(90)90115-8. [DOI] [PubMed] [Google Scholar]
- 25.Schnier J, Gewitz HS, Behrens SE, Lee A, Ginther C, Leighton T. Isolation and characterization of Bacillus stearothermophilus 30S and 50S ribosomal protein mutations. J Bacteriol. 1990;172:7306–7309. doi: 10.1128/jb.172.12.7306-7309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 27.von Rango U. Fetal tolerance in human pregnancy--a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115:21–32. doi: 10.1016/j.imlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 29.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003;1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 31.Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunol Rev. 2006;212:330–343. doi: 10.1111/j.0105-2896.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 32.Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS One. 2007;2:e382. doi: 10.1371/journal.pone.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terness P, Kallikourdis M, Betz AG, Rabinovich GA, Saito S, Clark DA. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 34.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 35.Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernandez N, Arck PC. Dendritic cells: key to fetal tolerance? Biol Reprod. 2007;77:590–598. doi: 10.1095/biolreprod.107.060632. [DOI] [PubMed] [Google Scholar]
- 36.Makrigiannakis A, Karamouti M, Drakakis P, Loutradis D, Antsaklis A. Fetomaternal immunotolerance. Am J Reprod Immunol. 2008;60:482–496. doi: 10.1111/j.1600-0897.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 37.Petroff MG. Immune interactions at the maternal-fetal interface. J Reprod Immunol. 2005;68:1–13. doi: 10.1016/j.jri.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betz AG. Immunology. Have you seen your mother, baby. Science. 2010;330:1635–1636. doi: 10.1126/science.1200406. [DOI] [PubMed] [Google Scholar]
- 41.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85:71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol. 2013;69:346–358. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn KH, Parast MM. Decidual Regulatory T Cells in Placental Pathology and Pregnancy Complications. Am J Reprod Immunol. 2013 doi: 10.1111/aji.12077. [DOI] [PubMed] [Google Scholar]
- 45.Linscheid C, Petroff MG. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am J Reprod Immunol. 2013;69:304–314. doi: 10.1111/aji.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer PS, Hakam SM, Laissue PP, Jabeen A, Jain P, Hayrabedyan S, Todorova K, Blanch A, McElhinney JM, Muhandiram N, Alkhatib S, Dealtry GB, Miranda-Sayago JM, Fernandez N. Key cellular components and interactive histocompatibility molecules regulating tolerance to the fetal allograft. Am J Reprod Immunol. 2012;68:95–99. doi: 10.1111/j.1600-0897.2012.01138.x. [DOI] [PubMed] [Google Scholar]
- 47.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 48.Redman CW. Immune factors and recurrent abortion: a review. Am J Reprod Immunol. 1983;4:179–181. doi: 10.1111/j.1600-0897.1983.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 49.Kohut KG, Anthony MN, Salafia CM. Decidual and placental histologic findings in patients experiencing spontaneous abortions in relation to pregnancy order. Am J Reprod Immunol. 1997;37:257–261. doi: 10.1111/j.1600-0897.1997.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 50.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin LP, Fan DX, Li DJ. Regulation of costimulatory signal in maternal-fetal immune tolerance. Am J Reprod Immunol. 2011;66:76–83. doi: 10.1111/j.1600-0897.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 52.Riley JK, Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 2008;59:371–387. doi: 10.1111/j.1600-0897.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 53.Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009;12:16–21. doi: 10.2350/07-04-0270.1. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, Yoo W, Chaiworapongsa T, Mittal P, Hassan SS, Kim CJ. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kilpatrick DC. Immune mechanisms and pre-eclampsia. Lancet. 1987;2:1460–1461. doi: 10.1016/s0140-6736(87)91156-1. [DOI] [PubMed] [Google Scholar]
- 58.Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49–61. doi: 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- 59.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 60.Saito S, Sakai M, Sasaki Y, Nakashima A, Shiozaki A. Inadequate tolerance induction may induce pre-eclampsia. J Reprod Immunol. 2007;76:30–39. doi: 10.1016/j.jri.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73:1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 62.Kim IK, Bedi DS, Denecke C, Ge X, Tullius SG. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation. 2008;86:889–894. doi: 10.1097/TP.0b013e318186ac4a. [DOI] [PubMed] [Google Scholar]
- 63.Howell WM, Carter V, Clark B. The HLA system: immunobiology, HLA typing, antibody screening and crossmatching techniques. J Clin Pathol. 2010;63:387–390. doi: 10.1136/jcp.2009.072371. [DOI] [PubMed] [Google Scholar]
- 64.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 65.Ho EK, Vlad G, Colovai AI, Vasilescu ER, Schwartz J, Sondermeijer H, Burke E, Marboe CC, Itescu S, Suciu-Foca N, Mancini D. Alloantibodies in heart transplantation. Hum Immunol. 2009;70:825–829. doi: 10.1016/j.humimm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, Terasaki PI, Rose ML. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 67.Zeevi A, Girnita A, Duquesnoy R. HLA antibody analysis: sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res. 2006;36:255–264. doi: 10.1385/IR:36:1:255. [DOI] [PubMed] [Google Scholar]
- 68.Rowshani AT, Bemelman FJ, Lardy NM, Ten Berge IJ. Humoral immunity in renal transplantation: clinical significance and therapeutic approach. Clin Transplant. 2008;22:689–699. doi: 10.1111/j.1399-0012.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 69.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000;69:1370–1374. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 70.Betkowski AS, Graff R, Chen JJ, Hauptman PJ. Panel-reactive antibody screening practices prior to heart transplantation. J Heart Lung Transplant. 2002;21:644–650. doi: 10.1016/s1053-2498(01)00422-3. [DOI] [PubMed] [Google Scholar]
- 71.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 72.Panigrahi A, Deka R, Bhowmik D, Tiwari SC, Mehra NK. Immunological monitoring of posttransplant allograft sensitization following living related donor renal transplantation. Transplant Proc. 2004;36:1336–1339. doi: 10.1016/j.transproceed.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 73.Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M. Post-transplant anti-HLA class II antibodies as risk factor for late kidney allograft failure. Am J Transplant. 2006;6:2316–2320. doi: 10.1111/j.1600-6143.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Ishida H, Yamaguchi Y, Tanabe K. Poor graft outcome in recipients with de novo donor-specific anti-HLA antibodies after living related kidney transplantation. Transpl Int. 2008;21:1145–1152. doi: 10.1111/j.1432-2277.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen HS, Witvliet MD, Steffensen R, Haasnoot GW, Goulmy E, Christiansen OB, Claas F. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J Reprod Immunol. 2010;87:67–73. doi: 10.1016/j.jri.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 77.Ionescu DN, Girnita AL, Zeevi A, Duquesnoy R, Pilewski J, Johnson B, Studer S, McCurry KR, Yousem SA. C4d deposition in lung allografts is associated with circulating anti-HLA alloantibody. Transpl Immunol. 2005;15:63–68. doi: 10.1016/j.trim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, Land W, Albert E. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 79.Troxell ML, Weintraub LA, Higgins JP, Kambham N. Comparison of C4d immunostaining methods in renal allograft biopsies. Clin J Am Soc Nephrol. 2006;1:583–591. doi: 10.2215/CJN.00900805. [DOI] [PubMed] [Google Scholar]
- 80.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudzinski E, Gilroy M, Newbill C, Morgan T. Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr Dev Pathol. 2013;16:7–13. doi: 10.2350/12-05-1195-OA.1. [DOI] [PubMed] [Google Scholar]
- 83.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 84.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 85.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, Lipsett J, Staples A. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31:292–295. doi: 10.1016/s0046-8177(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 87.Robb JA, Benirschke K, Barmeyer R. Intrauterine latent herpes simplex virus infection: I. Spontaneous abortion. Hum Pathol. 1986;17:1196–1209. doi: 10.1016/S0046-8177(86)80561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gersell DJ. Chronic villitis, chronic chorioamnionitis, and maternal floor infarction. Semin Diagn Pathol. 1993;10:251–266. [PubMed] [Google Scholar]
- 89.Uxa R, Baczyk D, Kingdom JC, Viero S, Casper R, Keating S. Genetic polymorphisms in the fibrinolytic system of placentas with massive perivillous fibrin deposition. Placenta. 2010;31:499–505. doi: 10.1016/j.placenta.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, Dong Z, Hassan SS, Chaiworapongsa T. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol. 2013;208:310 e311-310 e311. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 92.Fuggle SV, Martin S. Tools for human leukocyte antigen antibody detection and their application to transplanting sensitized patients. Transplantation. 2008;86:384–390. doi: 10.1097/TP.0b013e31817c90f5. [DOI] [PubMed] [Google Scholar]
- 93.Scornik JC, Ireland JE, Howard RJ, Pfaff WW. Assessment of the risk for broad sensitization by blood transfusions. Transplantation. 1984;37:249–253. doi: 10.1097/00007890-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Rebibou JM, Chabod J, Alcalay D, Coussediere MC, Deteix P, Touchard G, Dupont I, Thevenin C, Chalopin JM, Tiberghien P. Flow cytometric evaluation of pregnancy-induced anti-HLA immunization and blood transfusion-induced reactivation. Transplantation. 2002;74:537–540. doi: 10.1097/00007890-200208270-00018. [DOI] [PubMed] [Google Scholar]
- 95.Sunku Cuddapah CS, Gadi VK, de Laval de Lacoste B, Guthrie KA, Nelson JL. Maternal and fetal microchimerism in granulocytes. Chimerism. 2010;1:11–14. doi: 10.4161/chim.1.1.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bianchi DW. Fetomaternal cell trafficking: a new cause of disease? Am J Med Genet. 2000;91:22–28. doi: 10.1002/(sici)1096-8628(20000306)91:1<22::aid-ajmg4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 97.Berry SM, Hassan SS, Russell E, Kukuruga D, Land S, Kaplan J. Association of maternal histocompatibility at class II HLA loci with maternal microchimerism in the fetus. Pediatr Res. 2004;56:73–78. doi: 10.1203/01.PDR.0000129656.10005.A6. [DOI] [PubMed] [Google Scholar]
- 98.Yan Z, Aydelotte T, Gadi VK, Guthrie KA, Nelson JL. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism. Arthritis Rheum. 2011;63:640–644. doi: 10.1002/art.30160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gold BG, Densmore V, Shou W, Matzuk MM, Gordon HS. Immunophilin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J Pharmacol Exp Ther. 1999;289:1202–1210. [PubMed] [Google Scholar]
- 100.Lopes LB, Fabron-Jr A, Chiba AK, Ruiz MO, Bordin JO. Impact of using different laboratory assays to detect human leukocyte antigen antibodies in female blood donors. Transfusion. 2010;50:902–908. doi: 10.1111/j.1537-2995.2009.02523.x. [DOI] [PubMed] [Google Scholar]
- 101.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 102.Haas M. The significance of C4d staining with minimal histologic abnormalities. Curr Opin Organ Transplant. 2010;15:21–27. doi: 10.1097/MOT.0b013e3283342ebd. [DOI] [PubMed] [Google Scholar]
- 103.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, Shaked A, Wille K, Lama VN, Milstone A, Ware LB, Orens J, Weinacker A, Demissie E, Bellamy S, Kawut SM, Hancock WW, Christie JD. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9:389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rotondi M, Rosati A, Buonamano A, Lasagni L, Lazzeri E, Pradella F, Fossombroni V, Cirami C, Liotta F, La Villa G, Serio M, Bertoni E, Salvadori M, Romagnani P. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4:1466–1474. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 106.Matz M, Beyer J, Wunsch D, Mashreghi MF, Seiler M, Pratschke J, Babel N, Volk HD, Reinke P, Kotsch K. Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function. Kidney Int. 2006;69:1683–1690. doi: 10.1038/sj.ki.5000343. [DOI] [PubMed] [Google Scholar]
- 107.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lazzeri E, Rotondi M, Mazzinghi B, Lasagni L, Buonamano A, Rosati A, Pradella F, Fossombroni V, La Villa G, Gacci M, Bertoni E, Serio M, Salvadori M, Romagnani P. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation. 2005;79:1215–1220. doi: 10.1097/01.tp.0000160759.85080.2e. [DOI] [PubMed] [Google Scholar]
- 109.Mao Y, Wang M, Zhou Q, Jin J, Wang Y, Peng W, Wu J, Shou Z, Chen J. CXCL10 and CXCL13 Expression were highly up-regulated in peripheral blood mononuclear cells in acute rejection and poor response to anti-rejection therapy. J Clin Immunol. 2011;31:414–418. doi: 10.1007/s10875-010-9500-8. [DOI] [PubMed] [Google Scholar]
- 110.Tan J, Zhou G. Chemokine receptors and transplantation. Cell Mol Immunol. 2005;2:343–349. [PubMed] [Google Scholar]
- 111.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, Craddock C, Moss PA. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 113.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe DM. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 114.Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, Pfalzer B, Schneider A, Thaiss F, Mack M, Conrad S, Huland H, Helmchen U, Stahl RA. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–1350. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 115.Baker MS, Chen X, Rotramel AR, Nelson JJ, Lu B, Gerard C, Kanwar Y, Kaufman DB. Genetic deletion of chemokine receptor CXCR3 or antibody blockade of its ligand IP-10 modulates posttransplantation graft-site lymphocytic infiltrates and prolongs functional graft survival in pancreatic islet allograft recipients. Surgery. 2003;134:126–133. doi: 10.1067/msy.2003.213. [DOI] [PubMed] [Google Scholar]
- 116.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, Mittal P, Edwin S, Yoon BH, Kim CJ, Mazaki-Tovi S, Chaiworapongsa T, Hassan SS. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]