Abstract
IMPORTANCE
Clinical trials of prophylactic implantable cardioverter-defibrillators (ICDs) have included a minority of patients with a left ventricular ejection fraction (LVEF) between 30% and 35%. Because a large number of ICDs in the United States are implanted in such patients, it is important to study survival associated with this therapy.
OBJECTIVE
To characterize patients with LVEF between 30% and 35% and compare the survival of those with and without ICDs.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study of Medicare beneficiaries in the National Cardiovascular Data Registry ICD registry (January 1, 2006, through December 31, 2007) with an LVEF between 30% and 35% who received an ICD during a heart failure hospitalization and similar patients in the Get With The Guidelines–Heart Failure (GWTG-HF) database (January 1, 2005, through December 31, 2009) with no ICD. The analysis was repeated in patients with an LVEF less than 30%. There were 3120 patients with an LVEF between 30% and 35% (816 in matched cohorts) and 4578 with an LVEF less than 30% (2176 in matched cohorts). Propensity score matching and Cox models were applied.
MAIN OUTCOMES AND MEASURES
The primary outcome was all-cause mortality; data were obtained from Medicare claims through December 31, 2011.
RESULTS
There were no significant differences in the baseline characteristics of the matched groups (n = 408 for both groups). Among patients with an LVEF between 30% and 35%, there were 248 deaths in the ICD Registry group, within a median follow-up of 4.4 years (interquartile range, 2.7-4.9) and 249 deaths in the GWTG HF group, within a median follow-up of 2.9 years (interquartile range, 2.1-4.4). The risk of all-cause mortality in patients with an LVEF between 30% and 35% and an ICD was significantly lower than that in matched patients without an ICD (3-year mortality rates: 51.4% vs 55.0%; hazard ratio, 0.83 [95% CI, 0.69-0.99]; P = .04). Presence of an ICD also was associated with better survival in patients with an LVEF less than 30% (3-year mortality rates: 45.0% vs 57.6%; 634 and 660 total deaths; hazard ratio, 0.72 [95% CI, 0.65-0.81]; P < .001) (P = .20 for interaction).
CONCLUSIONS AND RELEVANCE
Among Medicare beneficiaries hospitalized for heart failure and with an LVEF between 30% and 35% and less than 30%, survival at 3 years was better in patients who received a prophylactic ICD than in comparable patients with no ICD. These findings support guideline recommendations to implant prophylactic ICDs in eligible patients with an LVEF of 35% or less.
Patients with heart failure attributable to left ventricular systolic dysfunction have a substantial risk of sudden cardiac death.1-3 Although randomized clinical trials have established the implantable cardioverter-defibrillator (ICD) as the best therapy currently available to prevent sudden cardiac death in patients with heart failure, some uncertainties remain regarding the use of prophylactic ICDs in patients seen in clinical practice.4-8 Several of these uncertainties involve the survival benefit associated with the ICD in patient groups not well represented in clinical trials.
One important group is patients with a left ventricular ejection fraction (LVEF) between 30% and 35%. Although most of the randomized clinical trials of prophylactic ICDs have required an LVEF of 35% or less for enrollment, the median LVEF of enrolled patients was well below 30%.4-8 Because a large number of prophylactic ICDs in the United States are implanted in patients with an LVEF between 30% and 35%, understanding outcomes associated with the ICDs in such patients is important.9,10 The Centers for Medicare & Medicaid Services have designated patients with an LVEF between 30% and 35% as an important subgroup for whom more data on ICD effectiveness are needed.9,10
Using the National Cardiovascular Data Registry (NCDR) ICD registry and the American Heart Association Get With The Guidelines–Heart Failure (GWTG-HF) database, we sought to characterize patients with an LVEF between 30% and 35% and to compare the survival of patients with an ICD with that of patients with no ICD.
Methods
Data Sources
NCDR ICD Registry
When the Centers for Medicare & Medicaid Services expanded coverage for prophylactic ICDs, it required that data on all prophylactic ICD implants in Medicare beneficiaries be entered into a national ICD registry. This led to the introduction of the NCDR ICD registry in June 2005. Because 78% of the 1448 participating hospitals submit data on all ICD implants and those are generally the larger participating hospitals, they account for 90% of all ICD implants entered into the registry.10,11
Processes of data collection in the NCDR ICD registry have been published.10,11 After formal training by the NCDR, participating hospitals submit data via a secure website. Submitted data undergo rigorous electronic quality checks, and each year up to 10% of participating sites are randomly selected for an on-site audit.12 In the 2010 audit, the average raw accuracy of data abstraction was 91.2%.12
GWTG-HF Database
The GWTG-HF project involves ongoing voluntary data collection that started in 2000 and is intended for quality improvement. Using a point-of-service Internet-based tool, participating hospitals submit clinical information on the in-hospital care and outcomes of patients admitted to the hospital for heart failure. Trained personnel at participating sites abstract data on consecutive eligible patients using standardized definitions and submit these data to the GWTG-HF database. Because data are mainly used at the local site for quality improvement, all sites were granted a waiver of informed consent by local institutional review boards under the Common Rule. The Duke Clinical Research Institute, the data analysis center for this database, has an agreement to analyze the aggregate deidentified data for research purposes.13 Outcome, a Quintiles company, is the data collection and coordination center for the American Heart Association/American Stroke Association GWTG programs.
Medicare Database
We used the Medicare 100% inpatient sample standard analytic files and related denominator files from 2005 through 2011. The 100% inpatient sample includes all inpatient claims filed during fee-for-service coverage periods by in patient hospital providers for a diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification codes 428.×, 402.×1, 404.×1, and 404.×3). The denominator file includes demographic and enrollment information for each beneficiary enrolled in Medicare during a calendar year. To be included in this analysis, a beneficiary had to be living in the United States and had to be 65 years or older on the date of cohort entry. We linked the registry data to Medicare claims data using a validated method that uses combinations of indirect identifiers including admission date, discharge date, patient sex, and patient date of birth or age.14
Study Population
Indications for prophylactic ICDs in patients with heart failure include (1) LVEF of 35% or less, prior myocardial infarction, and New York Heart Association (NYHA) class II or III, (2) nonischemic cardiomyopathy, LVEF of 35% or less, and NYHA class II or III, and (3) LVEF of 30% or less, prior myocardial infarction, and NYHA class I. The ICD group was derived from the NCDR ICD registry, and the group of patients with heart failure eligible for a prophylactic ICD but not treated was obtained from the GWTG-HF database. Both groups were limited to patients with an LVEF between 30% and 35% hospitalized for heart failure.
Outcomes
The primary outcome of this analysis was all-cause mortality. Vital status was obtained using Medicare claims data through December 31, 2011. When no record of death in the claims data was found, a patient was considered alive as of December 31, 2011, or the date at which the patient was no longer enrolled in Part A and Part B fee-for-service Medicare, whichever came first.
Statistical Analysis
The baseline characteristics of patients with an LVEF between 30% and 35% in the ICD group and the non-ICD group were compared using the Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test for categorical variables. Data are presented as medians and interquartile ranges (IQRs) for continuous variables and as counts with percentages for categorical variables. Variables with missing values for 15% or more of patients in either group were excluded from the analysis. The standardized difference between groups for each variable was defined as the absolute value of the difference in group means or proportions, divided by the average standard deviation, and expressed as a percentage.
The baseline characteristics of ICD and non-ICD patients were different. Thus, a matching process was implemented using the Rosenbaum and Rubin method to derive a set of non-ICD patients comparable with the sample of ICD patients (the smaller group).15 For continuous variables, we excluded non-ICD patients whose values were below the minimum or above the maximum for ICD patients. Missing data were handled by imputation, using the set-specific median for continuous variables and the mode for categorical variables. Missing rates were generally low: less than 1% for all variables in the NCDR ICD registry and less than 2% for most variables in the GWTG-HF database.
A propensity model was constructed using logistic regression in which the independent variables were baseline characteristics available in both groups and the dependent variable was an indicator of whether each patient was an ICD or a non-ICD patient. Variables included in the model were age, sex, race (white vs other), LVEF, ischemic heart disease, diabetes, hypertension, prior atrial arrhythmia, systolic blood pressure, and baseline use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, β-blocker, calcium channel blocker, digoxin, diuretic, or statin. In the GWTG-HF database, data were lacking on NYHA class and QRS duration. As a result, these variables were not included in the model. Using the logistic regression model, an estimated propensity score (the probability [p] of a patient having an ICD) and a corresponding logit for the propensity score (loge [p/(1 − p)]) were calculated for each patient.
For matching within calipers, a caliper width of 0.25 times the standard deviation of the logit was used. For a given ICD patient, all non-ICD patients were considered whose logit differed from the ICD patient’s logit by less than the caliper width. Among these patients, the non-ICD patient with the shortest distance (Mahalanobis distance) from the ICD patient was selected as a match. Variables used in calculating the Mahalanobis distance were all significant predictors from the propensity model. ICD patients for whom there were no non-ICD patients within the caliper width were omitted from the analysis (n = 2). Each non-ICD patient was matched only once.
The process of creating a matched cohort was repeated in the subgroup of patients with an LVEF less than 30%.
A Cox proportional hazards model was used to evaluate the association of the presence of an ICD with the risk of mortality among the matched patients. The model included patients with an LVEF between 30% and 35%, patients with an LVEF less than 30%, a term for the LVEF group, and a term for the interaction between LVEF group and presence of an ICD. The model was stratified by quartile of propensity score, and it also contained as covariates all baseline variables listed above. The proportional hazards assumption for the ICD term was assessed and determined to have been met. Risk relationships derived from the Cox model are expressed as hazard ratios (HRs) with 95% CIs. Mortality rates at 1 and 3 years are presented both as unadjusted Kaplan-Meier estimates and as adjusted rates derived from the Cox model. For the follow-up time, event rates, and matched sample size among patients with LVEF between 30% and 35% (the smaller cohort), we had 85% power to detect a 25% reduction in mortality risk with the ICD.
Differences were considered statistically significant at P < .05; all statistical tests were 2-sided. SAS version 9.2 (SAS Institute Inc) was used for all analyses.
Results
Derivation of the Study Population
From the NCDR ICD registry, we included patients who received a prophylactic ICD from January 1, 2006, through December 31, 2007, were 65 years or older, and whose primary insurance was Medicare (n = 2413). We then excluded records of patients with a potential contraindication to an ICD, including recent-onset of heart failure, recent myocardial infarction or coronary artery bypass graft surgery, or NYHA class IV heart failure symptoms (n = 919); patients who received a secondary prevention ICD (n = 90); patients who received an ICD with cardiac resynchronization therapy (n = 853); and patients who received device replacements (n = 18). After these exclusions, 533 records remained from the NCDR ICD registry group.
From the GWTG-HF database, we included patients with an LVEF between 30% and 35% who were hospitalized for heart failure from January 1, 2005, through December 31, 2009; did not receive an ICD; were at least 65 years of age; and whose primary insurance was Medicare (n = 5367). We excluded records of patients who had new-onset heart failure (n = 473); patients who left against medical advice (n = 16); patients transferred to another acute care facility (n = 80); and patients discharged to hospice, a skilled nursing facility, or a rehabilitation center (n = 1335). We also excluded records of patients with a contraindication or other physician-documented reason for not receiving an ICD, including recent-onset heart failure, recent myocardial infarction or coronary artery bypass graft surgery, NYHA class IV heart failure symptoms (entered as a reason for not receiving an ICD), and no reasonable expectation of survival to at least 1 year (n = 491). A total of 2972 records remained for analysis from the GWTG-HF group.
After these exclusions, qualifying records were matched with enrollment files and inpatient claims from the Medicare data to identify unique patients as described above. Patient data in the registries were merged with Medicare Part A inpatient claims, matching by admission and discharge dates, date of birth, sex, and hospital.14 Of the 3505 hospitalizations of patients 65 years or older, we matched 3354 to fee-for-service Medicare claims. We included only the first hospitalization for each patient among matching records and retained the NCDR ICD registry record for patients who appeared in both registries. As a result, our analysis included 3120 unique Medicare patients, 410 in the NCDR ICD registry and 2710 in the GWTG-HF database. The same process was used to obtain a study sample of patients with an LVEF less than 30% (1088 patients with an ICD and 1088 matched patients with no ICD).
Baseline Characteristics
As shown in Table 1, before matching, the majority of the baseline characteristics of patients in the NCDR ICD registry (ICD group) and GWTG-HF database (non-ICD group) were significantly different. Compared with patients in the ICD group, patients in the non-ICD group were older and less frequently men. Ischemic heart disease, prior atrial arrhythmias, diabetes, and hypertension were more common in the ICD group. The rates of use of β-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and digoxin were comparable between the 2 groups.
Table 1.
Baseline Characteristics of NCDR ICD Registry and GWTG-HF Database Patients With LVEF Between 30% and 35%
| All Patients Qualifying for Analysis |
1:1 Matched Patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Characteristic | GWTG-HF (n = 2710) |
NCDR (n = 410) |
Standardized Difference, % |
P Value |
GWTG-HF (n = 408) |
NCDR (n = 408) |
Standardized Difference, % |
P Value |
| Age, median (IQR), y | 79 (73-85) | 76 (71-80) | 44 | <.001 | 75 (71-80) | 76 (71-80) | 1 | .85 |
| Men, No. (%) | 1434 (53) | 279 (68) | 31 | <.001 | 268 (66) | 277 (68) | 5 | .50 |
| White race, No. (%) | 2189 (82) | 318 (78) | 12 | .02 | 310 (78) | 317 (78) | 0 | .99 |
| LVEF, median (IQR), % | 33 (30-35) | 30 (30-33) | 54 | <.001 | 30 (30-33) | 30 (30-33) | 1 | .99 |
| Ischemic heart disease, No. (%) | 1931 (71) | 312 (76) | 11 | .04 | 296 (73) | 310 (76) | 8 | .26 |
| Prior atrial arrhythmia, No. (%) | 940 (35) | 191 (47) | 24 | <.001 | 182 (45) | 189 (46) | 3 | .62 |
| Systolic blood pressure, median (IQR), mm Hg | 139 (120-158) | 132 (115-149) | 29 | <.001 | 132 (117-149) | 132 (115-149) | 1 | .87 |
| Diabetes, No. (%) | 1117 (41) | 205 (50) | 18 | <.001 | 202 (50) | 204 (50) | 1 | .89 |
| Hypertension, No. (%) | 2017 (74) | 351 (86) | 28 | <.001 | 350 (86) | 349 (86) | 1 | .92 |
| Baseline medication use, No. (%) | ||||||||
| ACE inhibitor or ARB | 1915 (71) | 285 (70) | 1 | .78 | 291 (71) | 284 (70) | 3 | .71 |
| β-Blocker | 2303 (85) | 350 (86) | 3 | .59 | 354 (87) | 349 (86) | 2 | .81 |
| CCB | 485 (20) | 49 (12) | 23 | <.001 | 47 (13) | 49 (12) | 3 | .67 |
| Digoxin | 662 (28) | 113 (28) | 0 | .95 | 99 (28) | 111 (27) | 0 | .98 |
| Diuretic | 2051 (82) | 313 (77) | 14 | .01 | 291 (77) | 312 (77) | 0 | .99 |
| Statin | 964 (36) | 261 (64) | 58 | <.001 | 266 (66) | 259 (64) | 4 | .54 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; GWTG-HF, Get With The Guidelines-Heart Failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NCDR, National Cardiovascular Data Registry.
Matching made the groups similar with no statistically significant differences remaining in the characteristics compared (Table 1); standardized differences in baseline characteristics did not exceed 8%. Matched patients were aged 75 to 76 years and were mostly men and white. The mean LVEF was 30%, and about 75% of the patients had ischemic cardiomyopathy. Guideline-recommended medications for heart failure were used for the majority of patients. A similar analysis of baseline characteristics of patients with an LVEF less than 30% before and after matching was performed (eTable in Supplement). After matching patients with an LVEF less than 30% from the ICD and non-ICD groups, the standardized difference in each measured variable did not exceed 6%.
All-Cause Mortality
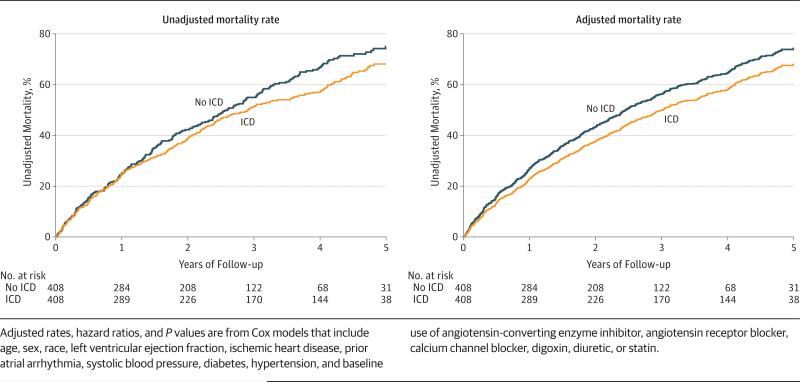
As shown in Table 2, during a median follow-up time of 4.4 years (IQR, 2.7-4.9), 248 matched ICD patients died, and during a median follow-up time of 2.9 years (IQR, 2.1-4.4), 249 matched non-ICD patients died. At 1 year, 24.5% of ICD patients died vs 24.9% of non-ICD patients. At 3 years, 51.4% of the ICD patients died, compared with 55.0% of the non-ICD patients. The risk of mortality in patients with an LVEF between 30% and 35% and an ICD was significantly lower than that of matched patients without an ICD (HR, 0.83 [95% CI 0.69-0.99]; P = .04). Likewise, in patients with an LVEF less than 30% and an ICD, the risk of mortality was significantly lower compared with that of matched patients with no ICD (HR, 0.72 [95% CI, 0.65-0.81]; P < .001) (Table 2). In the matched analysis, un-adjusted Kaplan-Meier estimates and adjusted model– derived estimates for mortality were generated and are shown in the Figure. There was no interaction of LVEF with the presence of an ICD in relation to mortality risk (P = .20), indicating that the association of ICD with mortality risk is similar for patients with an LVEF between 30% and 35% and for those with an LVEF less than 30%.
Table 2.
All-Cause Mortality in NCDR ICD Registry and GWTG-HF Database Patients, by LVEFa
| LVEF 30%-35% |
LVEF <30% |
|||
|---|---|---|---|---|
| ICD (NCDR) (n = 408) | No ICD (GWTG-HF) (n = 408) | ICD (NCDR) (n = 1088) | No ICD (GWTG-HF) (n = 1088) | |
| Follow-up duration among survivors, median (IQR), y | 4.4 (2.7 to 4.9) | 2.9 (2.1 to 4.4) | 4.6 (2.9 to 5.1) | 3.1 (2.0 to 4.2) |
| Total deaths | 248 | 249 | 634 | 660 |
| Deaths by 1 y | 97 | 99 | 234 | 322 |
| Unadjusted mortality rate at 1 y, % (95% CI) | 24.5 (20.5 to 29.0) | 24.9 (20.9 to 29.5) | 22.0 (19.6 to 24.6) | 30.7 (28.0 to 33.6) |
| Difference between no ICD and ICD in unadjusted mortality rates at 1 y, % (95% CI) | 0.4 (−5.6 to 6.5) | 8.7 (4.9 to 12.4) | ||
| Deaths by 3 y | 196 | 204 | 458 | 571 |
| Unadjusted mortality rate at 3 y, % (95% CI) | 51.4 (46.4 to 56.5) | 55.0 (49.9 to 60.2) | 45.0 (42.0 to 48.2) | 57.6 (54.5 to 60.8) |
| Difference between no ICD and ICD in unadjusted mortality rates at 3 y, % (95% CI) | 3.6 (−3.7 to 10.8) | 12.6 (8.2 to 17.1) | ||
| Adjusted mortality rate at 1 y, % (95% CI) | 22.8 (22.3 to 23.4) | 30.0 (29.4 to 30.6) | 22.3 (22.0 to 22.6) | 29.3 (29.0 to 29.7) |
| Adjusted mortality rate at 3 y, % (95% CI) | 47.1 (46.2 to 47.9) | 58.0 (57.1 to 58.8) | 46.1 (45.6 to 46.7) | 57.0 (56.4 to 57.5) |
| Adjusted HR (95% CI) for ICD vs no ICDb | 0.83 (0.69 to 0.99) | 0.72 (0.65 to 0.81) | ||
| P value for HR | .04 | <.001 | ||
| P value for interaction of LVEF group with ICD | .20 | |||
Abbreviations: GWTG-HF, Get With The Guidelines–Heart Failure; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NCDR, National Cardiovascular Data Registry.
Adjusted rates, hazard ratios, and P values are from Cox models that include age, sex, race, LVEF, ischemic heart disease, prior atrial arrhythmia, systolic blood pressure, diabetes, hypertension, and baseline use of angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, digoxin, diuretic, or statin.
C-index for the model = 0.78.
Figure.
Unadjusted Kaplan-Meier Estimates of Mortality, and Cox Model-Derived Adjusted Mortality Rates, for Patients With an LVEF Between 30% and 35% With and Without an Implantable Cardioverter-Defibrillator (ICD)
Discussion
Using the largest registry of ICD implants in the United States and studying “real-world” settings, we showed that the survival of patients with heart failure and an LVEF between 30% and 35% and an ICD was significantly better than that of patients with heart failure and no ICD. Patients with an LVEF less than 30% and an ICD also had better survival than patients with heart failure and no ICD. The difference in survival may appear to be greater in patients with an LVEF less than 30%; however, it was clearly significant in both groups of patients, and the interaction test showed no interaction of LVEF with the presence of an ICD in relation to mortality. Although the difference in absolute risk by 3 years was not large (3.6% at 3 years), it was significant and close in magnitude to what was observed in the clinical trials of prophylactic ICDs (5.3% at 3 years in the Sudden Cardiac Death in Heart Failure Trial8 and 5.6% at about 2 years in the Multicenter Automatic Defibrillator Implantation Trial II6). These results support guidelines’ recommendations to implant a prophylactic ICD in eligible patients with an LVEF of 35% or less.16
The association between the presence of an ICD and improved outcomes in patients with an LVEF between 30% and 35% has been largely implied. Although most of the randomized clinical trials of prophylactic ICDs demanded an inclusion LVEF of 35% or less, the median LVEF of enrolled patients was considerably lower.4-8 In a previous analysis of 3530 patients from 5 prophylactic ICD trials, our group found that an LVEF between 30% and 35% was present in only 389 patients, of whom only 184 had an ICD.17 Although a statistically nonsignificant difference in survival was found among ICD recipients with an LVEF between 30% and 35%, this benefit could not be confirmed, possibly because of the small sample size.17 Therefore, our findings in the current study fill an important gap in knowledge.
The Centers for Medicare & Medicaid Services identified understanding the outcomes of patients with LVEF between 30% and 35% and a prophylactic ICD as a priority that needs to be addressed within the NCDR ICD registry.9,10 This, coupled with the fact that a large number of ICDs are being implanted in patients with an LVEF between 30% and 35% in the United States, underscores the potential health policy implications of our findings9,10
In this analysis, we reported only on all-cause mortality. Although survival is a very important end point, other end points, such as quality of life, also may be of value to patients. Information on such end points as well as on procedural complications is crucial for clinical decision making. Although we were not able to examine other end points in our study, our research may provide the impetus for studying these end points in future trials. Second, the index event for all patients in our analysis was a hospitalization for heart failure, which has been associated with high risk of mortality.18-20 Patients in our analysis likely had other competing causes of death, which may have led to underestimation of the survival advantage associated with presence of an ICD.
Our study has several limitations. We restricted this analysis to Medicare patients; as such, our results may not apply to non-Medicare patients. Propensity score matching may limit the generalizability of our findings, especially because patients who could not be matched and those who did not survive to receive an ICD were excluded. Our inability to adjust for unknown confounders or for clinical factors with missing values in 15% or more of patients, such as creatinine, or for factors not collected by the NCDR ICD registry and the GWTG-HF database, such as QRS width and NYHA class, may partially explain the differences in outcomes. Interaction tests are often underpowered, and it is possible that a difference exists in the risk relationship between the groups that we did not detect. The associations of device use with outcomes may not reflect causality. Data quality was dependent on the accuracy and completeness of documentation and abstraction in the NCDR ICD registry and the GWTG-HF database and of coding in the Medicare Claims database. However, in a recent audit of the NCDR ICD registry, the majority of fields accurately reflected data in medical charts.12 Some patients considered eligible for treatment who were not treated may have had contraindications or other reasons that prevented treatment but were not documented in the medical record. Because trials of prophylactic ICDs predated dissemination of cardiac resynchronization therapy, they included patients eligible for that therapy. Excluding such patients from our study may have led to inclusion of healthier patients with fewer competing mortality risks. Although the comparators came from different data sources, the overlap between the centers participating in these registries is likely substantial because all centers involved in implanting prophylactic ICDs in Medicare beneficiaries are required to participate in the NCDR ICD registry.
Conclusions
Using the NCDR ICD registry and the GWTG-HF database, we demonstrated that in Medicare beneficiaries with an LVEF between 30% and 35%, survival associated with a prophylactic ICD was significantly better than survival associated with no ICD. This was also observed in patients with an LVEF less than 30%. These findings support the use of prophylactic ICDs in eligible patients with an LVEF of 35% or less.
Supplementary Material
Acknowledgments
Funding/Support: This analysis was funded by grant 1R01-HL093071-01A1 from the National Heart, Lung, and Blood Institute.
Role of the Sponsors: The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Al-Khatib and Ms Hellkamp had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Al-Khatib, Fonarow, S. Hammill.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Al-Khatib, Hellkamp, S. Hammill.
Critical revision of the manuscript for important intellectual content: Fonarow, Mark, Curtis, Hernandez, Anstrom, Peterson, Sanders, Al-Khalidi, B. Hammill, Heidenreich, S. Hammill.
Statistical analysis: Hellkamp, Anstrom, Peterson, Al-Khalidi.
Obtained funding: Al-Khatib, Mark. Administrative, technical, or material support: Peterson, B. Hammill, S. Hammill.
Study supervision: Peterson, S. Hammill.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Fonarow reported serving as a consultant for Novartis, Medtronic, Gambro, Bayer, Johnson & Johnson, Takeda, and The Medicines Company; receiving grants or grants pending from the National Institutes of Health; being employed by the Ahmanson Foundation ; and serving as the Eliot Corday chair of Cardiovascular Medicine and Science, UCLA. Dr Mark reported receiving grants from Medtronic, Eli Lilly, Gilead, and AstraZeneca and receiving personal fees from Janssen Pharmaceuticals. Dr Curtis reported receiving grants from Medtronic and Boston Scientific; receiving research and salary support from Johnson & Johnson, GlaxoSmithKline, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute; Dr Curtis also has made a detailed listing of financial disclosures available at (http://www.dcri.org/research/coi.jsp). Dr Hernandez reported receiving consulting fees from Boston Scientific and a grant from Medtronic. Dr Anstrom reported receiving consulting fees from Abbott Vascular, AstraZeneca, Bristol-Meyers Squibb, Pfizer, GlaxoSmithKline, and Ikaria; receiving research support from AstraZeneca; and serving on data monitoring committees for Pfizer and Vertex, Dr Peterson reported receiving grants from Eli LIlly and Janssen Pharmaceuticals and receiving personal fees from Janssen Pharmaceuticals and Boehringer Ingelheim. No other authors reported disclosures.
Supplemental content at jama.com
Disclaimer: Dr Peterson, associate editor for JAMA, was not involved in the editorial review of or the decision to publish this article. Views expressed in this article are those of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute. The manuscript was reviewed by the American College of Cardiology-NCDR ICD registry Research and Publications Committee and by the American Heart Association-Get With The Guidelines Publications Committee. The views expressed in this manuscript represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies identified at http://www.ncdr.com/webncdr/. The ICD registry is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
REFERENCES
- 1.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7(1):119–125. doi: 10.1016/j.ejheart.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Weir RA, McMurray JJ. Epidemiology of heart failure and left ventricular dysfunction after acute myocardial infarction. Curr Heart Fail Rep. 2006;3(4):175–180. doi: 10.1007/s11897-006-0019-5. [DOI] [PubMed] [Google Scholar]
- 3.MERIT HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, et al. Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341(25):1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 7.Kadish A, Dyer A, Daubert JP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 8.Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 9.Hammill SC, Stevenson LW, Kadish AH, et al. Review of the registry's first year, data collected, and future plans. Heart Rhythm. 2007;4(9):1260–1263. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10(4):e59–e65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry's third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6(9):1397–1401. doi: 10.1016/j.hrthm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Messenger JC, Ho KK, Young CH, et al. NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60(16):1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157(6):995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 16.Epstein AE, DiMarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation. 2008;117(21):e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 17.Al-Khatib SM, Han JY, Edwards R, et al. Do patients with a left ventricular ejection fraction between 30% and 35% benefit from a primary prevention implantable cardioverter defibrillator? Int J Cardiol. 2014;172(1):253–254. doi: 10.1016/j.ijcard.2013.12.278. [DOI] [PubMed] [Google Scholar]
- 18.Curtis LH, Greiner MA, Hammill BG, et al. Early and long-term outcomes of heart failure in elderly persons, 2001-2005. Arch Intern Med. 2008;168(22):2481–2488. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsson F, Torp-Pedersen C, Brendorp B, Seibaek M, Burchardt H, Køber L. DIAMOND Study Group. Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J. 2003;24(9):863–870. doi: 10.1016/s0195-668x(02)00845-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.