Abstract
BACKGROUND:
There is a wide variability in measurement methodology of physical activity. This study investigated the effect of different analysis techniques on the statistical power of physical activity outcomes after pulmonary rehabilitation.
METHODS:
Physical activity was measured with an activity monitor armband in 57 patients with COPD (mean ± SD age, 66 ± 7 years; FEV1, 46 ± 17% predicted) before and after 3 months of pulmonary rehabilitation. The choice of the outcome (daily number of steps [STEPS], time spent in at least moderate physical activity [TMA], mean metabolic equivalents of task level [METS], and activity time [ACT]), impact of weekends, number of days of assessment, postprocessing techniques, and influence of duration of daylight time (DT) on the sample size to achieve a power of 0.8 were investigated.
RESULTS:
The STEPS and ACT (1.6-2.3 metabolic equivalents of task) were the most sensitive outcomes. Excluding weekends decreased the sample size for STEPS (83 vs 56), TMA (160 vs 148), and METS (251 vs 207). Using 4 weekdays (STEPS and TMA) or 5 weekdays (METS) rendered the lowest sample size. Excluding days with < 8 h wearing time reduced the sample size for STEPS (56 vs 51). Differences in DT were an important confounder.
CONCLUSIONS:
Changes in physical activity following pulmonary rehabilitation are best measured for 4 weekdays, including only days with at least 8 h of wearing time (during waking hours) and considering the difference in DT as a covariate in the analysis.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00948623; URL: www.clinicaltrials.gov
In patients with COPD, physical inactivity is believed to play a crucial role in the development of comorbidities (ie, skeletal muscle weakness, osteoporosis, depression, exercise intolerance, cardiovascular disease).1‐3 Moreover, physical inactivity is an independent predictor of adverse outcome4,5 and affects quality of life.6,7 Increasing physical activity has become a patient-centered goal for the treatment of patients with COPD. Unfortunately, the literature suggests that after following a pulmonary rehabilitation program, an enhancement of physical activity is not guaranteed.8,9 The lack of statistically significant improvements can be due to a failure of interventions to achieve behavioral changes or to the conduct of underpowered studies unable to account for the variability in the outcome measure.
Physical activity is characterized by large variability because it is measured under unstandardized conditions. Recommendations identify the need for optimal activity monitor schedules in field research.10 Factors affecting standardization are related to intrinsic differences in physical activity levels from day-to-day, extrinsic variability (ie, climatologic conditions, seasons11,12); the measurement itself (ie, the monitor, the number of days of assessment, the number of hours of measurement); and postprocessing (ie, days and time use in the analysis). Minimizing the noise around the measure of physical activity can enhance the statistical power of studies, whereas minimizing the number of days and hours of assessment reduces the burden to patients, which may contribute to study compliance.
The aim of the present study was to find a standardized method of physical activity measurement and data analysis to improve the power of physical activity-related outcomes, predominantly by reducing the variability of the outcomes and optimizing the effect size. We explored the following research questions: What is the impact of (1) the chosen outcome, (2) the exclusion of weekends, (3) increasing the number of days of assessment, and (4) altering the postprocessing analysis techniques (eg, time set used, definition of valid days according to wearing time, correction for daylight time)?
We hypothesized that the variability in physical activity can be reduced by excluding weekends, using more assessment days, comparing the same days of the week at both time points, using a fixed time frame for physical activity analysis (eg, 7:00 am-8:00 pm), and omitting days with a low monitor wearing time. Previous studies13‐17 identified the number of days of assessment through cross-sectional data analysis. The present study compared the impact of different techniques of analysis on the intervention effect after rehabilitation.
Materials and Methods
Study Subjects and Design
The baseline and 3-month data of a rehabilitation study (Clinical Trials registry No.: NCT00948623; approved by UZ Leuven Medical Ethics Committee [B32220095599]) were used to investigate variability in physical activity. Patients with stable COPD2 (no exacerbations in preceding 4 weeks) referred for outpatient pulmonary rehabilitation were randomly assigned to a conventional rehabilitation group (described in detail elsewhere18) or a conventional rehabilitation plus counseling group. In the present analysis, both groups are combined. The sample size calculation of the original study was based on the primary aim of the rehabilitation study; hence, the present sample should be seen as a convenience sample and was judged to be appropriate for the present (sub)analysis. This judgment was based on a sensitivity analysis where random patients were left out of the analysis, which showed no change in mean and SD of the effect (e-Appendix 1 (773.2KB, pdf) ). In addition, the present study sample is one of the larger samples analyzing the (objectively measured) physical activity of patients undergoing pulmonary rehabilitation.9 Fifty-seven of the subjects already had accelerometer data files both at baseline and after 3 months. These data were retrieved for this investigation. Informed consent was obtained prior to the start of the study. More information can be found in e-Appendix 1 (773.2KB, pdf) .
Clinical Measurements
All subjects underwent spirometry (Jaeger MasterScreen Body; CareFusion Corp) according to European Respiratory Society and American Thoracic Society standards. The results were referred to the predicted normal values proposed by Quanjer et al.19 A 6-min walk test was performed in a 50-m corridor, and the best of two tests was used.20
Physical activity was measured before and immediately after rehabilitation for 7 consecutive days with the SenseWear Pro armband (BodyMedia, Inc), which detects wearing time directly by skin contact.21 The SenseWear Pro armband has been thoroughly validated.22‐24
Subjects were asked to wear the monitor whenever awake. They refrained from their rehabilitation program in the week of the physical activity assessment. The minute-by-minute output of the number of steps and metabolic equivalents of task (METs) was exported for further analysis using SenseWear Professional, version 6.0 software (BodyMedia, Inc).
The variables chosen for this analysis were the total daily number of steps (STEPS); daily time spent in at least moderate physical activity (TMA), defined as any activity ≥ 3 METs25; and daily mean METs level (METS). Active time was also defined at lower thresholds (between 1 and 3 METs) using a 0.1-MET increase (e-Appendix 1 (773.2KB, pdf) ).
Data Treatment
The full analysis set comprised all available minute-by-minute data before and after rehabilitation. From the full analysis set, several datasets were constructed to test various hypotheses (e-Appendix 1 (773.2KB, pdf) ).
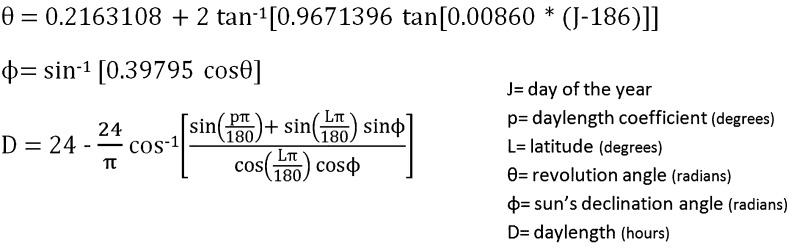
Duration of daylight time (DT) has been suggested as a proxy for seasonality. DT of each measured day was predicted using the CSIRO (Commonwealth Scientific and Industrial Research Organisation) Biosphere model, with a day length coefficient of 0.8333 and latitude of 50.78° (Fig 1).26
Figure 1 .
– Formula used to calculate daylight time based on the day of the year and latitude (CSIRO [Commonwealth Scientific and Industrial Research Organisation] Biosphere model).26 Α day length coefficient of 0.8333° (US government definition of day length) and a latitude of 50.78° (Belgium) were used to predict the daylight time. Northern latitudes are positive, southern latitudes are negative, and daylight time is calculated in hours (and converted to minutes).
Statistics
Minute-by-minute datasets were analyzed with SAS, version 9.3 (SAS Institute Inc) statistical software. Two different approaches were used to identify the impact of standardization:
1. Similar to previous cross-sectional research,13‐17 the intraclass correlation coefficient (ICC) of the baseline measurement was used to assess reliability and identify the optimal number of days of measurement. The usually desired ICC value for multiple days is 0.80.17 In addition, the repeatability coefficient of the baseline measurement was calculated as a measure of absolute reliability.27
2. Based on data of the intervention effect, robustness of the measurement was expressed as the sample size needed to achieve a power of 0.8 with a significance level α = .05. Sample size calculation (two-tailed) was done with an a priori power calculation using G*Power 3.1.3.28
To identify a difference between the analysis techniques, a paired t test was performed. The influence of a difference in DT on the intervention effect was studied using a mixed-model analysis. The corrected intervention and interaction effects (ΔPA × ΔDT [where PA = physical activity]) were retrieved. P < .05 was considered statistically significant.
Results
Subjects
Fifty-seven subjects with COPD were analyzed. The subject characteristics are shown in Table 1.
TABLE 1 .
] Baseline Subject Characteristics
| Characteristic | Baseline Value |
| Sex | |
| Male | 47 (82) |
| Female | 10 (18) |
| Age, y | 66 ± 7 |
| BMI, kg/m2 | 26 ± 7 |
| FEV1, L | 1.28 ± 0.46 |
| FEV1, % predicted | 46 ± 17 |
| FVC, L | 3.10 ± 0.79 |
| FVC % predicted | 88 ± 22 |
| FEV1/FVC, % | 41 ± 11 |
| GOLD stage | |
| I | 4 (7) |
| II | 15 (26) |
| III | 29 (51) |
| IV | 9 (16) |
| 6MWD, m | 427 ± 105 |
| 6MWD, % predicted | 67 ± 17 |
Data are presented as No. (%) or mean ± SD. 6MWD = 6-min walk distance; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Impact of Outcome Used
A summary of the effect and variability of the intervention is provided in Table 2. The choice of the physical activity outcome used (STEPS, TMA, METS) had important implications in identifying intervention effects. Use of a 7-day measurement (mean effect compared with baseline, 21% for STEPS, 29% for TMA, and 4% for METS) resulted in a calculated sample size of 83 subjects for STEPS, 160 for TMA, and 215 for METS. The use of an activity threshold between 1.3 and 2.3 METs lowered the sample size (lowest at 1.3 METs, 69 subjects) (e-Appendix 1 (773.2KB, pdf) ).
TABLE 2 .
] Influence of the Outcome Used, Exclusion of Weekends, and Number of Days of Measurement on the Calculated Sample Size
| Outcome | Baseline | 3 mo | Δ | p5-p95 | P Value |
| STEPS, No./d | |||||
| 2 d | 4,038 ± 2,783 | 4,837 ± 3,333 | 799 ± 2,625 | −2,683 to 7,629 | .0253 |
| 3 d | 3,989 ± 2,639 | 4,814 ± 3,222 | 824 ± 2,495 | −2,126 to 4,815 | .0156 |
| 4 d | 3,966 ± 2,636 | 4,932 ± 3,381 | 967 ± 2,505 | −1,775 to 5,212 | .0051 |
| 5 d | 3,931 ± 2,566 | 4,846 ± 3,257 | 916 ± 2,392 | −1,599 to 4,948 | .0055 |
| 7 d | 3,892 ± 2,656 | 4,692 ± 3,284 | 800 ± 2,560 | −1,771 to 9,183 | .0218 |
| TMA, min/d | |||||
| 2 d | 51 ± 64 | 62 ± 64 | 11 ± 67 | −116 to 134 | .2199 |
| 3 d | 50 ± 58 | 64 ± 77 | 14 ± 69 | −76 to 176 | .1309 |
| 4 d | 50 ± 58 | 66 ± 78 | 16 ± 67 | −67 to 161 | .0784 |
| 5 d | 50 ± 55 | 64 ± 75 | 15 ± 63 | −58 to 158 | .0855 |
| 7 d | 49 ± 52 | 63 ± 76 | 14 ± 63 | −66 to 153 | .0976 |
| METS | |||||
| 2 d | 1.333 ± 0.28 | 1.375 ± 0.33 | 0.042 ± 0.29 | −0.445 to 0.478 | .2792 |
| 3 d | 1.331 ± 0.26 | 1.379 ± 0.34 | 0.048 ± 0.31 | −0.417 to 0.464 | .2447 |
| 4 d | 1.332 ± 0.26 | 1.388 ± 0.35 | 0.056 ± 0.30 | −0.402 to 0.401 | .1640 |
| 5 d | 1.327 ± 0.26 | 1.383 ± 0.34 | 0.055 ± 0.28 | −0.371 to 0.485 | .1450 |
| 7 d | 1.319 ± 0.24 | 1.374 ± 0.35 | 0.055 ± 0.29 | −0.271 to 0.424 | .1524 |
Data are presented as mean ± SD. Δ = found intervention effect; METS = mean metabolic equivalents of task level; p5-p95 = percentile 5 to percentile 95 of the found intervention effect; STEPS = daily number of steps; TMA = time spent in at least moderate physical activity.
Impact of Days of Assessment
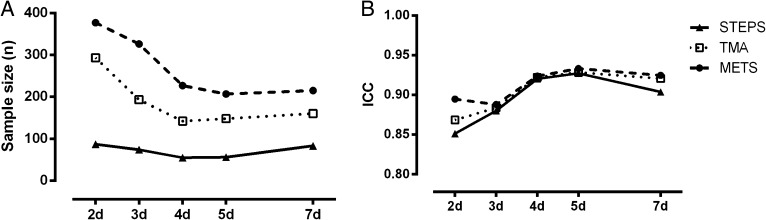
Excluding weekends did not have an impact on the intervention effect but did decrease the sample size to achieve the intended power (Fig 2) (33% [56 subjects vs 83 subjects] for STEPS, 4% [207 subjects vs 215 subjects] for METS, and 8% [148 subjects vs 160 subjects] for TMA) because of a reduction of the SD of the effect (Table 2). The sample size decreased gradually until 4 days of measurement for STEPS and TMA and until 5 days for METS (Fig 2). Comparing the same weekdays prerehabilitation and postrehabilitation did not enhance the robustness for any of the outcome measurements and resulted in a loss of subjects with no valid comparable data before and after intervention.
Figure 2 .
– Influence of the number of days of measurement and exclusion of weekend days on the calculated sample size and ICC. A, Sample size needed to achieve a power of 0.8 with a significance level of .05 (STEPS, TMA, and METS) in 2 to 5 (random) weekdays and a whole week of measurement. B, ICCs (STEPS, TMA, and METS) in 2 to 5 (random) weekdays and a whole week of measurement. ICC = intraclass correlation coefficient; METS = mean metabolic equivalents of task level; TMA = time spent in at least moderate physical activity; STEPS = daily number of steps.
Solely based on achieving an acceptable reliability (ICC > 0.8), one would conclude that 2 days for STEPS (ICC = 0.85), TMA (ICC = 0.87), and METS (ICC = 0.89) would be sufficient. The ICC further increased up until 5 weekdays of measurement (STEPS, 0.93; TMA, 0.93; METS, 0.93) but slightly worsened when including the weekend days (STEPS, 0.90; TMA, 0.92; METS, 0.92) for all outcomes (Fig 2). Analysis of the repeatability coefficient showed a comparable result with a gradual decrease from 2 to 5 weekdays (STEPS, 1,157 steps/d vs 497 steps/d; TMA, 66 min vs 41 min; METS, 0.25 vs 0.19 METs).
Difference in Postprocessing Analysis
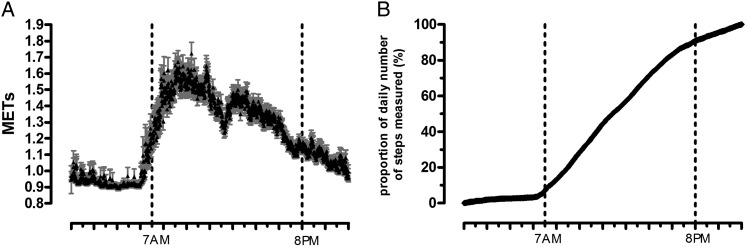
Subjects were most active between 7:00 am and 8:00 pm (Fig 3A). During this period, 84% of steps were captured (Fig 3B). The influence of the various analysis techniques is shown in Table 3. Altering the postprocessing technique had little to no impact on the statistical significance of the intervention’s effect for any of the outcomes.
Figure 3 .
– Whole-day physical activity pattern. Mean min-by-min physical activity pattern (all data, 7 d) of all patients. A, Mean METs per minute, presented as mean ± SEM (gray). B, Proportion of total number of steps measured presented min by min. METs = metabolic equivalents of task.
TABLE 3 .
] Influence of Various Analysis Techniques on the Calculated Sample Size (Measurement Based on 5 Weekdays)
| Technique | Baseline | 3 mo | Δ | p5-p95 | P Value | Sample Size |
| STEPS, No./d | ||||||
| All data | 3,931 ± 2,565 | 4,846 ± 3,257 | 916 ± 2,392 | −1,599 to 4,948 | .0055 | 56 |
| 7:00 am-8:00 pm | 3,664 ± 2,456 | 4,529 ± 3,143 | 866 ± 2,298 | −1,430 to 4,963 | .0062 | 58 |
| > 480 min | 3,981 ± 2,602 | 4,927 ± 2,364 | 946 ± 2,364 | −1,631 to 4,948 | .0038 | 51 |
| > 600 min | 4,026 ± 2,666 | 4,988 ± 3,253 | 962 ± 2,445 | −1,631 to 4,948 | .0044 | 53 |
| Combination | 3,711 ± 2,464 | 4,652 ± 2,272 | 941 ± 2,272 | −1,352 to 4,963 | .0028 | 48 |
| TMA, min/d | ||||||
| All data | 50 ± 55 | 64 ± 74 | 15 ± 63 | −58 to 158 | .0855 | 148 |
| 7:00 am-8:00 pm | 48 ± 53 | 61 ± 69 | 13 ± 60 | −58 to 115 | .0957 | 158 |
| > 480 min | 50 ± 56 | 65 ± 75 | 15 ± 63 | −61 to 158 | .0873 | 150 |
| > 600 min | 51 ± 57 | 66 ± 76 | 15 ± 65 | −61 to 158 | .0895 | 152 |
| Combination | 48 ± 53 | 62 ± 69 | 14 ± 60 | −58 to 115 | .0903 | 153 |
| METS | ||||||
| All data | 1.327 ± 0.26 | 1.375 ± 0.34 | 0.055 ± 0.28 | −0.445 to 0.478 | .1450 | 207 |
| 7:00 am-8:00 pm | 1.395 ± 0.29 | 1.466 ± 0.36 | 0.071 ± 0.30 | −0.428 to 0.626 | .0763 | 140 |
| > 480 min | 1.330 ± 0.26 | 1.387 ± 0.34 | 0.057 ± 0.29 | −0.371 to 0.485 | .1349 | 197 |
| > 600 min | 1.330 ± 0.26 | 1.388 ± 0.34 | 0.058 ± 0.29 | −0.371 to 0.488 | .1418 | 204 |
| Combination | 1.395 ± 0.29 | 1.467 ± 0.36 | 0.072 ± 0.30 | −0.428 to 0.626 | .0780 | 141 |
| Wearing time, min/d | ||||||
| All data | 890 ± 808 | 916 ± 812 | … | … | … | … |
| No. d | 4.75 | 4.37 | … | … | … | … |
| 7:00 am-8:00 pm | 689 ± 62 | 680 ± 83 | … | … | … | … |
| No. d | 4.72 | 4.37 | … | … | … | … |
| > 480 min | 901 ± 223 | 924 ± 255 | … | … | … | … |
| No. d | 4.67 | 4.25 | … | … | … | … |
| > 600 min | 912 ± 233 | 932 ± 256 | … | … | … | … |
| No. d | 4.53 | 4.09 | … | … | … | … |
| Combination | 698 ± 57 | 697 ± 65 | … | … | … | … |
| No. d | 4.58 | 4.12 | … | … | … | … |
Data are presented as mean ± SD unless otherwise indicated. 7:00 am-8:00 pm = data exclusion before 7:00 am and after 8:00 pm; > 480 min = exclusion of days with ≤ 480 min of wearing time; > 600 min = exclusion of days with ≤ 600 min of wearing time; combination = included data between 7:00 am and 8:00 pm of days with minimum of 480 min of wearing time in this period. See Table 2 legend for expansion of other abbreviations.
Excluding data recorded before 7:00 am and after 8:00 pm reduced the sample size needed for METS. Analyzing days with a valid wearing time (a minimum of 480 min) reduced the sample size needed for STEPS (Table 3). Excluding days of < 12 h wearing time had no further impact on the sample size but resulted in the loss of seven subjects (12%) who lacked valid days of assessment in this scenario.
Sixteen subjects (28%) started in winter, 19 (33%) in spring, 16 (28%) in summer, and six (11%) in autumn, with a mean DT measurement of 758 ± 180 min at baseline and 731 ± 167 min at 3 months. For all outcome measurements, the difference in DT had a significant influence on the intervention effect. The impact of DT remained in all scenarios of postprocessing. Correcting for the difference in DT resulted in slightly larger intervention effects, which further reduced the sample size requirements (Table 4).
TABLE 4 .
] The Influence of Daylight Time on the Intervention Effect and Calculated Sample Size (Measurement Based on 5 Weekdays)
| Intervention Effect (Δ) Unadjusteda | Interaction Effectb | Intervention Effect (Δ) Adjustedc | ||||
| Technique | Mean ± SD | P Value | P Value | Mean ± SD | P Value | Sample Size |
| STEPS, No./d | ||||||
| All data | 916 ± 2,392 | .0055 | .0129 | 990 ± 2,291 | .0019 | 44 |
| 7:00 am-8:00 pm | 866 ± 2,298 | .0062 | .0217 | 932 ± 2,219 | .0025 | 47 |
| > 480 min | 946 ± 2,364 | .0038 | .0079 | 1,024 ± 2,247 | .0011 | 40 |
| > 600 min | 962 ± 2,445 | .0044 | .0023 | 1,055 ± 2,276 | .0009 | 39 |
| Combination | 941 ± 2,272 | .0028 | .0203 | 1,007 ± 2,192 | .0010 | 40 |
| TMA, min/d | ||||||
| All data | 15 ± 63 | .0855 | .0016 | 17 ± 58 | .0314 | 94 |
| 7:00 am-8:00 pm | 13 ± 60 | .0957 | .0030 | 16 ± 56 | .0395 | 99 |
| > 480 min | 15 ± 63 | .0873 | .0014 | 17 ± 59 | .0316 | 97 |
| > 600 min | 15 ± 65 | .0895 | .0006 | 18 ± 59 | .0284 | 87 |
| Combination | 14 ± 60 | .0903 | .0027 | 16 ± 56 | .0361 | 99 |
| METS | ||||||
| All data | 0.055 ± 0.28 | .1450 | .0101 | 0.065 ± 0.27 | .0767 | 138 |
| 7:00 am-8:00 pm | 0.071 ± 0.30 | .0763 | .0028 | 0.080 ± 0.28 | .0297 | 99 |
| > 480 min | 0.057 ± 0.29 | .1349 | .0092 | 0.067 ± 0.27 | .0696 | 130 |
| > 600 min | 0.058 ± 0.29 | .1418 | .0052 | 0.068 ± 0.28 | .0686 | 136 |
| Combination | 0.072 ± 0.30 | .0780 | .0024 | 0.083 ± 0.28 | .0298 | 92 |
Discussion
The present study showed that the choice of outcome and the processing of physical activity data had important implications for the statistical significance of the results. These methodologic choices, thus, affect the number of subjects required in a study. Excluding weekends improved the robustness, as did the inclusion of more weekdays. A difference in DT had an important influence on the observed change in physical activity. We hypothesized that this effect would be counteracted by limiting the assessment time to between 7:00 am and 8:00 pm, but this was not the case. The methodology of data processing has a significant impact on the outcome and estimates of effect, which was also highlighted by the American College of Sports Medicine.10
Data provided by activity monitors are commonly used to measure daily physical activity. In patients with COPD, validation studies highlighted the usability and validity of measures of physical activity compared with a gold standard methodology.23,24 Promoting physical activity has become an increasingly important part of the treatment of patients with COPD. The measurement of physical activity, therefore, is of indisputable clinical importance. Activity monitor-based counseling programs are proven to have a beneficial effect in patients with chronic diseases,29 and clear physical activity guidelines for promoting health are widely used.30 For clinicians, standardization of measurements, as proposed in the present study, can provide a clear benchmark of normal and abnormal physical activity and can be used for clinical comparisons before and after interventions. In some paradigms (eg, coaching patients toward a more active lifestyle), full data including weekends may be valuable. The main outcome chosen for the present study was the sample size needed to achieve a power of 0.80. The statistical power is the probability that the null hypothesis will be rejected when it is truly false (type II error).28 Analyzing the power of a study depends on the significance level α, the sample size, and the effect size. The latter depends on the average difference and the variability of the measurement. The present study took several approaches into account to reduce variability.
Comparing the same days (ie, Monday to Monday) was hypothesized to control for differences in patients’ weekly routines and, hence, decrease the variability. The data suggest that a systematic day-to-day pattern is absent, similar to observations in healthy subjects comparably aged31 and patients with COPD.32 Therefore, we confidently suggest that correcting for weekly routines is not necessary. The exclusion of weekends could decrease the variability of the physical activity measurement and did not affect the intervention effect.
Previously, at least 2,14 3,15,16 5,17 and 713 days of assessment were suggested for physical activity measurement. These cross-sectional studies were mainly based on the obtained ICC and assumed the reliability of the baseline measurement only. Similarly, we would have concluded a minimum of 2 days of measurement needed for reliable data (ICC > 0.8). In a longitudinal design, however, including more days clearly enhanced the robustness. The reliability coefficient is a useful index for quantifying absolute reliability using the same units as the measurement tool.27 The reliability coefficient is also referred to as the smallest real difference and shows that more days of measurement can halve the minimal detectable difference needed to prove that an effective intervention can increase the number of steps.
In healthy adults, at least 3 weekdays are needed to achieve a sufficient ICC for physical activity level, and 5 weekdays are needed for STEPS.21 The present results showed no difference in ICC among the various outcomes. Based on the sample sizes, the most sensitive outcome to find differences is STEPS, which is explained by (1) a reasonable change (20% vs only 5% in average METs) and (2) a manageable SD. It seems that motion-related outcomes (ie, number of steps) better reflect changes in physical activity following rehabilitation compared with other physical activity outcomes (ie, energy expenditure). The use of a light-intensity activity threshold (1.6-2.3 METs) instead of the defined moderate physical activity level reduced the needed sample size and almost approached the sample size needed for STEPS. TMA is an outcome measurement that is difficult to alter and is probably out of reach for many patients with COPD, even following rehabilitation (e-Appendix 1 (773.2KB, pdf) ).
Because of the association with mortality and morbidity in patients with chronic diseases (often independent of physical activity levels) increasing attention is given to the measurement of sedentary behavior.33,34 Sedentary behavior has been defined as any waking behavior characterized by an energy expenditure of ≤ 1.5 METs while in a sitting or reclining posture.35 The SenseWear armband does not record information on changes in posture; therefore, the results discussed in the present study only relate to physical activity measurement and not to standardization of sedentary behavior data collection. In healthy elderly people, more days of measurement are needed to reliably (ICC > 0.80) assess sedentary data (7 days) in cross-sectional analyses.31,36 Thus, we hypothesize that more days and hours of wearing time are needed to analyze sedentary behavior in patients with COPD, which is a direction for future studies.
DT and weather conditions affect physical activity levels in elderly people.12 The difference in DT as a marker of the measured season influences the effects on physical activity seen in repeated measures and can be included as a confounder in intervention studies. This is in line with previous literature showing that seasonal variation has an important impact on the change in physical activity after rehabilitation11 and concluding that season affects the change in STEPS.37 This finding may be less relevant to certain regions, such as equatorial Brazil where the mean temperature difference in winter compared with summer is less pronounced than in most European countries, seasonal effects have been shown to be less present,38 and differences in DT are less pronounced compared with Belgium (latitude 51°N).
The present study confirms that patients with COPD perform most activities between 7:00 am and 8:00 pm.14 Compliance with wearing an activity monitor is a prerequisite for obtaining an accurate physical activity measurement. Research has concluded an excellent compliance with wearing the monitors day and night, supporting feasibility.39 In overweight adults, reliable and comparable results were observed with 8, 10, and 12 h of assessment.40 This finding can be explained by the very low activity pattern of the studied population, which is also common in the present COPD population.41 When physical activity is only measured during waking hours, we recommend defining days as noncompliant when wearing time is < 8 h. If data are available during night and day, we propose defining valid days as those with a minimum of 8 h of wearing time between 7:00 am and 8:00 pm. Exclusion of days with < 12 h of wearing time is not recommended because of the large drop in the number of subjects.
Aside from decreasing the variability of the measurement, increasing the effect size (and subsequently the power) of the study can be achieved by increasing the magnitude of the intervention effect itself. The fact that enhanced physical activity is not guaranteed after pulmonary rehabilitation programs can be due to a lack of effective behavior change. This behavior change probably needs more-specific interventions aimed at an increase in physical activity instead of exercise programs that primarily aim for an increase in functional capacity. More research on these programs is warranted, but pilot data using a combination of a step counter and online feedback seem promising.42 Various strategies of processing physical activity data have implications for the perceived impact of an intervention but do not change the magnitude of the effect itself. The latter is limited because no minimal clinically important difference has been established yet.
A few limitations should be considered when interpreting the present data. First, the SenseWear armband provides accurate information on identifying minutes of moderate to intense activity but is less reliable as a step counter.22 This multisensory activity monitor, worn at the arm and validated against both indirect calorimetry and doubly labeled water, is less accurate in identifying the number of steps. When expressed as the difference between steps measured by an activity monitor vs actual steps measured by manual counting, accelerometers worn at the arm less accurately represent the actual step count compared with leg activity monitors.22,43 The number of steps measured in the present study may be underestimated, but although this may affect cross-sectional values, it is not likely to affect the interventional changes or the conclusions. Second, the activity measurement was restricted to 7 days. Whether more days of measurement could have further decreased the sample size is not known. Increasing the assessment time is not feasible because patients may refuse to wear the monitors for longer periods. The majority of patients appear to be willing to wear activity monitors for up to 1 week.23 In addition, measurement data at 5 days was almost identical to that at 4 days, so it is unlikely that longer periods would have changed the present conclusions. Third, it should be highlighted that through the proposed standardized way of measuring physical activity, we attempted to provide an optimal analysis of activity data from a statistical standpoint, but this does not necessarily provide a more accurate representation or validation of the outcomes. Finally, although the main findings apply to pulmonary rehabilitation, the proposed way of analyzing physical activity data likely will also be applicable to studies of other interventions because the measurement methods mainly had an effect on the SD. Similarly, we suggest that our way of analysis can be generalized to other validated activity monitoring devices, in as far as their activity outcomes, (eg, step count), are validated.
Conclusions
Outcome measurements of number of steps and time in light activity (1.6-2.3 METs) assessed on at least 4 weekdays with a minimum of 8 h of monitor wearing time (during waking hours) with proper correction for a difference in DT reduce the noise in physical activity data and improve intervention responsiveness. These findings have implications for trial design and data processing and would potentially be cost saving for researchers on the one hand and helpful in measuring physical activity in the clinical routine for clinicians on the other hand by reducing the burden on patients.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: T.T. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. T. T. contributed to the study concept; H. D. and T. T. contributed to data analysis; C. B., H. V. R., D. L., M. D., R. G., W. J. and T. T. contributed to protocol development; C. B., H. V. R., and D. L. contributed to data collection; H. D. contributed to the writing of the manuscript; and C. B., H. V. R., M. H., D. L., M. D., R. G., W. J., and T. T. contributed to the critical review of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank physiotherapists V. Barbier, MSc, I. Coosemans, BSc, and I. Muylaert, MSc; the staff of the Respiratory Rehabilitation Department, the Pulmonary Function Department, and the Clinical Trial Unit at the University Hospitals Gasthuisberg (Leuven, Belgium) for providing the exercise training and performing the clinical tests; and Hans Scheers, MSc, for the statistical support.
Additional information. The e-Appendix can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- DT
duration of daylight time
- ICC
intraclass correlation coefficient
- METs
metabolic equivalents of task
- METS
mean metabolic equivalents of task level
- STEPS
daily number of steps
- TMA
time spent in at least moderate physical activity
Footnotes
Part of this article has been presented in abstract form at the American Thoracic Society 2012 International Conference, May 18-23, 2012, San Francisco, CA.
FUNDING/SUPPORT: This work was supported by the Flemish Research Foundation [Grant G.0871.13] and PROactive Innovative Medicines Initiative Joint Undertaking [Grant IMI-JU 115011]. Drs Langer and Janssens are postdoctoral research fellows of the Flemish Research Foundation.
References
- 1.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences—clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235-256 [DOI] [PubMed] [Google Scholar]
- 2.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, updated 2014. Global Initiative for Chronic Obstructive Lung Disease website. http://www.goldcopd.org. Accessed March 11, 2014.
- 3.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177(7):743-751 [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest. 2012;142(2):338-346 [DOI] [PubMed] [Google Scholar]
- 5.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331-342 [DOI] [PubMed] [Google Scholar]
- 6.Arne M, Lundin F, Boman G, Janson C, Janson S, Emtner M. Factors associated with good self-rated health and quality of life in subjects with self-reported COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:511-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jehn M, Schindler C, Meyer A, et al. Daily walking intensity as a predictor of quality of life in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2012;44(7):1212-1218 [DOI] [PubMed] [Google Scholar]
- 8.Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19(115):24-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng LWC, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9(1):17-26 [DOI] [PubMed] [Google Scholar]
- 10.Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(suppl 1):S68-S76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sewell L, Singh SJ, Williams JEA, Morgan MD. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil Prev. 2010;30(5):329-333 [DOI] [PubMed] [Google Scholar]
- 12.Sumukadas D, Witham M, Struthers A, McMurdo M. Day length and weather conditions profoundly affect physical activity levels in older functionally impaired people. J Epidemiol Community Health. 2009;63(4):305-309 [DOI] [PubMed] [Google Scholar]
- 13.Hecht A, Ma SY, Porszasz J, Casaburi R; COPD Clinical Research Network. Methodology for using long-term accelerometry monitoring to describe daily activity patterns in COPD. COPD. 2009;6(2):121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977 [DOI] [PubMed] [Google Scholar]
- 15.Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117(5):1359-1367 [DOI] [PubMed] [Google Scholar]
- 16.Sugino A, Minakata Y, Kanda M, et al. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration. 2012;83(4):300-307 [DOI] [PubMed] [Google Scholar]
- 17.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262-272 [DOI] [PubMed] [Google Scholar]
- 18.Burtin C, Saey D, Saglam M, et al. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J. 2012;40(2):338-344 [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5-40 [PubMed] [Google Scholar]
- 20.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14(2):270-274 [DOI] [PubMed] [Google Scholar]
- 21.Scheers T, Philippaerts R, Lefevre J. Variability in physical activity patterns as measured by the SenseWear Armband: how many days are needed? Eur J Appl Physiol. 2012;112(5):1653-1662 [DOI] [PubMed] [Google Scholar]
- 22.Langer D, Gosselink R, Sena R, Burtin C, Decramer M, Troosters T. Validation of two activity monitors in patients with COPD. Thorax. 2009;64(7):641-642 [DOI] [PubMed] [Google Scholar]
- 23.Rabinovich RA, Louvaris Z, Raste Y, et al. ; PROactive Consortium. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J. 2013;42(5):1205-1215 [DOI] [PubMed] [Google Scholar]
- 24.Van Remoortel H, Raste Y, Louvaris Z, et al. ; PROactive Consortium. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7(6):e39198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423-1434 [DOI] [PubMed] [Google Scholar]
- 26.Forsythe WC, Rykiel EJ, Stahl RS, et al. A model comparison for daylength as a function of latitude and day of year. Ecol Modell. 1995;80(1):87-95 [Google Scholar]
- 27.Vaz S, Falkmer T, Passmore AE, et al. The case for using the repeatability coefficient when calculation test-retest reliability. PLoS One. 2013;8(9):e73990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191 [DOI] [PubMed] [Google Scholar]
- 29.Vaes AW, Cheung A, Atakhorrami M, et al. Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: a systematic review and meta-analysis. Ann Med. 2013;45(5-6):397-412 [DOI] [PubMed] [Google Scholar]
- 30.Garber CE, Blissmer B, Deschenes MR, et al. ; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359 [DOI] [PubMed] [Google Scholar]
- 31.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376-1381 [DOI] [PubMed] [Google Scholar]
- 32.Lores V, García-Río F, Rojo B, Alcolea S, Mediano O. Recording the daily physical activity of COPD patients with an accelerometer: an analysis of agreement and repeatability [in Spanish]. Arch Bronconeumol. 2006;42(12):627-632 [DOI] [PubMed] [Google Scholar]
- 33.Atkin AJ, Gorely T, Clemes SA, et al. Methods of measurement in epidemiology: sedentary behaviour. Int J Epidemiol. 2012;41(5):1460-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henson J, Yates T, Biddle SJ, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56(5):1012-1020 [DOI] [PubMed] [Google Scholar]
- 35.Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37(3):540-542 [DOI] [PubMed] [Google Scholar]
- 36.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitta F, Breyer MK, Hernandes NA, et al. Comparison of daily physical activity between COPD patients from Central Europe and South America. Respir Med. 2009;103(3):421-426 [DOI] [PubMed] [Google Scholar]
- 39.Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522-530 [DOI] [PubMed] [Google Scholar]
- 40.Miller GD, Jakicic JM, Rejeski WJ, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity (Silver Spring). 2013;21(1):32-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorrink SNW, Kort HSM, Troosters T, Lammers JW. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moy ML, Janney AW, Nguyen HQ, et al. Use of pedometer and Internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev. 2010;47(5):485-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Remoortel H, Giavedoni S, Raste Y, et al. ; PROactive Consortium. Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Act. 2012;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement