Abstract
Background
Given the established relation between testosterone and aging in older adults, we tested whether buccal telomere length (TL), an established cellular biomarker of aging, was associated with testosterone levels in youth.
Methods
Children, mean age 10.2 years, were recruited from the greater New Orleans area and salivary testosterone was measured during both an acute stressor and diurnally. Buccal TL was measured using monochrome multiplex quantitative real-time PCR (MMQ-PCR). Testosterone and telomere length data was available on 77 individuals. The association between buccal TL and testosterone was tested using multivariate Generalized Estimating Equations (GEE) to account for clustering of children within families.
Results
Greater peak testosterone levels (β=-0.87, p < 0.01) and slower recovery (β=-0.56, p < 0.01) and reactivity (β = -1.22, p < 0.01) following a social stressor were significantly associated with shorter buccal TL after controlling for parental age at conception, child age, sex, sociodemographic factors and puberty. No association was initially present between diurnal measurements of testosterone or morning basal testosterone levels and buccal TL. Sex significantly moderated the relation between testosterone reactivity and buccal TL.
Conclusions
The association between testosterone and buccal TL supports gonadal maturation as a developmentally sensitive biomarker of aging within youth. As stress levels of testosterone were significantly associated with buccal TL, these findings are consistent with the growing literature linking stress exposure and accelerated maturation. The lack of association of diurnal testosterone or morning basal levels with buccal TL bolsters the notion of a shared stress-related maturational mechanism between cellular stress and the hypothalamic pituitary gonadal (HPG) axis. These data provide novel evidence supporting the interaction of aging, physiologic stress and cellular processes as an underlying mechanism linking negative health outcomes and early life stress.
Keywords: testosterone, telomere length, aging, puberty, stress
Introduction
“Getting older” is considered a negative process for adults. The link between advanced aging and negative health outcomes is well established. A significant body of recent research suggests that the divergence between chronological and biological aging may underlie the negative health outcomes associated with early adversity and stress, thus highlighting the need for greater examination of the underlying biological processes. More recently, a downward extension of the aging process suggests that the biological trajectories of accelerated aging are established during early developmental windows typically characterized by greatest health, i.e. childhood. The ability to understand the aging process from a life course perspective and measure the impact of stress on maturational processes is critical as we seek to disentangle the factors contributing to accelerated aging early in development when maturation likely has different implications and associations. As such the impetus for this present study is to expand the number of biological indicators of aging with applicability across the life course and explore the relationship among them. The long term goal of this line of research is to inform novel intervention and prevention efforts directed at ameliorating the negative effect of stress as early as possible in development.
Telomere length as a marker of aging across the life course
Telomere length (TL) is an established cellular marker of aging that is gaining validity as a marker of stress and cumulative adversity [1, 2]. Shorter TL, in adults, is associated with a range of aging-related negative health outcomes including cardiovascular disease, cancer, cognitive decline, diabetes, and psychopathology [3, 4]. TL serves as a biological cellular clock and figures prominently in cellular differentiation and senescence [5]. Factors that trigger DNA damage such as toxins, oxidative stress, and radiation preferentially effect telomeres thereby accelerating TL decline [6]. In youth, shorter TL has been associated with early life adversity, socioeconomic levels, prenatal exposure to tobacco, and neighborhood and community violence in multiple studies [2, 7-9].
Testosterone and Aging
Testosterone has historically been thought of as a masculinizing hormone that rises (especially in boys) and triggers secondary sexual characteristics related to pubertal maturation as well as behavioral changes more commonly seen after puberty. As a lipid-soluble steroid hormone, testosterone easily crosses through cell membranes, travels into the nucleus of the cell to directly alter gene expression, passes through the acinar cells and into saliva via passive diffusion, and crosses the blood-brain-barrier to influence neural function [10]. Receptors for testosterone are found throughout the brain, [11, 12] particularly within limbic structures. Acute changes in tesosterone modifies an individual's responses to salient emotional stimuli involving largely fear [13], anger [14], and reward cues [11, 15], suggesting that acute testosterone enhances an individual's reactivity, both physiologically and behaviorally, to emotional and stressful stimuli within a brief time course. Most previous studies have examined the impact of exogenously administered testosterone on behavior and physiology to understand acute testosterone changes, yet this is challenging in pediatric populations. Endogenous testosterone release can be triggered by exposure to stressful challenges [16] such as the Trier Social Stress Test for Children (TSST-C) [17] which takes advantage of testosterone's response to social evaluative threat to acutely alter testosterone release within 20-60 min [13, 18]. To more closely capture maturational processes, morning testosterone levels are often utilized as an index of basal testosterone levels [19] as testosterone values are typically at their highest in the morning. In addition, testosterone's diurnal change across the day is expected to capture the flexibility or change in this axis from nadir to zenith and has been suggested to be a useful index of the range of testosterone to which the child must adjust [20, 21] . While all of these measure the hypothalamic pituitary gonadal axis (HPG), these measurements each capture unique aspects of HPG development, including acute stress-responsivity, level of development, and changing maturation processes [22]. All three are measured in the present study.
Testosterone, similar to TL, is also related to aging. For example, testosterone levels decline in older adults, and testosterone replacement therapy has been thought to slow down the aging process [23]. In youth, who represent the other end of the lifespan, elevated levels of testosterone advance maturational processes [19, 24]. Puberty and testosterone are highly correlated, especially within boys [20] and testosterone is responsible for many secondary sexual characteristics [25, 26] including physical growth spurts [27]. Administration of testosterone results in rapid physical growth and pubertal advancement in boys with delayed puberty or constitutional growth delays [28, 29]. In adolescence, testosterone rise associated with stress and competition is especially robust in box sexes, however sex differences in the behavioral patterns associated with testosterone reactivity are notable [30]. Together, these findings indicate that testosterone, similar to TL, is reflective of aging and maturational processes across the life course. Concurrent exploration of both testosterone reactivity and diurnal measurements is expected to provide novel insight into the links between stress, aging, and gonadal maturation.
Linking testosterone and telomere length
Testosterone, similar to TL, is associated with a range of health problems [31], even within young populations and indirect evidence of their association exists [20]. Studies have demonstrated an association between testosterone and telomerase activity in spermatogonia that develop adjacent to testosterone-producing leydig cells [32, 33]. Elevated levels of reactive oxygen species and oxidative stress result in decreased testosterone and decreased telomerase with subsequent decreased TL [34]. Oocyte TL shortening has also been related to ovarian testosterone levels suggesting that the relationship between testosterone and TL may be evident in both males and females [35]. One previous study in elderly males showed an association between aging and both leukocyte TL and basal testosterone levels. However, in this study testosterone basal levels were not directly associated with TL, suggesting that if a direct relationship exists between TL and testosterone, testosterone stress reactivity may better capture this association [36]. No previous study, to our knowledge, has examined the relation between TL and testosterone in youth.
Capitalizing on an existing cohort of children recruited from the greater New Orleans area, the association between TL and testosterone reactivity during a social stressor, morning basal levels, and diurnal testosterone measures were tested. We hypothesized that elevated testosterone would be associated with shorter TL. However, to better refine our competing hypotheses related to the potential association of testosterone and TL, we tested the association of TL with three overarching testosterone indices in the same child: basal, diurnal, and stress-reactive testosterone. Demonstration of an association between TL and testosterone would increase the utility of both biomarkers and provide additional evidence that the divergence of biological from chronological aging as a result of stress underlies the lasting negative health outcomes associated with early adversity.
Methods
Subjects
Children, ages 5-15 years old, were recruited from the greater New Orleans area. Families were recruited using street outreach techniques, including ethnographic mapping and targeted sampling and through schools in these communities. Recruitment neighborhoods were identified using the community identification process, a mapping method to record epidemiological indicators of the prevalence and incidence of community violence and other selected social and health conditions. Interested families contacted the research site to schedule an appointment. Transportation was provided and families were compensated. This study was approved by the Tulane University Institutional Review Board.
Data
Parental caregivers provided information about multiple levels of the child's social ecology (i.e., household and neighborhood) using an interview-assisted computer survey administered face-to-face at the research site (Questionnaire Development System, QDS, Nova Research, Bethesda, MD). Oral responses were recorded by trained interviewers on the computer. Buccal swabs were collected from the child for DNA analysis of telomere length. BMI was measured at the research site.
Telomere length
DNA was collected using Isohelix SK1 buccal swabs (Cell Projects, Kent, UK) and extracted using the QIAamp® DNA mini kit protocol (Qiagen, Valencia, CA, USA). Concentration of extracted DNA was quantified with a Qubit dsDNA BR assay kit (Invitrogen, Carlsbad, CA, USA), purity of the DNA was determined using a NanoDrop 1000® spectrophotometer (Thermo Fisher Scientific, Delaware, USA), and DNA integrity was confirmed by gel electrophoresis. DNA was stored at -80°C. The average relative buccal cell TL was determined from the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio using an adapted monochrome multiplex quantitative real-time PCR (MMQ-PCR) and a BioRad CFX96 [37]. 10μl DNA sample, containing ~0.1-0.5ng of DNA, was combined with 15μl of PCR mixture, for a final volume of 25μl per reaction. The PCR reaction consisted of 0.75X Sybr Green I (Invitrogen, Carlsbad, CA, USA), 1X Gene Amp Buffer II (Applied Biosystems, Foster City, CA, USA), 0.8mM dNTPs, 10mM MgCl2, 3mM DTT, 1M Betaine, 2.5U AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA, USA), 0.9μM telg primer (ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT), 0.9μM telc primer (TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA), 0.6μM albd2 primer (GCGGGCCCGCGTGGCGGAGCGAGGCCGGAAAAGCATGGTCGCCTGT) and 0.6μM albu2 primer (GCCTCGCTCCGGGAGCGCCGCGCGGCCAAATGCTGCACAGAATCCTTG). The reaction proceeded for 1 cycle at 95C for 15 minutes, 2 cycles at 94C for 15 seconds and 49C for 1 min, 4 cycles at 94C for 15 sec and 59C for 30 sec, followed by 19 cycles at 85C for 15 seconds, and 73.5C for 30 sec, then 30 cycles at 94C for 15 sec and 84C for 30 sec. All samples were performed in triplicate, with a 7-point standard curve (0.0313ng to 2ng) using pooled control DNA. Triplicate plates were repeated with all samples in a different well position. Thus, six replicates were available for each individual. PCR efficiency criteria for telomere and albumin reactions were 90-110%. Coefficients of variations were calculated within each triplicate (CV criteria ≤10%) and between plates (CV criteria ≤6%). Samples with unacceptably high CVs (10% intra- and 6% inter-assay CV) were removed from analysis or repeated (N=6), resulting in a final sample of 80 individuals with buccal TL. Buccal TL ratio was determined by the average of the triplicates from both plates. Children without buccal TL data did not differ significantly (p > 0.05) from children with buccal TL data on study measures.
Trier Social Stressor for Children (TSST-C)
We administered the TSST-C thirty minutes after arrival at the research center with all sessions beginning in the early afternoon (M=2:03 pm, SD=57min). The TSST-C is a modified form of the adult version of the TSST specifically validated and designed for children. A familiar experimenter took the child to a separate room. After a 5 min preparation period, the child was led into a new room where unfamiliar confederates were waiting (i.e., the committee). The child was told a 2-sentence introduction to a story and then instructed to finish telling the story in as exciting a manner as possible in front of the committee. The child was asked directly to try to perform better than all the other children participating in the study, which is expected to induce testosterone reactivity through competition. The child stood in front of a 2-person committee with a microphone and obtrusive video cameras poised at them. The child was asked to finish the story in a free speech for 5 minutes. Next the committee asked the children to serially subtract numbers orally. For children younger than 12 they were asked to subtract the number 7 from 758, while for youth older than 12 they were asked to subtract the number 13 from 1023. Again, an important component for testosterone is that the child was asked to be as fast and accurate as possible to be better than other children in the study to induce a competitive emotion. On every failure, the committee interfered, stating “That is incorrect. Please start from the beginning at (the number to initially subtract from, e.g. 758 or 1023).” Of the sample 1 child refused the TSST-C entirely and was not included in the analysis. Two children refused the narrative section of the story, however saliva samples were collected and they were included in the analysis.
Testosterone
Saliva was collected using passive drool [10]. For the acute testosterone measurement saliva was collected a mean of 39 minutes after arrival to the research site, mean 23 minutes after the TSST-C and mean of 85 minutes post TSST-C to capture stress reactivity and recovery. Diurnal testosterone was collected across 2 days. Saliva was collected upon awakening (mean 8:01am), 30-min later (mean 8:35am), in the early afternoon (mean 3:57pm) and at bedtime (mean 8:17pm) to capture waking basal levels and the diurnal rhythm. With each sample, a daily diary questionnaire filled out by the participant's caregiver measured time of awakening, time of sample collection, and relevant control variables [10]. TSST-C samples were frozen immediately; basal and diurnal samples were picked up at the home of the participant the day after the samples were collected. Samples were stored in at -80°C freezer for up to 1 month. Samples were delivered frozen by courier to the laboratory of Dr. Elizabeth Shirtcliff. On the day of testing, saliva was thawed and centrifuged at 3,000 rpm for 15 min. The clear top-phase was pipetted into appropriate test wells. Testosterone was assayed using a commercially-available enzyme immunoassay specifically designed for use with saliva (Salimetrics, PA). The test uses 25 μl of saliva (per singlet determination) and has a minimum detection limit of 6.1 pg/mL. Average intra-and inter-assay coefficients of variation (CVs) are 4.6 and 9.83%, respectively. All samples were tested in duplicate; samples from the same subject will be assayed within the same run. Duplicate test values that varied by more than 7% were repeat tested with reagents from the same lot.
To manage the inherent nesting of samples within participants (up to 12 testosterone scores per youth), we utilized hierarchical linear modeling to capture basal levels and change scores using a slopes-as-outcome approach. TSST-C acute reactivity was modeled separately from the diurnal rhythm. Each testosterone score was the outcome. Testosterone reactivity was captured using time (in minutes) until the individual achieved their peak testosterone level after the stressor (B=.02, p=.029). Testosterone recovery was time (in minutes) after the peak testosterone during which testosterone dropped (B=-.16, p=.002). Consequently, the intercept captures the peak testosterone level achieved after the TSST-C (B=3.45, p<.002). Empirical Bayes estimates were extracted which conservatively shrink extreme scores to derive a “best estimate” of each youth's peak testosterone level, reactivity and recovery score, all of which were examined as key exposures in the present analysis.
A similar approach was utilized for testosterone's diurnal rhythm. The decline in testosterone level across the day was captured using time-since-waking (in minutes) as a predictor of testosterone level (B =-.026, p<.001). Consequently, basal testosterone was captured as testosterone level upon awakening (B =3.59, p<.001) when values are typically highest during the day. Empirical Bayes estimates were extracted for basal testosterone level and diurnal rhythm, similar to our other work [19].
Data Analysis
Buccal TL data was available on 80 children. Buccal TL and at either testosterone reactivity or diurnal measurements of testosterone were available on 77 individual children (38 female, 39 males). Seven non-overlapping children did not have either testosterone reactivity or diurnal measurements. Children with buccal TL but missing testosterone data in any of the measures did not significantly differ from children with both testosterone measurements. Telomere length was adjusted for paternal and maternal age at conception and the child's age, given the known association of these factors with TL.[38]
Analyses were performed using SAS version 9.3 (SAS, Cary, NC). Bivariate analyses examined crude associations, including Pearson or Spearman correlations. Sixty-two percent of enrolled families had one child participate (range 1-5). To account for correlation between siblings or children living in the same household and ensure correlated data did not inflate findings, generalized estimating equations (GEE) analyses were employed using an unstructured correlation structure using PROC GENMOD. Three primary multivariate analyses were conducted, including comparison of buccal TL across measurements of acute testosterone levels crudely and controlling for potential confounders.
All analyses controlled for child sex, age, body mass index (BMI), maternal and paternal age at conception, pubertal stage, race and maternal education as a marker of socioeconomic status (SES). Race was self- reported and categorized as Black (91% of the sample) or Other (7%). Maternal education was classified as less than high school, high school degree, some college, and a college or associates degree or more. Pubertal status was determined by parent and child report using the Pubertal Development Scale [39]. Discrepancies in parent and child report were reconciled. To be developmentally appropriate, children less than 8 years of age were administered the PDS by parent-report only, consistent with standard practice [40]. Our prior work has indicated that this measure is reliably linked with sex hormones [19]. Child's age at DNA collection was calculated from their birthdate. Maternal and paternal age at child's conception was determined from parent-report. Missing paternal age (n=11) scores were replaced with mean imputation [41].
Results
Demographics
Demographic data and crude correlations with testosterone and buccal TL are presented in Table 1. TL was not associated with age, sex, race, BMI, or maternal education However, when controlling for puberty, TL was negatively associated with age (β = - 0.0458, z= - 2.32, p=0.02,). Child sex, race and maternal education were not significantly associated with testosterone levels. However, age and puberty were significantly associated with testosterone levels. Specifically, older age and more advanced puberty were both associated with increased testosterone levels. BMI was correlated with acute testosterone levels but not diurnal testosterone levels.
Table 1.
Sample Characteristics and Crude Association with Predicted Buccal Telomere Lengtha (TL), Acute and Diurnal Testosterone using Pearson Correlations (N=77)
| Mean (sd) or Frequency (%) | TLa r (p-value) | Acute Testosterone Peak r (p-value) | Acute Testosterone Recovery r (p-value) | Acute Testosterone Reactivity r (p-value) | Diurnal Testosterone r (p-value) | Basal Testosterone r (p-value) | |
|---|---|---|---|---|---|---|---|
| Age (years) | 10.22 (2.83) | −.06 (0.58) | 0.66 (0.0001) | 0.43 (0.0002) | 0.66 (<0.0001) | 0.04 (0.72) | 0.58 (<0.0001) |
| Sex (female vs. male) | 38 (49.35) | −0.056 (0.63) | −0.085 (0.48) | 0.05 (0.68) | −0.09 (0.49) | 0.07 (0.58) | −0.09 (0.44) |
| Race (Black vs. other) | 73 (94.81) | −0.031 (0.79) | 0.09 (0.45) | 0.07 (0.54) | 0.09 (0.45) | −0.16 (0.18) | 0.17 (0.15) |
| Maternal Education (N=76) | 0.040 (0.73) | 0.054 (0.66) | −0.07 (0.56) | 0.05 (0.66) | −0.18(0.14) | −0.01 (0.96) | |
| Grade School | 18 (23.68) | ||||||
| High School graduate or GED | 18 (23.68) | ||||||
| Some college | 27 (35.53) | ||||||
| College/associates degree or | 13 (17.10) | ||||||
| more | |||||||
| Puberty Stageb | −0.66 (<0.0001) | 0.52 (<0.0001) | 0.20 (0.10) | 0.52 (<0.0001) | 0.09 (0.44) | 0.51(<0.0001) | |
| Mean (sd) TL | |||||||
| Pre-pubertal | 32 (41.56) | 1.72 (0.03) | |||||
| Beginning pubertal | 11 (14.29) | 1.72 (0.06) | |||||
| Mid-pubertal | 20 (25.97) | 1.67 (0.05) | |||||
| Advanced pubertal | 10 (12.99) | 1.64 (0.04) | |||||
| Post pubertal | 4 (5.19) | 1.66 (0.02) | |||||
| Body Mass Index (BMI) | 19.85 (4.80) | −0.31 (0.01) | 0.24 (0.04) | 0.17 (0.17) | 0.24 (0.04) | 0.16(0.20) | 0.16 (0.20) |
| (Range is 13.47- 34.76) | |||||||
| Mean (sd, range ) a | 1.69 (0.05) | 3.45 (0.57) | −0.15 (0.08) | 0.02 (0.03) | −0.03 (0.02) | 3.59 (0.64) | |
| (1.58, 1.84) | (1.80,4.90) | (−0.53, −0.02) | (−0.08, 0.10) | (−0.09, 0.01) | (1.99, 5.14) |
Average buccal cell telomere length as represented by the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio. Note that the correlation with TL utilizes the predicted value of telomere length corrected for parental age at conception and child age. The correlations reported with age and TL do not account for child age.
Puberty Stage classifies participants into one of five pubertal status categories based on level of development reported on the three relevant indices of pubertal change. For females, staging is based on pubic hair growth, breast development, and menarche. For males staging is based on development of pubic hair, facial hair and voice change.
Values reflect overall means, sd and range of values, not correlations.
Acute testosterone
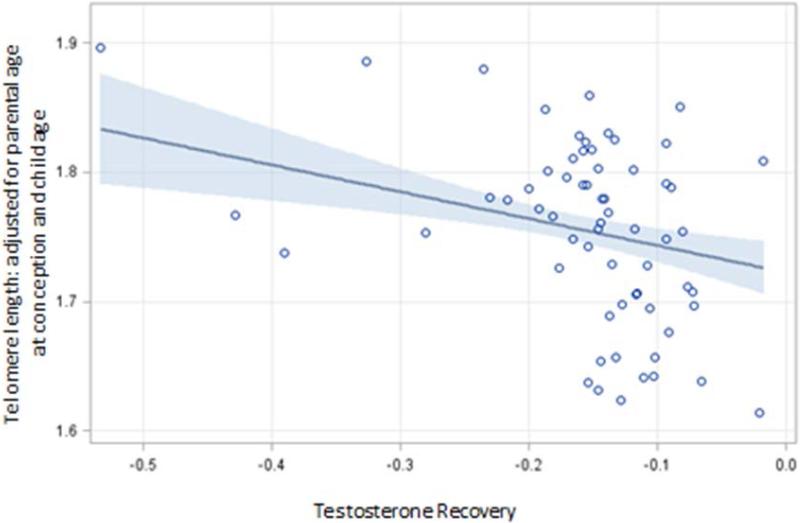
The final multivariate models for the acute measurements of testosterone are presented in Table 2. Overall testosterone levels significantly increased during the TSST-C, and there were no sex or age effects on testosterone. A significant association between buccal TL and all three measures of acute testosterone responsivity was detected (Table 2). Specifically, higher peak levels of testosterone, increased rise to peak, and flattened recovery slope after the TSST-C were all significantly associated with shorter buccal TL. These effects persisted even after controlling for morning testosterone diurnal levels as a measure of adjusting for individual differences in the basal testosterone levels. Across all models sex and pubertal stage significantly added to the final model. Figure 1 presents the scatter plot of the association between buccal TL and testosterone. The association between testosterone recovery and buccal TL is presented in Figure 1 which depicts the inverse relation between the 2 so that greater recovery or time needed to recover from the TSST-C was significantly associated with lower buccal TL. [Table 2]/[Figure 1]
Table 2.
Generalized Estimating Equation Models of the Association between Testosterone and Average Buccal Cell Telomere Lengtha (TL) in children (N=70)
| Beta (SE) * | ||||
|---|---|---|---|---|
| Variables | Acute Testosterone Peak | Acute Testosterone Recovery | Acute Testosterone Reactivity | Diurnal testosterone |
| Testosterone | −0.087 (0.029)** | −0.560 (0.160)** | −1.22 (0.472)** | −0.014 (0.024) |
| Sex | −0.093 (0.074) | 0.070 (0.020)** | 0.002 (0.011) | −0.040 (0.055) |
| Testosterone *Sex | −0.035 (0.022) | 0.27 (0.11)* | 0.599 (0.320) | 0.015 (0.016) |
| Mother's Education | 0.002 (0.003) | 0.002 (0.003) | 0.003 (0.005) | 0.001 (0.005) |
| Puberty Stageb | −0.039 (0.007)** | −0.038 (0.007)** | −0.024 (0.006)** | −0.027 (0.005)** |
| BMI | −0.002 (0.001) | −0.001 (0.001) | −0.001 (0.001) | −0.001 (0.001) |
| Diurnal Testosterone | 0.026 (0.017) | 0.0002 (0.011) | 0.020 (0.011) | ---- |
P-value < 0.05
P-value < 0.01
Average buccal cell telomere length as represented by the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio corrected for parental age at conception and child age.
Puberty stage classifies participants into one of five pubertal status categories based on level of development reported on the three relevant indices of pubertal change. For females, staging is based on pubic hair growth, breast development, and menarche. For males staging is based on development of pubic hair, facial hair and voice change.
Figure 1.
Graph of relation between stress and buccal telomere length
Diurnal testosterone
No direct association was found between buccal TL and diurnal measures of testosterone. However, when examining the association between buccal TL and peak measurements of awakening testosterone, a significant independent effect of morning testosterone levels was found in only the male subjects.
Sex effects
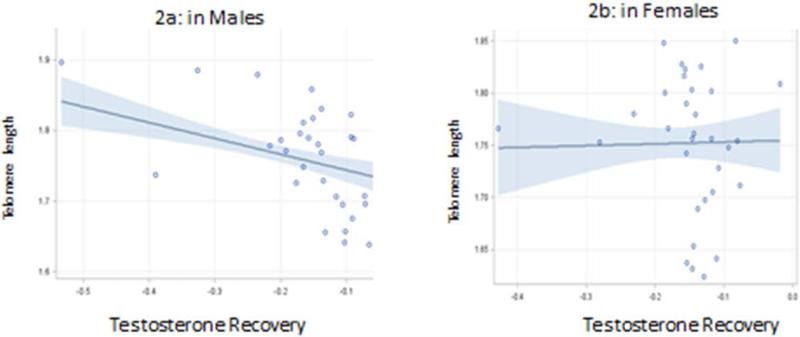
The effects of sex on the models are presented in Table 3 and graphically in Figure 2a and 2b. Significant effect modification by sex was observed. Although only significant at p < 0.05 for testosterone recovery (Table 2), all other interaction terms had p values < 0.15 and with the small sample size, likely a result of limited power as once stratified differences were evident. In boys, when controlling for awakening testosterone levels, both acute and diurnal testosterone levels were significant and independent predictors of buccal TL, with the strongest effect observed for the stress response. No association between acute testosterone or diurnal testosterone was observed in girls. Pubertal status was a significant predictor of buccal TL in both boys and girls, with more advanced pubertal status associated with decreased buccal TL. These effects are shown in Table 3 and Figure 2.
Table 3.
Generalized Estimating Equation Models of the Association between Testosterone and Average Buccal Telomere Lengtha (TL) in children, Stratified by Sex (N=70)
| Beta (SE) * | ||||||
|---|---|---|---|---|---|---|
| Variables | Acute Testosterone Peak | Acute Testosterone Recovery | Acute Testosterone Reactivity | |||
| Males | Females | Males | Females | Males | Females | |
| Testosterone | −0.047 (0.015)** | −0.003 (0.027) | −0.225 (0.053)** | 0.017 (0.088) | −0.81 (0.25)** | −0.052 (0.47) |
| Mother's Education | −0.004 (0.004) | 0.005 (0.005) | −0.003 (0.005) | 0.005 (0.005) | −0.004 (0.004) | 0.005 (0.005) |
| Puberty Stageb | −0.054 (0.011)** | −0.031 (0.008)** | −0.060 (0.009)** | −0.030 (0.008)** | −0.054 (0.011)** | −0.031 (0.008)** |
| BMI | −0.003 (0.002) | −0.001 (0.002) | −0.003 (0.001) | −0.001 (0.002) | −0.003 (0.002) | −0.001 (0.002) |
| Diurnal Testosterone | 0.043 (0.015** | −0.009 (0.029) | 0.018(0.013) | −0.012 (0.023) | 0.043 (0.015)+ | −0.010 (0.029) |
P-value < 0.05
P-value < 0.01
Average buccal cell telomere length as represented by the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio corrected for parental age at conception and child age when DNA collected.
Puberty stage classifies participants into one of five pubertal status categories based on level of development reported on the three relevant indices of pubertal change. For females, staging is based on pubic hair growth, breast development, and menarche. For males staging is based on development of pubic hair, facial hair and voice change.
Figure 2.
Association between Testosterone Stress Recovery and buccal telomere length
Discussion
These are the first findings, to our knowledge, to demonstrate an association between buccal TL and testosterone levels. In youth a significant association was demonstrated between higher peak testosterone levels and slower testosterone recovery during an acute social stressor even after accounting for sex, age, parental age at conception, maternal highest educational achievement, and puberty. Demonstrating that 2 distinct biological factors impacted by stress, TL and testosterone, are related hints toward a shared mechanism though which biological age may diverge from chronological age following early adversity. Given the increasing evidence of testosterone's role in the aging process as well as in the sculpting of the stress response systems, these findings offer data in support of a complex integrated system that includes the HPG axis as part of the biological mechanisms linking accelerated maturation and stress.
It is important to demonstrate that the process of aging spans across development, being initiated long before “getting older” may be considered problematic. Individual differences in age-related developmental biomarkers (both TL and testosterone) are apparent early, while the individual may still be “growing up” or, perhaps more accurately, “growing up too soon.” There is a wealth of literature demonstrating the challenges of early maturational events in childhood and adolescents [42, 43], such as limiting the flexibility of the child to learn, play, and adapt to a wider range of environments [44]. Our findings diverge somewhat from the interpretation of aging studies which emphasize the youth-enhancing properties of elevated testosterone, yet this has only been seen in adults [34]. It is possible that the link between TL and testosterone is limited to youth [i.e., with testosterone operating as a developmental switch-point, 45], or that the relationship apparent in adults is inverted from that observed in youth.
This interpretation is supported by theoretical evolutionary models such as life-history theory which emphasizes that there are inherent trade-offs in development and no single developmental trajectory is ideal for every context [46]. Effort directed toward growth and development cannot be simultaneously directed toward mating or parenting, so development shifts according to the costs and benefits of meeting these respective goals [47]. Early environment provides cues about the cost-benefit tradeoffs [42]. For example, a fast life-history strategy may be beneficial in an unpredictable or unstable environment as development would be directed towards early maturation, early mating and low parental investment [48]; the costs of such early maturation are largely incurred later in development [49, 50]. An individual's developmental trajectories do not consciously shift, but rather are guided by a suite of psychobiological changes including stress-responsive [45, 51] and gonadal hormone changes [40] which are responsive to the environment, longitudinally informative, and related to health [52]. Like testosterone, TL appears to fulfill each of these criteria [53]. We speculate that testosterone and TL may be operating as life-history relevant biomarkers, shifting an individual towards a fast life history strategy that favors early maturation and development while incurring the long-term costs in terms of early morbidity, mortality, and gonadal senescence [32, 54]. If so, then the link between higher testosterone and shorter TL within youth which we observed would be expected to invert or shift within older adults.
In addition to demonstrating convergence of two developmentally sensitive biomarkers of aging (testosterone reactivity and TL), the present study adds to the literature by showing that TL was specifically linked with peak testosterone and recovery from the TSST-C, beyond basal testosterone levels or the diurnal fluctuations of the gonadal axis across the day. This finding dovetails with life-history theory's emphasis on development and maturation as being stress-sensitive. That is, TL and testosterone, in youth, represent overlapping stress-sensitive biomarkers of both development and aging. Further evidence is that both are sensitive to oxidative stress, are altered by psychosocial stress, and interface with the HPA axis. There are at least two other alternative explanations for why TL and testosterone are linked. First, there is the possibility that both operate as developmentally sensitive biomarkers of aging. If that were solely the explanation, then the prediction would be that morning testosterone levels would have been linked closest with TL as those values are the highest point in the day and the most sensitive to youth's developmental stage. Second is the possibility that both TL and testosterone changes indicate maturational processes. If so, the prediction would be that TL would be most correlated with testosterone's diurnal rhythm as this captures the greatest change in testosterone values within a short time frame [55]. Our findings of the strongest association between stress testosterone and TL are most supportive of the hypothesis that TL and testosterone are biological indicators of stress-related changes in development and aging.
In adults, testosterone has been hypothesized to attenuate the stress response; however, in pre-pubertal male rodents who demonstrated a prolonged cortisol response following restraint stress, testosterone was not able to attenuate that stress, suggesting that there may be a protracted shaping of the neuroendocrine stress response system that spans puberty, and that only in adults is the HPG axis able to tighten regulation of the HPA axis [56]. Adolescent humans, who often show stress reactive testosterone, [57] and a positive link between stress and sex hormones [58], switch to a more adult-like profile as they age [59]. These studies provide additional support for the interpretation of testosterone and TL from the life-history theoretical framework given that the pattern of associations switch from adolescence into adulthood. Future studies are needed to explore whether stressors beyond the acute laboratory setting (e.g., naturalistic stressors or chronic stressors) alter developmental trajectories of these reactive biomarkers of aging. Our prediction is that testosterone and TL would be most tightly linked within individuals experiencing early and chronic psychosocial stress based on the literature that shows these experiences speed up pubertal (and perhaps gonadal) maturation [40, 42], and that testosterone and cortisol reactivity are accentuated following chronic stress [60].
Our findings were especially strong for boys in whom both basal testosterone and acute testosterone were related to TL. Several explanations for this sex difference that partially rely on the adolescent development literature exist. First, it may be that the gonadal axis is not stress-responsive in girls. This is unlikely because pubertal timing and maturation (events driven by gonadal axis hormones) is stress sensitive in girls [42]. Second, it is possible that testosterone more directly captures gonadal maturation for boys. While girls also produce testosterone in measurable quantities, much of girls' testosterone is of adrenal origin [10] and girls' gonadal maturation may be more directly tied to estradiol or progesterone [19]. Third, it may be the case that the initiation of a developmental switch-point is most stress-responsive for girls, whereas the tempo of maturation is the most stress responsive aspect for boys. Our observations that pubertal timing (i.e. the physical aspects of puberty when controlling for age and basal testosterone levels) was related to TL in girls, while testosterone reactivity was related to TL in boys, fits with this last interpretation as pubertal timing indirectly captures the developmental switch-point of adolescence. Research finds that pubertal timing is more relevant for girls whereas pubertal tempo, (i.e. the rate at which an individual progresses through puberty) is more relevant for boys in terms of the sensitivity to early stress, health risk outcomes [40, 43, 61], and testosterone changes [28, 62]. This interpretation also fits with the extant literature which finds that adjustment is challenging for boys across all maturational stages [22]. Together they suggest that although developmental events unfold differently within boys and girls, stress exposure impacts developmental trajectories in both sexes.
There are several limitations to this study. First, this represents a relatively small sample spanning a wide age range including pre-pubertal youth. This limitation was assuaged, to a certain extent, by exclusion of children whose gonadal axis was not yet activated (i.e., testosterone below detection levels). Second, this was a cross-sectional study; as such the direction of effect and developmental trajectory of this relationship can only be speculative. Longitudinal studies, particularly ones that assess this relationship across puberty, would be of significant benefit in better defining the underlying mechanism and would be especially of value for measuring pubertal tempo or the testosterone rise within boys. Third, diurnal testosterone was collected at home, and consequently, there may be random variation in accurate reporting of collection times or ecological conditions within the home on those days for which we cannot account. That the acute and diurnal testosterone scores were distinct, however, argues against diurnal testosterone variation being driven by daily stressors. Fourth, as this sample is almost entirely self-reportedly identified as black, the generalizability to other racial groups is uncertain. Given that the life history theory emphasizes developmental shifts in a high stress, unpredictable environment (which is, unfortunately, often characteristic of African-Americans [63]), however, there may be advantages in focusing on the present sample. Lastly, TL was measured from buccal cell DNA. To date there is no empirical evidence to suggest that one peripheral source of DNA for TL measurement is superior to other sources (e.g. leukocyte, buccal, saliva). Previous studies in youth have measured TL using blood, buccal and saliva, however recent data suggests that buccal cells may be the best surrogate for global epigenetic effects due to their more homogenous cell type[2, 7, 8, 64].
Our results provide the first association of a cellular marker of stress and aging with acute testosterone reactivity in youth. We found that sex significantly modulated the association between telomere length and testosterone, with testosterone reactivity being significantly associated with buccal TL in males only. The association of telomere length with pubertal timing in girls, coupled with the significant findings in males, suggests that the relation between stress, gonadal maturation, and cellular aging are different between males and females. Further our data indicate that future studies examining the association between early adversity and TL in youth need to consider sex differences. These data build on the existing literature associating telomere length with stress, and provide novel evidence of sex specific differences in the relationship between puberty, hormone stress responses, and cellular aging in youth.
Acknowledgments
Sources of support: National Institutes of Health, NIH (1R01ES020447-01 to K.P.T., R21MH094688-01 and 2K12HD043451-06 to SSD) and Tulane University Oliver Scholars Fund (SSD). The project described was also supported by Award Number K12HD043451 (SSD) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health
Footnotes
Disclaimers: None.
References
- 1.Shalev I, et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theall K, et al. Neighborhood disorder and telomeres: Connecting children's exposure to community level stress and cellular response. Social Science & Medicine. 2013;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epel E, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Hamel FG. Telomeres and type 2 diabetes. Translational Research. 2010;155(4):164–165. doi: 10.1016/j.trsl.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Dabouras V, et al. Exogenous application of glucose induces aging in rat cerebral oligodendrocytes as revealed by alteration in telomere length. Neuroscience Letters. 2004;368(1):68–72. doi: 10.1016/j.neulet.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Rhee DB, et al. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair. 2011;10:34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drury S, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2012:719–27. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalev I, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular Psychiatry. 2013;18:576–81. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needham BL, et al. Socioeconomic status and cell aging in children. Social Science & Medicine. 2012;74:1948–1951. doi: 10.1016/j.socscimed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Granger DA, et al. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29(10):1229–40. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Op de Macks ZA, et al. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev Cogn Neurosci. 2011;1(4):506–16. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peper JS, Koolschijn PC. Sex steroids and the organization of the human brain. J Neurosci. 2012;32(20):6745–6. doi: 10.1523/JNEUROSCI.1012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans EJ, et al. A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry. 2006;59(9):872–4. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiol Behav. 2007;90(2-3):496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Hermans EJ, et al. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52(1):277–83. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Schoofs D, Wolf OT. Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST). Int J Psychophysiol. 2011;80(1):36–43. doi: 10.1016/j.ijpsycho.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Buske-Kirschbaum A, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59(4):419–26. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 18.van Honk J, et al. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm Behav. 1999;36(1):17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- 19.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–37. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger DA, et al. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003;15(2):431–49. [PubMed] [Google Scholar]
- 21.Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007;24(5):969–90. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111(1):62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Egea E, et al. Testosterone in newly diagnosed, antipsychotic-naive men with nonaffective psychosis: a test of the accelerated aging hypothesis. Psychosom Med. 2011;73(8):643–7. doi: 10.1097/PSY.0b013e318230343f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorn LD, et al. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- 25.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 26.Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153(8):974–84. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 27.Hiort O. Androgens and puberty. Best Pract Res Clin Endocrinol Metab. 2002;16(1):31–41. doi: 10.1053/beem.2002.0178. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein JW, et al. Self-assessment of physical sexual maturation in boys and girls with delayed puberty. J Adolesc Health. 1999;25(6):379–81. doi: 10.1016/s1054-139x(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 29.Kulin HE, et al. Diversity of pubertal testosterone changes in boys with constitutional delay in growth and/or adolescence. J Pediatr Endocrinol Metab. 1997;10(4):395–400. doi: 10.1515/jpem.1997.10.4.395. [DOI] [PubMed] [Google Scholar]
- 30.Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology. 2005;30(1):58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Booth A, Johnson DR, Granger DA. Testosterone and men's health. J Behav Med. 1999;22(1):1–19. doi: 10.1023/a:1018705001117. [DOI] [PubMed] [Google Scholar]
- 32.Fujisawa M, et al. Telomerase activity in the testis of infertile patients with selected causes. Hum Reprod. 1998;13(6):1476–9. doi: 10.1093/humrep/13.6.1476. [DOI] [PubMed] [Google Scholar]
- 33.Meeker AK, Sommerfeld HJ, Coffey DS. Telomerase is activated in the prostate and seminal vesicles of the castrated rat. Endocrinology. 1996;137(12):5743–6. doi: 10.1210/endo.137.12.8940411. [DOI] [PubMed] [Google Scholar]
- 34.Desai N, et al. Free radical theory of aging: implications in male infertility. Urology. 2010;75(1):14–9. doi: 10.1016/j.urology.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Meldrum DR. Aging gonads, glands, and gametes: immutable or partially reversible changes? Fertil Steril. 2013;99(1):1–4. doi: 10.1016/j.fertnstert.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 36.Bekaert S, et al. Telomere length versus hormonal and bone mineral status in healthy elderly men. Mechanisms of Ageing and Development. 2005;126(10):1115–1122. doi: 10.1016/j.mad.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Cawthon RM, Cawthorn RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Research. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott J, et al. Paternal age at birth is associated with offspring leukocyte telomere length in the Nurses’ Health Study. Human Reproduction. 2012 doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen A, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 40.Ellis BJ, et al. Quality of early family relationships and the timing and tempo of puberty: effects depend on biological sensitivity to context. Dev Psychopathol. 2011;23(1):85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Meyer T, et al. Paternal age at birth is an important determinant of offspring telomere length. Human Molecular Genetics. 2007;16(24):3097–3102. doi: 10.1093/hmg/ddm271. [DOI] [PubMed] [Google Scholar]
- 42.Ellis BJ. Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull. 2004;130(6):920–58. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- 43.Ge X, et al. It's about timing and change: pubertal transition effects on symptoms of major depression among African American youths. Dev Psychol. 2003;39(3):430–9. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- 44.Bjorklund DF. The role of immaturity in human development. Psychol Bull. 1997;122(2):153–69. doi: 10.1037/0033-2909.122.2.153. [DOI] [PubMed] [Google Scholar]
- 45.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–92. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive srategy: An evolutionary theory of socialization. Child Development. 1991;62:642–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 47.Sefcek JA, Figueredo AJ. A life-history model of human fitness indicators. Biodemography Soc Biol. 2010;56(1):42–66. doi: 10.1080/19485561003709214. [DOI] [PubMed] [Google Scholar]
- 48.Sherman RA, Figueredo AJ, Funder DC. The behavioral correlates of overall and distinctive life history strategy. J Pers Soc Psychol. 2013;105(5):873–88. doi: 10.1037/a0033772. [DOI] [PubMed] [Google Scholar]
- 49.Korte SM, et al. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29(1):3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 51.Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78(6):1799–817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 52.Lupien SJ, et al. Beyond the stress concept: Allostatic Load--A developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. John Wiley & Sons; Hoboken, NJ: 2006. pp. 578–628. [Google Scholar]
- 53.Theall KP, et al. Neighborhood disorder and telomeres: Connecting children's exposure to community level stress and cellular response. Soc Sci Med. 2013;85:50–8. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simm A, et al. Potential biomarkers of ageing. Biol Chem. 2008;389(3):257–65. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
- 55.Shirtcliff E, et al. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. J Child Psychol Psychiatry. 2007;48(6):580–91. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- 56.Romeo R, et al. Testosterone Cannot Activate an Adult-Like Stress Response in Prepubertal Male Rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- 57.Eatough EM, et al. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson MM, et al. Psychopathy's influence on the coupling between hypothalamic-pituitary-adrenal and -gonadal axes among incarcerated adolescents. Developmental Psychobiology. 2013 doi: 10.1002/dev.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruttle PL, et al. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol. 2013 doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore M, Thompson C, Marler C. Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard,Urosaurus ornatus. General and Comparative Endocrinology. 1991;81(2):217–226. doi: 10.1016/0016-6480(91)90006-r. [DOI] [PubMed] [Google Scholar]
- 61.Marceau K, et al. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 2011;47(5):1389–409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raivio T, et al. Serum androgen bioactivity in adolescence: a longitudinal study of boys with constitutional delay of puberty. J Clin Endocrinol Metab. 2004;89(3):1188–92. doi: 10.1210/jc.2003-031655. [DOI] [PubMed] [Google Scholar]
- 63.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58:201–25. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lowe R, et al. Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics. 2013;8:1–10. doi: 10.4161/epi.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]