Abstract
Purpose of Review
This review summarizes significant advances in the epidemiology, pathophysiology, and treatment of congenital hypothyroidism (CH), with a focus on thyroid dysfunction in preterm infants.
Recent Findings
CH appears to be increasing in incidence, primarily due to increased stringency of screening strategies, with smaller contributions from changing demographics and improved survival of increasingly premature infants. The greatest increase has been in mildly affected infants. Although many such cases are transient, some eventually prove to be severe and/or permanent. In preterm infants, transient hypothyroidism is common and may be delayed in onset. The etiology is probably multifactorial, and inadequate iodine intake may contribute to some cases. Transient hypothyroxinemia of prematurity (THOP), also common in premature infants, is correlated with markers of inflammation. Despite concern about the potential morbidity of THOP, the benefits and safety of treatment have not been established. Novel genetic causes of CH continue to be identified, and accumulating data support the sensitivity of infants with severe CH to small changes in levothyroxine formulation.
Summary
Changes in newborn screening strategies have increasingly identified thyroid function abnormalities of unclear clinical significance. Novel causes of CH continue to be identified, and new data continue to emerge regarding optimal therapy.
Keywords: Thyroid, congenital hypothyroidism, prematurity, preterm, low birth weight
Introduction
Thyroid hormone is critical for normal growth and brain development, and hypothyroidism in infancy is the leading cause of intellectual impairment worldwide. This update will discuss significant new contributions to this field since the subject was last reviewed in February 2010 [1]. Particular attention will be given to the emerging understanding of thyroid dysfunction in preterm infants.
Incidence of Congenital Hypothyroidism
Beginning in the 1980’s, newborn screening programs have reported a rise in the incidence of CH to 1:1,400–1:2,800 infants from the rate of 1:3,000–1:4,000 when screening was first introduced [2–5]. This increase has been attributed to the widespread shift from primary T4 to primary TSH screening strategies and to the diagnosis of milder cases of CH [6]. Deladoey et al. retrospectively analyzed 1.6 million infants screened in Quebec over 20 years and found that the increase in overall incidence of CH (from 1:2,898 to 1:2,450) was entirely attributable to a decrease in the screening TSH threshold [4]. The additional cases identified were milder, with a lower median TSH concentration (18 mIU/L vs. 106.5 mIU/L) and a higher median T4 concentration (15.0 μg/dL vs. 5.9 μg/dL). Because 90% of CH patients in Quebec undergo thyroid scintigraphy, the investigators were further able to assess changes in CH incidence by etiology. They observed a stable incidence of thyroid dysgenesis and dyshormonogenetic goiter (generally more severe forms of CH); a rise was seen only in cases with a normal thyroid gland in situ, consistent with their milder phenotype [4].
An important clinical question is whether these milder cases of CH are transient or require permanent treatment. Two recent retrospective studies from Italy directly address this question. Of note, both studies used a standardized assessment of the need for ongoing treatment after age 2–3 years, overcoming a limitation of many prior studies. Olivieri et al. reviewed 1,676 cases of CH and found that 21.6% of patients with permanent CH had only mild TSH elevation (<15 mIU/L) on screening. Surprisingly, 19.6% of these patients had thyroid dysgenesis [7*]. Similar results were obtained by Rabbiosi et al. who found that 34% of 84 babies with a normal-appearing, eutopic thyroid gland had permanent hypothyroidism irrespective of whether the initial TSH elevation was mild (<20 mIU/L) or severe (≥20 mIU/L) [8*]. Furthermore, in 20% of initially mild cases the serum TSH concentration eventually rose above 100 mIU/L. These data support the conclusion that newborns with mild abnormalities on neonatal screening nonetheless have a significant risk of permanent CH that may become more severe in the future.
Another factor in the increasing incidence of CH is changing demography, as initially suggested by data from the United States [9]. Albert et al. reviewed all cases of CH diagnosed in New Zealand over 17 years and documented a rising incidence (1:3,846 to 1:2,778) due entirely to an increased rate of dyshormonogenesis, with no change in the rate of thyroid dysgenesis [5]. Based on national demographic data and the increased risk of dyshormonogenesis in Asians and Pacific Islanders, the investigators concluded that the rise in CH incidence was due solely to increased birth rates in these ethnic populations.
In recent years, survival has improved dramatically for low birth weight (LBW, 1500–2500 g) and very low birth weight (VLBW, <1500 g) infants. While these infants are at high risk of thyroid dysfunction, they are probably not the major reason for the overall increase in CH incidence. Mitchell et al. reviewed 304 cases of CH diagnosed by the New England regional screening program over a recent decade (Figure 1) [3]. The proportion of CH patients with LBW rose (5% to 17%), but the proportion with VLBW actually fell slightly (23% to 19%); overall, the increase in LBW and VLBW infants accounted for only a small fraction of the near-doubling in overall CH incidence (1:3,010 to 1:1,660). Notably, a similar conclusion was reached by the group from Quebec [4].
Figure 1.
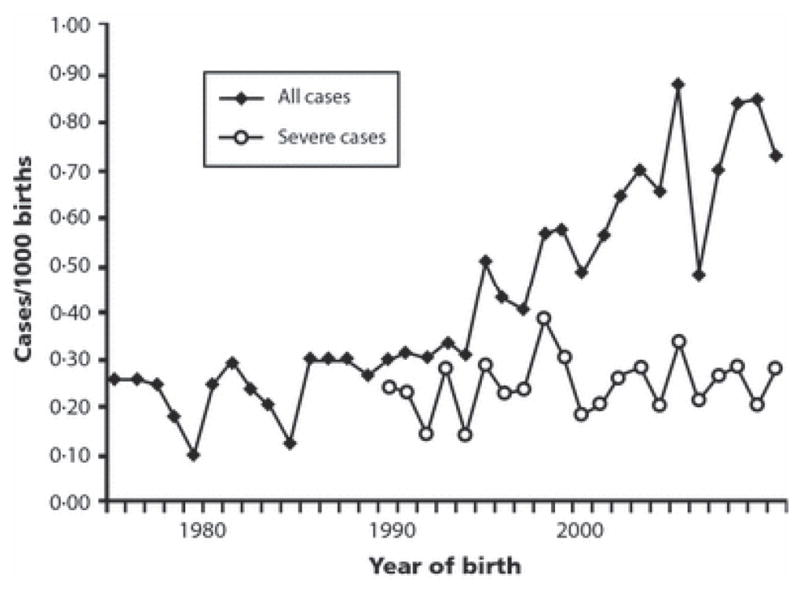
The increasing incidence of CH in New England is due to a rise in mild cases, with no change in the rate of severe CH [3]. (Reproduced with permission of John Wiley & Sons, Inc.)
Thyroid Function in Preterm Infants
Although advances in neonatal care have dramatically improved the survival of extremely preterm newborns, many such infants suffer from complications that include cerebral palsy, developmental delay, and blindness. In addition to their medical morbidity, caring for these life-long complications imposes significant costs on the medical system (estimated at $1 million per patient with cerebral palsy) [10]. Thyroid hormone is essential for normal neurodevelopment, and hypothyroxinemia is correlated with poor outcomes in preterm infants [11]. It remains unclear whether thyroid dysfunction actually contributes to neurodevelopmental deficits in these patients, but if so, treatment might both improve outcomes and reduce health care costs.
Several patterns of thyroid dysfunction are seen in preterm infants. The most common pattern is transient hypothyroxinemia of prematurity (THOP; low T4 with normal TSH), which is observed in up to 50% of infants born before 28 weeks [10]. The only interventional trial to assess the effect of L-thyroxine treatment of THOP on developmental outcome showed an initial benefit at 2 years of age in infants born < 27 weeks gestation, but a lower IQ in more mature infants, with no significant difference in either group by the age of 10 years [12, 13]. A recent study from Japan also suggests that treatment of THOP may not be benign. In a nationwide case control study, the risk of circulatory collapse was higher in VLBW infants treated with L-thyroxine than in untreated controls (4.2% vs. 1.8%) [14]. This finding, along with previous concerns for an increased risk of necrotizing enterocolitis [15], warrants close attention in future interventional trials of therapy for THOP.
In addition to THOP, VLBW infants have a risk of primary hypothyroidism (low T4 with elevated TSH) about 14 times higher than that of normal birth weight babies (1:250) [16]. Of VLBW infants with CH detected on newborn screening, nearly two-thirds exhibit a pattern of “delayed TSH rise,” in which the TSH is initially normal but later becomes elevated [16, 17]. Woo et al. retrospectively studied 22 patients with CH and delayed TSH rise identified by screening 92,800 infants over 7 years in Rhode Island, USA. The incidence of CH with delayed TSH rise increased strikingly with lower birth weight: 1:58 in extremely low birth weight (ELBW, <1000 g) infants, 1:95 in VLBW infants, and 1:30,329 in infants with birth weight ≥1500 g [18]. In 19 ELBW/VLBW infants, delayed rise in TSH was detected at a mean age of 22 days, the mean initial T4 concentration was low (4.7 μg/dL), and the mean peak TSH concentration was 62.3 mμIU/L (Table 1). Three of 19 infants (15.8%) had a peak TSH concentration >100 mIU/L and were treated with L-thyroxine, while the remaining patients were untreated. All cases of CH with delayed TSH rise were transient and resolved at an average age of 51 days, in contrast to another series in which 30% of cases were permanent [3]. Follow up data, including developmental assessments, were obtained at 18 months of age in 9/16 surviving patients (55%). Compared to matched controls, patients with delayed TSH rise showed no difference in neurological exam, or mental, or psychomotor development but had an increased incidence of small head circumference <10th percentile (33% vs. 0%), a finding of unclear clinical significance [18].
Table 1.
Clinical characteristics of infants with delayed TSH rise detected on newborn screening. Dash (—) indicates data not reported. Modified from [18].
| VLBW and ELBW | ≥1500 grams | |
|---|---|---|
| Number of cases | 19 | 3 |
| Gestational age, mean (weeks) | 25.9 | — |
| Birth weight, mean (grams) | 790 | — |
| Age at initial TSH elevation, mean (days) | 22 | 25 |
| Initial T4, mean (μg/dL) | 4.7 | 8.1 |
| Initial T4 < 5 μg/dL, n (%) | 10 (52.6) | 1 (33.3) |
| Peak TSH, mean (mIU/L) | 62.28 | 26.05 |
| Peak TSH > 40 mIU/L, n (%) | 4 (21.1) | 0 (0) |
| Peak TSH > 100 mIU/L, n (%) | 3 (15.8) | 0 (0) |
| Age at resolution, mean (days) | 51.1 | — |
The high incidence of thyroid dysfunction in preterm infants has multiple causes. These infants are often critically ill and therefore may have low serum T4 and T3 concentrations due to non-thyroidal illness (NTI). Because inflammatory cytokines have been implicated in the pathophysiology of NTI, Dilli et al. investigated the relationship of markers of systemic inflammation with thyroid function in 148 infants born at <33 weeks gestational age [19]. The authors confirmed a negative correlation between serum T3 concentration and levels of inflammatory markers (IL-6 and CRP), as well as a significantly higher rate of sepsis in patients with a low T3 concentration (<65 ng/dL).
Because the ability to escape from the Wolff-Chaikoff effect does not mature until 36 weeks, preterm infants are at risk of hypothyroidism from excess iodine exposure. Recognition of this risk has led to the removal of iodine-containing antiseptics from most intensive care nurseries and has greatly reduced this cause of hypothyroidism. However, because preterm infants have lower iodine stores and greater iodine requirements than term infants, they are also at risk for hypothyroxinemia due to iodine deficiency. The potential importance of this risk is highlighted by a study of iodine content in the nutrition provided to hospitalized preterm infants [20*]. Belfort et al. demonstrated that a hypothetical 1 kg preterm infant would receive less than the recommended 30 μg/kg/day of iodine with nearly any standard nutritional regimen. The commercial preterm formulas tested contained only 16–26 μg/kg/day of iodine, even when concentrated to maximum caloric density. Samples of pooled donor breastmilk contained surprisingly little iodine (median 9.0 μg/kg/day, range 5.0 – 17.6 μg/kg/day), and even fortification with human mild fortifier (HMF) failed to raise their iodine content to recommended levels. Notably, the parenteral nutrition solutions tested contained virtually no iodine (0.2–0.3 μg/kg/day) [20*].
Genetics of Congenital Hypothyroidism
CH may be caused by mutations in a variety of genes involved in thyroid development or thyroid hormone biosynthesis. The dual oxidase (DUOX) enzymes expressed in thyroid follicular cells generate the hydrogen peroxide necessary for the organification of iodide. Defects in DUOX2 cause a broad spectrum of thyroid dysfunction ranging from permanent or transient CH to euthyroid goiter in adults. A suggested explanation for this phenotypic variability is partial compensation of deficient DUOX2 function by the closely related DUOX1. In a mouse model, Grasberger et al. showed that while DUOX2 deficiency causes mild hypothyroidism, animals lacking both DUOX2 and DUOX1 function are severely hypothyroid [21]. Since isolated DUOX1 deficiency has never been found to cause thyroid dysfunction, this report represents the first in vivo evidence of a physiologic role for DUOX1 in the thyroid.
Although most CH is due to defects of the thyroid gland, a small proportion of CH is central in origin. Sun et al. recently described a novel genetic cause of central hypothyroidism due to mutations in IGSF1 [22]. Deficiency of IGSF1 results in X-linked central CH, either in isolation or in combination with prolactin or growth hormone deficiency. Patients with IGSF1 deficiency also develop macroorchidism and may have pubertal delay, although gonadotropin deficiency has not been described.
Treatment of Congenital Hypothyroidism
Since the introduction of newborn screening 40 years ago, early detection and treatment has essentially eradicated severe intellectual impairment due to CH in the developed world. However, individuals with CH may still have subtle neurodevelopmental problems including motor delays, behavioral difficulties, attention deficit, and alterations in memory [11]. Whether these deficits are simply due to inadequate postnatal treatment was addressed by a recent Dutch study. Van der Sluijs Veer et al. studied 95 toddlers with CH in whom L-thyroxine treatment had been started at a median age of 9 days, with normalization of the serum free T4 concentration within 2.1 days and of the serum TSH level within 18.6 days [23*]. Nevertheless, at age 2 years patients with severe CH had delays in mental development relative to the general population (mental development index 88 vs. 100, p < 0.001), while similar delays in psychomotor development were evident regardless of the severity of CH (psychomotor development index 89 vs. 100, p < 0.001). Deficits were more pronounced in patients with lower initial free T4 levels, but were unrelated to the initial L-thyroxine dose or to timing of treatment. The authors argue that prenatal hypothyroxinemia may be important in the long-term sequelae of CH, but further studies are needed to determine whether these deficits are sustained at an older age, when cognitive testing is more reliable.
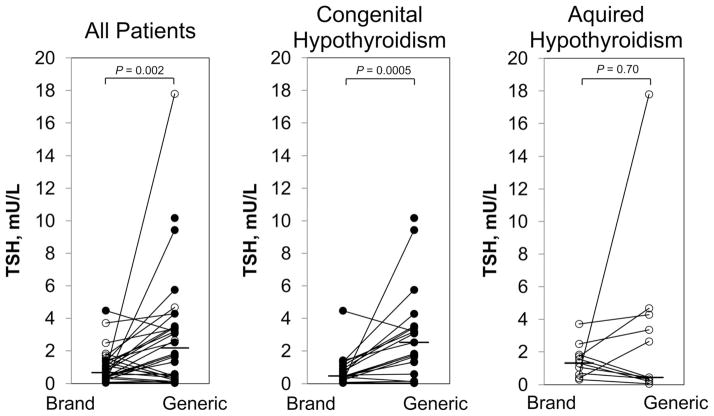
A longstanding question in the treatment of hypothyroidism is the bioequivalence of various formulations of L-thyroxine. This issue is especially relevant to young children with CH in whom maintaining consistent euthyroidism is critical to normal brain developmental. A study directly assessing this question in children with severe CH was recently performed by Carswell et al. [24*]. These investigators conducted a randomized, controlled crossover trial to assess control of hypothyroidism in individual patients when using a brand name L-thyroxine formulation vs. a single generic formulation that are deemed “bioequivalent” by regulatory standards. They demonstrated that TSH values were significantly lower (by 1.4 mIU/L) on brand name than on generic L-thyroxine, with no difference in the serum free T4 or total T3 concentration (Figure 2).
Figure 2.
Serum TSH concentration after 8 weeks of brand name LT4 vs. generic formulation. Closed circles denote patients with congenital hypothyroidism; open circles indicate patients with acquired hypothyroidism. Lines connect paired data. The horizontal bar indicates the median value. The serum TSH concentration was significantly greater after generic vs. brand name product only in patients with CH [24*]. (Republished with permission of The Endocrine Society from Carswell JM, Gordon JH, Popovsky E et al. Generic and brand-name L-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J Clin Endocrinol Metab 2013; 98:610–617; permission conveyed through Copyright Clearance Center, Inc.)
Apparently contradictory findings were reported by Lomenick et al., who retrospectively studied 62 CH patients under age 3 years taking a generic L-thyroxine product or a specific brand name formulation [25*]. They found no difference in the number of TSH checks or L-thyroxine dose adjustments but noted a possible decrease in variance of the serum TSH concentration in the generic group. The authors concluded that generic and brand name L-thyroxine are equally effective in young children with CH. A limitation of this study was that only 19% of subjects changed from one formulation to another; therefore, the interchangeability of L-thyroxine formulations in a given patient was not assessed. It is also notable that the majority of patients studied by Carswell et al. had thyroid dysgenesis, resulting in more severe CH (median serum TSH concentration >200 mIU/L) than those studied by Lomenick et al. (median TSH 58 mIU/L and 81 mIU/L in the generic and brand name groups, respectively). In patients with severe acquired hypothyroidism, Carswell et al. found no significant difference between treatment groups (Figure 2), similar to the result of Lomenick et al. Taken together, both studies support the conclusion that brand name and generic L-thyroxine formulations may not be interchangeable in patients with little or no thyroid reserve, but that the small difference between preparations is less important in patients with milder functional impairment.
Liquid formulations of L-thyroxine have traditionally been avoided for treating CH in the United States due to concerns about variable bioavailability. Cassio et al. investigated a new liquid formulation of L-thyroxine by randomizing infants with CH to receive a standard dose of L-thyroxine in either liquid or tablet form [26*]. The two preparations resulted in equally effective treatment of hypothyroidism in patients with mild or moderate CH, but the liquid formulation caused significantly more TSH suppression. These data suggest that liquid and tablet formulations of L-thyroxine are not equally bioavailable. In addition, the liquid formulation contains ethanol as an excipient, and the effect on neonates of prolonged exposure to this dose of ethanol is unknown.
Conclusion
Recent data continue to illuminate the incidence, etiology, optimal treatment and outcome of CH, including the disorders of thyroid function seen predominantly in infants born preterm. However, new data have raised new questions. Increased understanding of the basic pathophysiology of thyroid dysfunction and of variables underlying developmental complications in CH will hopefully lead to further optimization of treatment and improvement in outcomes in the future.
KEY POINTS.
The incidence of congenital hypothyroidism has risen over the last several decades, primarily due to changes in newborn screening strategies.
Preterm newborns are at high risk for thyroid dysfunction, including THOP and CH with delayed TSH rise, but the clinical significance of these conditions remains uncertain.
Patients with severe CH remain at risk for subtle neurocognitive deficits and may be sensitive to small variations in L-thyroxine dose.
Acknowledgments
This work was supported by National Institutes of Health Grant K12 HD052896-06 (to AJW).
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose relevant to the content of this review.
References
- 1.Raymond J, LaFranchi SH. Fetal and neonatal thyroid function: review and summary of significant new findings. Current opinion in endocrinology, diabetes, and obesity. 2010;17:1–7. doi: 10.1097/MED.0b013e328333b0b2. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DA, Dussault JH, Foley TP, Jr, et al. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979;94:700–705. doi: 10.1016/s0022-3476(79)80133-x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell ML, Hsu HW, Sahai I. The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf) 2011;75:806–810. doi: 10.1111/j.1365-2265.2011.04128.x. [DOI] [PubMed] [Google Scholar]
- 4.Deladoey J, Ruel J, Giguere Y, Van Vliet G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J Clin Endocrinol Metab. 2011;96:2422–2429. doi: 10.1210/jc.2011-1073. [DOI] [PubMed] [Google Scholar]
- 5.Albert BB, Cutfield WS, Webster D, et al. Etiology of increasing incidence of congenital hypothyroidism in New Zealand from 1993–2010. J Clin Endocrinol Metab. 2012;97:3155–3160. doi: 10.1210/jc.2012-1562. [DOI] [PubMed] [Google Scholar]
- 6.Corbetta C, Weber G, Cortinovis F, et al. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH) Clin Endocrinol (Oxf) 2009;71:739–745. doi: 10.1111/j.1365-2265.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 7*.Olivieri A, Corbetta C, Weber G, et al. Congenital Hypothyroidism due to Defects of Thyroid Development and Mild Increase of TSH at Screening: Data From the Italian National Registry of Infants With Congenital Hypothyroidism. J Clin Endocrinol Metab. 2013;98:1403–1408. doi: 10.1210/jc.2012-3273. A substantial proportion of neonates with mild hypothyroidism at screening may require permanent therapy. [DOI] [PubMed] [Google Scholar]
- 8*.Rabbiosi S, Vigone MC, Cortinovis F, et al. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after reevaluation. J Clin Endocrinol Metab. 2013;98:1395–1402. doi: 10.1210/jc.2012-3174. CH in infants with a normal-appearing thyroid gland in situ may later prove to be permanent and/or severe even if initial TSH elevation is mild. [DOI] [PubMed] [Google Scholar]
- 9.Hinton CF, Harris KB, Borgfeld L, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125 (Suppl 2):S37–47. doi: 10.1542/peds.2009-1975D. [DOI] [PubMed] [Google Scholar]
- 10.La Gamma EF, Paneth N. Clinical importance of hypothyroxinemia in the preterm infant and a discussion of treatment concerns. Curr Opin Pediatr. 2012;24:172–180. doi: 10.1097/MOP.0b013e32835067cc. [DOI] [PubMed] [Google Scholar]
- 11.Delahunty C, Falconer S, Hume R, et al. Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J Clin Endocrinol Metab. 2010;95:4898–4908. doi: 10.1210/jc.2010-0743. [DOI] [PubMed] [Google Scholar]
- 12.van Wassenaer AG, Kok JH, de Vijlder JJ, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N Engl J Med. 1997;336:21–26. doi: 10.1056/NEJM199701023360104. [DOI] [PubMed] [Google Scholar]
- 13.van Wassenaer AG, Westera J, Houtzager BA, Kok JH. Ten-year follow-up of children born at <30 weeks’ gestational age supplemented with thyroxine in the neonatal period in a randomized, controlled trial. Pediatrics. 2005;116:e613–618. doi: 10.1542/peds.2005-0876. [DOI] [PubMed] [Google Scholar]
- 14.Kawai M, Kusuda S, Cho K, et al. Nationwide surveillance of circulatory collapse associated with levothyroxine administration in very-low-birthweight infants in Japan. Pediatrics international: official journal of the Japan Pediatric Society. 2012;54:177–181. doi: 10.1111/j.1442-200X.2011.03518.x. [DOI] [PubMed] [Google Scholar]
- 15.La Gamma EF, van Wassenaer AG, Ares S, et al. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks’ gestation. Pediatrics. 2009;124:e258–268. doi: 10.1542/peds.2008-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson C, Hermos R, Delaney A, et al. Risk factors associated with delayed thyrotropin elevations in congenital hypothyroidism. J Pediatr. 2003;143:587–591. doi: 10.1067/S0022-3476(03)00332-9. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell ML, Walraven C, Rojas DA, et al. Screening very-low-birthweight infants for congenital hypothyroidism. Lancet. 1994;343:60–61. doi: 10.1016/s0140-6736(94)90918-0. [DOI] [PubMed] [Google Scholar]
- 18.Woo HC, Lizarda A, Tucker R, et al. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: incidence and growth and developmental outcomes. J Pediatr. 2011;158:538–542. doi: 10.1016/j.jpeds.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Dilli D, Dilmen U. The role of interleukin-6 and C-reactive protein in non-thyroidal illness in premature infants followed in neonatal intensive care unit. J Clin Res Pediatr Endocrinol. 2012;4:66–71. doi: 10.4274/jcrpe.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Belfort MB, Pearce EN, Braverman LE, et al. Low iodine content in the diets of hospitalized preterm infants. J Clin Endocrinol Metab. 2012;97:E632–636. doi: 10.1210/jc.2011-3369. Nutritional regimens for hospitalized preterm infants may provide less than the recommended dietary intake of iodine, which may contribute to their high rate of thyroid dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasberger H, De Deken X, Mayo OB, et al. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol. 2012;26:481–492. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Bak B, Schoenmakers N, et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nature Genetics. 2012;44:1375–1381. doi: 10.1038/ng.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.van der Sluijs Veer L, Kempers MJ, Wiedijk BM, et al. Evaluation of cognitive and motor development in toddlers with congenital hypothyroidism diagnosed by neonatal screening. J Dev Behav Pediatr. 2012;33:633–640. doi: 10.1097/DBP.0b013e3182690727. Patients with severe CH may have mild cognitive and psychomotor delays despite early and apparently adequate treatment. [DOI] [PubMed] [Google Scholar]
- 24*.Carswell JM, Gordon JH, Popovsky E, et al. Generic and brand-name L-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J Clin Endocrinol Metab. 2013;98:610–617. doi: 10.1210/jc.2012-3125. This randomized crossover study demonstrated that generic and brand-name L-thyroxine are not bioequivalent in children with severe CH but are equally effective in children with acquired hypothyroidism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Lomenick JP, Wang L, Ampah SB, et al. Generic levothyroxine compared with synthroid in young children with congenital hypothyroidism. J Clin Endocrinol Metab. 2013;98:653–658. doi: 10.1210/jc.2012-3558. This retrospective study found no difference between generic and brand-name L-thyroxine in the treatment of children with less severe CH. [DOI] [PubMed] [Google Scholar]
- 26*.Cassio A, Monti S, Rizzello A, et al. Comparison between Liquid and Tablet Formulations of Levothyroxine in the Initial Treatment of Congenital Hypothyroidism. J Pediatr. 2013;162:1264–1269. doi: 10.1016/j.jpeds.2012.11.070. This randomized, controlled trial showed that liquid and tablet formulations of L-thyroxine are not completely bioequivalent in infants with CH. [DOI] [PubMed] [Google Scholar]