Abstract
Pulmonary hypertension (PH) is an uncommon but progressive condition, and much of what we know about it comes from specialized disease registries. With expanding research into the diagnosis and treatment of PH, it is important to provide updated surveillance on the impact of this disease on hospitalizations and mortality. This study, which builds on previous PH surveillance of mortality and hospitalization, analyzed mortality data from the National Vital Statistics System and data from the National Hospital Discharge Survey between 2001 and 2010. PH deaths were identified using International Classification of Diseases, Tenth Revision codes I27.0, I27.2, I27.8, or I27.9 as any contributing cause of death on the death certificate. Hospital discharges associated with PH were identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes 416.0, 416.8, or 416.9 as one of up to seven listed medical diagnoses. The decline in death rates associated with PH among men from 1980 to 2005 has reversed and now shows a significant increasing trend. Similarly, the death rates for women with PH have continued to increase significantly during the past decade. PH-associated mortality rates for those aged 85 years and older have accelerated compared with rates for younger age groups. There have been significant declines in PH-associated mortality rates for those with pulmonary embolism and emphysema. Rates of hospitalization for PH have increased significantly for both men and women during the past decade; for those aged 85 years and older, hospitalization rates have nearly doubled. Continued surveillance helps us understand and address the evolving trends in hospitalization and mortality associated with PH and PH-associated conditions, especially regarding sex, age, and race/ethnicity disparities.
Pulmonary hypertension (PH) is an uncommon but progressive condition. In the past, it has been called an orphan disease because it affects small numbers of individuals, is associated with many diseases, and is often overlooked by doctors.1 Previous surveillance from the Centers for Disease Control and Prevention2 from 1980 to 2002 identified decreasing mortality rates associated with PH among men, but increasing mortality rates among women, along with stable rates among whites but increasing rates among blacks. Increasing rates of hospitalization associated with PH were identified as well. The symptoms of PH during the initial stage of the disease are common to many other medical conditions (eg, difficulty breathing, fatigue), often resulting in a delayed diagnosis until more severe symptoms arise (eg, dizziness, chest pain, ankle swelling, palpitations).3,4
PH is characterized by increased pressure in the pulmonary arteries (resting mean pulmonary artery pressure ≥ 25 mm Hg) and increased pulmonary arterial resistance but it is associated with many underlying conditions.5 The World Health Organization classification of PH, known as the Dana Point classification, was last updated in 20086 (Table 1), after the most recent PH surveillance summary from the Centers for Disease Control and Prevention.2 Some common underlying causes include pulmonary arterial hypertension (PAH) from congenital heart disease, connective tissue disease, or persistent PH of the newborn; PH due to left-sided heart disease; chronic lung diseases and hypoxemia; and chronic thromboembolic pulmonary disease. Genetics also plays a role in PH, and although PH occurs at all ages, the incidence increases with age. Registries from France and the United Kingdom report an incidence rate of 1.1 to 2.4 cases per million population per year, a prevalence of 6.6 to 15.0 cases per million population per year for PAH, and a 5-year mortality of approximately 40%.7
TABLE 1 .
] World Health Organization Dana Point Classification of Pulmonary Hypertension (2008)
| Classification |
| 1. Pulmonary arterial hypertension |
| 1.1. Idiopathic |
| 1.2. Heritable |
| 1.2.1. BMPR2 |
| 1.2.2. ALK1 endoglin (with or without hereditary hemorrhagic telangiectasia |
| 1.2.3. Unknown |
| 1.3. Drug and toxin induced |
| 1.4. Associated with |
| 1.4.1. Connective tissue diseases |
| 1.4.2. HIV infection |
| 1.4.3. Portal hypertension |
| 1.4.4. Congenital heart diseases |
| 1.4.5. Shistosomiasis |
| 1.4.6. Chronic hemolytic anemia |
| 1.5. Persistent pulmonary hypertension of the newborn |
| 1.6. Pulmonary venoocclusive disease |
| 2. Pulmonary hypertension owing to left-sided heart disease |
| 2.2. Systolic dysfunction |
| 2.2. Diastolic dysfunction |
| 2.3. Valvular disease |
| 3. Pulmonary hypertension owing to lung diseases and/or hypoxia |
| 3.1. COPD |
| 3.2. Interstitial lung disease |
| 3.3. Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4. Sleep-disordered breathing |
| 3.5. Alveolar hypoventilation disorders |
| 3.6. Chronic exposure to high altitude |
| 3.7. Development abnormalities |
| 4. Chronic thromboembolic pulmonary hypertension |
| 5. Pulmonary hypertension with unclear multifactorial mechanisms |
| 5.1. Hematologic disorders: myeloproliferative disorders, splenectomy |
| 5.2. Systemic disorders: sarcoidosis, pulmonary Langerhans’ cell histiocytosis |
| 5.3. Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4. Others: tumoral obstruction fibrosing mediastinitis, chronic renal failure or dialysis |
ALK1 = activin receptor-like kinase type 1; BMPR2 = bone morphogenetic protein receptor type 2. (Adapted with permission from Simonneau et al.6)
Much of what we know about PH comes from specialized disease registries.8‐10 With expanding research into the diagnosis and treatment of PH, it is important to provide updated surveillance on the impact of this disease on hospitalizations and mortality. The surveillance report by Hyduk et al2 described trends in mortality and hospitalization rates associated with PH among adults aged 45 years and older from 1980 through 2002 by demographic characteristics. This study builds on previous PH surveillance of mortality and hospitalization using data from the National Vital Statistics System and the National Hospital Discharge Survey (NHDS).11 The purpose of our report is to describe trends in diagnosed PH-related mortality and hospitalizations during the period 2001 to 2010. Because PH is frequently reported as a secondary diagnosis, our report presents data for PH as any contributing cause of death or as any listed hospital diagnosis.
Materials and Methods
Mortality
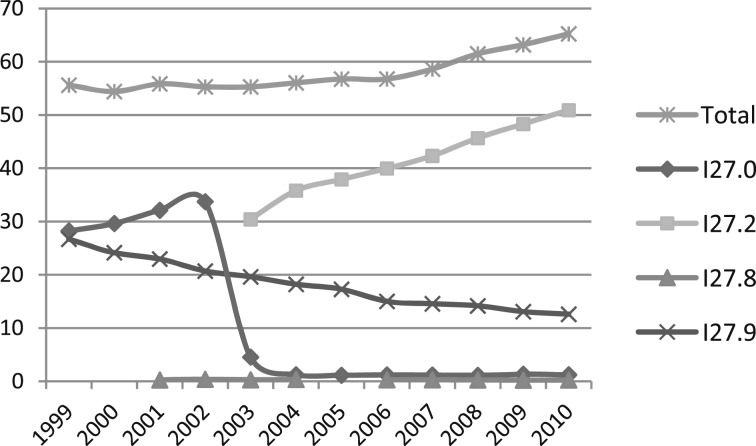
Mortality data from the National Vital Statistics System for the period 2001 to 2010 were analyzed. Bridged-race July 1 population estimates produced by the US Census Bureau in collaboration with the National Center for Health Statistics were compiled using intercensal estimates for the period 2001 to 2009 and postcensal estimates for 2010. For this report, all diseases and conditions reported on death certificates were classified according to codes from the International Classification of Diseases, 10th Revision (ICD-10). For this analysis, PH deaths are defined as those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including underlying cause) on the death certificate. An ICD coding change for mortality occurred in 2003 with the addition of ICD-10 code I27.2 for secondary PH. This resulted in a shift from coding most cases of death related to PH from primary PH (ICD-10 I27.0) to other secondary PH (Fig 1,12 Table 2). These changes require careful interpretation of PH surveillance data over time when relying on individual ICD-10 codes.13
Figure 1 .
– International Classification of Diseases coding for pulmonary hypertension mortality: United States, 2001-2010. I27.0 = primary pulmonary hypertension; I27.2 = other secondary pulmonary hypertension; I27.8 = other specified pulmonary heart diseases; and I27.9 = pulmonary heart disease, unspecified. Data are from the Multiple Cause of Death Files, 1999-2010, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. (Reprinted from the Centers for Disease Control and Prevention, National Center for Health Statistics.12)
TABLE 2 .
] ICD-CM Coding for Diagnosis During Hospitalizations and ICD for Mortality Used in the United States Since 2001
| ICD-CM Diagnoses During Hospitalizations | ICD for Mortality | |||
| ICD-9-CM 2001 | ICD-9-CM 2010 | ICD-10-CM 2013 | ICD-10 2001 | ICD-10 2003 |
| 416. Pulmonary heart disease | 416. Pulmonary heart disease | I27. Other pulmonary heart diseases | I27. Other pulmonary heart disease | I27. Other pulmonary heart disease |
| 416.0. Primary pulmonary hypertension; idiopathic pulmonary arteriosclerosis; pulmonary hypertension (essential) (idiopathic) (primary) | 416.0. Primary pulmonary hypertension; idiopathic pulmonary arteriosclerosis; pulmonary hypertension (essential) (idiopathic) (primary) | I27.0. Primary pulmonary hypertension | I27.0. Primary pulmonary hypertension | I27.0. Primary pulmonary hypertension |
| 416.1 Kyphoscoliotic heart disease | 416.1. Kyphoscoliotic heart disease | I27.1. Kyphoscoliotic heart disease | I27.1. Kyphoscoliotic heart disease | I27.1. Kyphoscoliotic heart disease |
| 416.2. Chronic pulmonary embolism | I27.2. Other secondary pulmonary hypertension (pulmonary hypertension NOS) | I27.2. Other secondary pulmonary hypertension | ||
| 416.8. Other specified diseases of pulmonary circulation (pulmonary arteritis, endarteritis, rupture or stricture of pulmonary vessel | 416.8. Other specified diseases of pulmonary circulation (pulmonary arteritis, endarteritis, rupture or stricture of pulmonary vessel | I27.8. Other specified pulmonary heart diseases | I27.8. Other specified pulmonary heart diseases | I27.8. Other specified pulmonary heart diseases |
| I27.81. Cor pulmonale (chronic) | ||||
| I27.82. Chronic pulmonary embolism | ||||
| I27.89. Other specified pulmonary heart diseases (Eisenmenger’s complex and syndrome) | ||||
| 417.9. Unspecified disease of pulmonary circulation | 417.9. Unspecified disease of pulmonary circulation | I27.9. Pulmonary heart disease, unspecified (chronic cardiopulmonary disease) | I27.9. Pulmonary heart disease, unspecified | I27.9. Pulmonary heart disease, unspecified |
ICD = International Classification of Diseases; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10 = International Classification of Diseases, Tenth Revision; ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification; NOS = not otherwise specified.
Rates are expressed per 100,000 population and were directly age standardized to the 2000 US standard population and eight age groups (0-12 months followed by those aged 1 to 34 years, 35 to 44 years, 45 to 54 years, 55 to 64 years, 65 to 74 years, 75 to 84 years, and ≥ 85 years). Age-standardized death rates and 95% CIs were calculated by sex, race/ethnicity (ie, non-Hispanic [NH] white, NH black, NH American Indian or Alaska native (AI/AN), NH Asian/Pacific Islander, Hispanic), and the decedent’s state of residence at time of death. Age-specific death rates were calculated. It should be noted that race and ethnicity may not be captured accurately on the death certificate, especially for Hispanics and races other than black or white.14 This may affect the observed death rates for Hispanics and other nonwhite/nonblack individuals, especially AI/AN decedents.
Trend analyses and comparability tests for age-standardized or age-specific death rates plotted over time by year were conducted using Joinpoint software, developed by the National Cancer Institute. (Joinpoint version 4.0.3; National Cancer Institute). The number of trend segments is based on a segmented line regression analysis of best fit with the smallest number of “joinpoints” or points (0 or 1) at which the direction of the trend line changes. Annual percent change (APC) was calculated for each of the trend segments. Average annual percent change (AAPC) was calculated for the time period 2001 to 2010 to quantify the average trend over this time period. To determine whether the trend lines were parallel or coincident, tests were conducted to assess pairwise differences between race/ethnicity, with NH white as the referent; age-specific differences, with 0 to 12 months as the referent; and sex differences, with men as the referent. Tests determined whether (1) two joinpoint regression functions were identical (test of coincidence) or (2) two regression mean functions were parallel (test of parallelism) at P < .05.
The distribution of selected disease categories reported as the underlying cause of death (UCOD) among decedents with reported PH was examined for 2001 through 2010 by race/ethnicity and sex. Rates and trends for these distributions were calculated over that time period.
Hospitalizations
The NHDS, conducted annually from 1965 to 2010 by the Centers for Disease Control and Prevention’s National Center for Health Statistics, is a national probability sample survey of hospital discharge information abstracted from the medical records of inpatients from nonfederal, short-stay general hospitals in the United States.15 Only hospitals with an average length of stay of fewer than 30 days for all patients, and general and children’s general hospitals, were included in the survey. Excluded were federal, military, and Veterans Affairs hospitals, as well as hospital units of institutions, such as prison hospitals, and hospitals with fewer than six beds staffed for patient use. NHDS data from 2001 to 2010 were analyzed in five 2-year increments (2001-2002, 2003-2004, 2005-2006, 2007-2008, and 2009-2010). Annual discharge and population estimates were combined in 2-year intervals to increase the reliability of rate estimates. This report provides estimates of the number and rates of hospitalization discharges attributed to PH, defined as having any one of up to seven listed medical diagnoses with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 416.0, 416.8, or 416.9. In 2010, a change occurred in the ICD-9-CM codes for PH, with the addition of a code for chronic pulmonary embolism (416.2). Estimates of the number and rate of hospitalizations associated with PH were calculated by using weights (ie, inflation factors) that allowed national estimation from the sample. More details about the design of NHDS have been published elsewhere.15 Because people with multiple discharges during the year may be sampled more than once, estimates are for hospital discharges, not persons. Data for newborn infants, defined as patients admitted to a hospital by birth, were excluded from this report.
Intercensal estimates of the US noninstitutionalized civilian population from 2001 to 2002 to 2009 to 2010 were provided by the US Bureau of the Census and were used to calculate age- and sex-specific hospitalization rates per 100,000 people. Age-specific hospitalization rates were calculated for those aged < 35 years, 35 to 44 years, 45 to 54 years, 55 to 64 years, 65 to 74 years, 75 to 84 years, and ≥ 85 years. Hospitalization rates were also directly age standardized to the 2000 US standard population using seven age groups.16 Race-specific estimates are not provided because of incomplete reporting of race on hospital records.
In addition, the distribution of selected disease categories as the principal (ie, first listed) diagnosis was examined for each period among hospitalizations with any listed PH, and the average lengths of stay for PH-related hospitalizations were compared by age group. Average length of stay was estimated as the ratio of total days of care per discharge to the total number of discharges.
A weighted least-squares regression method17 was used to test for linear trends in hospitalization rates. A P value of < .05 indicated statistical significance. Differences among subgroups were evaluated using unrounded numbers with two-tailed t tests. Data analyses were performed using the statistical packages SAS, versions 9.2 and 9.3 (SAS Institute Inc), and SUDAAN, versions 10.0 and 11.0 (Research Triangle Institute).
Results
Mortality
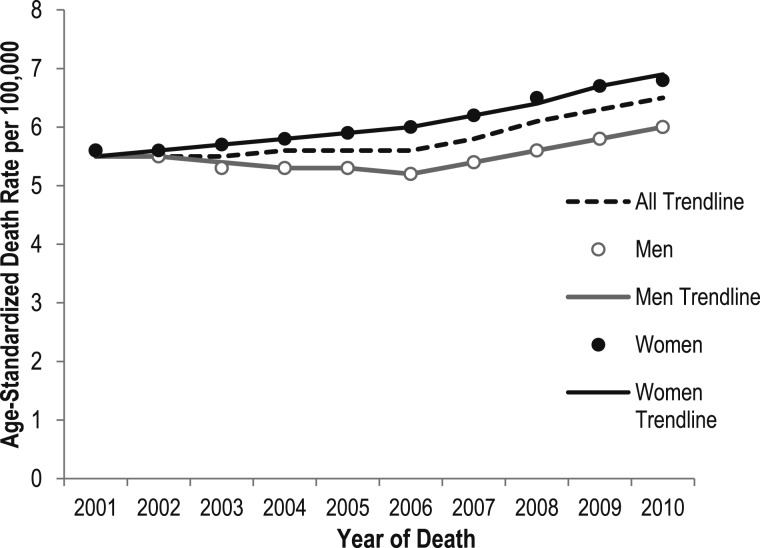
The death rate for PH as any contributing cause of death was 5.5 per 100,000 in 2001 and 6.5 per 100,000 in 2010 (Tables 3, 4). PH death rates for men and women were the same in 2001 (5.6 per 100,000). Although rates have increased for both men and women, the AAPC in death rates from 2001 to 2010 shows that rates rose more sharply for women (AAPC, 2.5; P < .05) than for men (AAPC, 0.9; P < .05) (Fig 2, Table 5). Although there was a 1.3% annual decrease in PH death rates among men from 2001 to 2006, this was followed by a significant increase (APC, 3.7; P < .05) from 2006 to 2010. PH death rates among women show significant increases during both time segments (APC, 1.7 in 2001-2006 and 3.5 in 2006-2010). Overall, trends in PH deaths increased significantly only from 2006 to 2010, with an APC of 3.6 (P < .05) and a total AAPC from 2001 to 2010 of 1.9 (95% CI, 1.5-2.4) (Fig 2, Table 5).
TABLE 3 .
] ASDRs (per 100,000) for Pulmonary Hypertension as Any Cause of Death Among Americans of All Ages by Sex and Race/Ethnicity: United States, 2001-2005
| Sex and Race/Ethnicity | 2001 | 2002 | 2003 | 2004 | 2005 | |||||
| Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | |
| Total | 15,596 | 5.5 (5.4-5.6) | 15,667 | 5.5 (5.4-5.6) | 15,910 | 5.5 (5.4-5.6) | 16,385 | 5.6 (5.5-5.6) | 16,880 | 5.6 (5.5-5.7) |
| Sex | ||||||||||
| Male | 6,513 | 5.6 (5.4-5.7) | 6,483 | 5.5 (5.3-5.6) | 6,357 | 5.3 (5.2-5.4) | 6,524 | 5.3 (5.2-5.4) | 6,649 | 5.3 (5.2-5.5) |
| Female | 9,083 | 5.6 (5.5-5.7) | 9,184 | 5.6 (5.4-5.7) | 9,553 | 5.7 (5.6-5.8) | 9,861 | 5.8 (5.7-5.9) | 10,231 | 5.9 (5.8-6.0) |
| Race | ||||||||||
| NH white | 12,648 | 5.5 (5.4-5.6) | 12,730 | 5.5 (5.4-5.6) | 12,876 | 5.5 (5.4-5.5) | 13,118 | 5.5 (5.4-5.6) | 13,503 | 5.6 (5.5-5.7) |
| NH black | 2,022 | 7.6 (7.2-7.9) | 2,055 | 7.5 (7.2-7.9) | 2,064 | 7.6 (7.2-7.9) | 2,205 | 7.8 (7.5-8.2) | 2,304 | 8.2 (7.8-8.5) |
| NH AI/AN | 66 | 4.2 (3.1-5.2) | 69 | 4.2 (3.1-5.3) | 74 | 4.9 (3.7-6.1) | 83 | 4.8 (3.7-6.0) | 88 | 5.2 (4.0-6.3) |
| NH API | 215 | 2.6 (2.3-3.0) | 196 | 2.2 (1.9-2.5) | 245 | 2.7 (2.3-3.0) | 244 | 2.7 (2.3-3.0) | 246 | 2.5 (2.2-2.8) |
| Hispanic | 645 | 3.2 (2.9-3.5) | 617 | 3.1 (2.8-3.4) | 651 | 3.2 (2.9-3.5) | 735 | 3.2 (3.0-3.5) | 739 | 3.2 (2.9-3.4) |
AI/AN = American Indian/Alaska native; API = Asian/Pacific Islander; ASDR = age-standardized death rate; NH = non-Hispanic.
TABLE 4 .
] ASDRs (per 100,000) for Pulmonary Hypertension as Any Cause of Death Among Americans of All Ages by Sex and Race/Ethnicity: United States, 2006-2010
| Sex and Race/Ethnicity | 2006 | 2007 | 2008 | 2009 | 2010 | |||||
| Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | |
| Total | 17,214 | 5.6 (5.5-5.7) | 18,164 | 5.8 (5.7-5.9) | 19,374 | 6.1 (6.0-6.2) | 20,330 | 6.3 (6.2-6.4) | 21,292 | 6.5 (6.4-6.5) |
| Sex | ||||||||||
| Male | 6,570 | 5.2 (5.0-5.3) | 7,047 | 5.4 (5.3-5.5) | 7,400 | 5.6 (5.5-5.7) | 7,792 | 5.8 (5.6-5.9) | 8,261 | 6.0 (5.9-6.1) |
| Female | 10,644 | 6.0 (5.9-6.1) | 11,117 | 6.2 (6.0-6.3) | 11,974 | 6.5 (6.4-6.6) | 12,538 | 6.7 (6.6-6.8) | 13,031 | 6.8 (6.7-6.9) |
| Race | ||||||||||
| NH white | 13,771 | 5.6 (5.5-5.7) | 14,516 | 5.8 (5.7-5.9) | 15,485 | 6.1 (6.0-6.2) | 16,116 | 6.2 (6.2-6.3) | 16,938 | 6.5 (6.4-6.6) |
| NH black | 2,345 | 8.0 (7.7-8.4) | 2,488 | 8.4 (8.1-8.8) | 2,595 | 8.6 (8.3-9.0) | 2,812 | 9.0 (8.7-9.4) | 2,862 | 9.1 (8.7-9.4) |
| NH AI/AN | 88 | 5.3 (4.1-6.5) | 82 | 4.7 (3.6-5.8) | 87 | 4.8 (3.7-5.9) | 102 | 5.6 (4.5-6.8) | 121 | 6.7 (5.4-8.0) |
| NH API | 256 | 2.6 (2.2-2.9) | 301 | 2.8 (2.5-3.2) | 342 | 3.1 (2.7-3.4) | 374 | 3.2 (2.9-3.6) | 399 | 3.3 (3.0-3.7) |
| Hispanic | 754 | 3.3 (3.0-3.5) | 777 | 3.1 (2.9-3.4) | 865 | 3.4 (3.1-3.6) | 926 | 3.5 (3.2-3.7) | 972 | 3.6 (3.4-3.8) |
See Table 3 legend for expansion of abbreviations.
Figure 2 .
– Age-standardized death rates for pulmonary hypertension as a contributing cause of death and trend lines among individuals of all ages, by sex: United States, 2001-2010. The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having International Classification of Diseases, 10th Revision (ICD-10) codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). All trend lines are compared with the referent group, men; P < .05. Both parallelism and coincident comparison of trend lines were rejected. Rates are per 100,000 population and are age standardized to the 2000 US standard population (eight age groups). See Table 5 for additional data.
TABLE 5 .
] Change in ASDRs (per 100,000) for Pulmonary Hypertension as Contributing Cause of Death Among Individuals of All Ages, by Sex: United States, 2001-2010
| Joinpoint Segment | Sex | APC | AAPC (95% CI) (2001-2010) |
| 2001-2006 | All | 0.6 | 1.9b (1.5-2.4) |
| 2006-2010 | All | 3.6a | … |
| 2001-2006 | Men | −1.3a | 0.9b (0.3-1.5) |
| 2006-2010 | Men | 3.7a | … |
| 2001-2006 | Women | 1.7a | 2.5b (1.9-3.1) |
| 2006-2010 | Women | 3.5a | … |
The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). AAPCs are provided for the time period 2001 to 2010; the joinpoint varies and is based on the joinpoint regression analysis of best fit with the smallest number of deaths. Rates are per 100,000 population and are age standardized to the 2000 US standard population (eight age groups). AAPC = average annual percent change; APC = annual percent change. See Table 2 and 3 legends for expansion of other abbreviations.
APC is significantly different from zero at α = 0.05.
AAPC is significantly different from zero at α = 0.05.
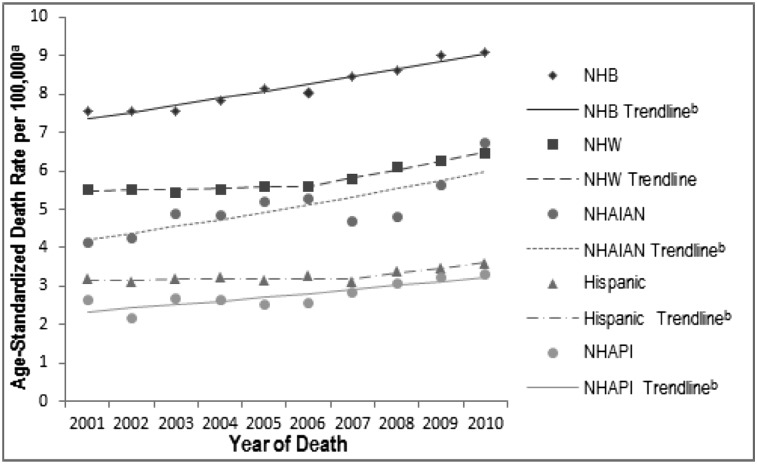
From 2001 to 2010, PH death rates were consistently higher for NH blacks than for NH whites (2010 rate, 9.1 per 100,000 for NH blacks vs 6.5 per 100,000 for NH whites) (Tables 3, 4). When compared with those for NH whites, PH death rates for NH AI/AN, NH Asian/Pacific Islanders, and Hispanics were consistently either lower or not statistically significantly different. However, it should be noted that because of concerns regarding the accuracy of coding race/ethnicity on death certificates, the comparisons of NH whites with race/ethnic groups other than NH blacks are provided with caution on interpretation.18 PH death rates increased over time for all races/ethnicities (Fig 3, Table 6). For NH whites, PH deaths did not increase during the first time segments from 2001 to 2006 (APC, 0.5) but increased significantly from 2006 to 2010 (APC, 3.8; P < .05). NH blacks experienced a significant increase in death rates during the entire time period from 2001 to 2010 (APC, 2.3; P < .05), as did NH AI/AN and NH Asian/Pacific Islanders (APC, 4.0 and 3.7, respectively; P < .05). Although there was no significant change in PH death rates among Hispanics from 2001 to 2007 (APC, 0.2), there was a significant increase from 2007 to 2010 (APC, 4.0; P < .05). The AAPC in the PH death rate for 2001 to 2010 shows significant increases for all races/ethnicities. Compared with NH whites, all race/ethnicity trend lines were parallel but not coincident (P < .05).
Figure 3 .
– Age-standardized death rates for pulmonary hypertension as a contributing cause of death and trend lines among individuals of all ages, by race/ethnicity: United States, 2001-2010. The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). aRates are per 100,000 population and are age standardized to the 2000 US standard population (eight age groups); ball race/ethnicity trend lines are parallel to the referent group of NHW, P < .05. There are no statistically significant coincident trend lines to the referent group of NHW. See Table 6 for additional data. NHAIAN = non-Hispanic American Indian/Alaska native; NHAPI = non-Hispanic Asian/Pacific Islander; NHB = non-Hispanic black; NHW = non-Hispanic white. See Figure 2 legend for expansion of other abbreviation.
TABLE 6 .
] Change in ASDRs for Pulmonary Hypertension as Contributing Cause of Death Among Individuals of All Ages, by Race/Ethnicity: United States, 2001-2010
| Joinpoint Segment | Race/Ethnicity | APC | AAPC (95% CI) (2001-2010) |
| 2001-2006 | NH white | 0.5 | 1.9a (1.5-2.4) |
| 2006-2010 | NH white | 3.8b | |
| 2001-2010 | NH black | 2.3b | 2.3a (1.9-2.8) |
| 2001-2010 | NH AI or AN | 4.0b | 4.0a (1.6-6.4) |
| 2001-2010 | NH API | 3.7b | 3.7a (1.9-5.5) |
| 2001-2007 | Hispanic | 0.2 | 1.5a (0.8-2.2) |
| 2007-2010 | Hispanic | 4.0b |
The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). See Table 2, 3, and 5 legends for expansion of abbreviations.
AAPC is significantly different from zero at α = 0.05.
APC is significantly different from zero at α = 0.05.
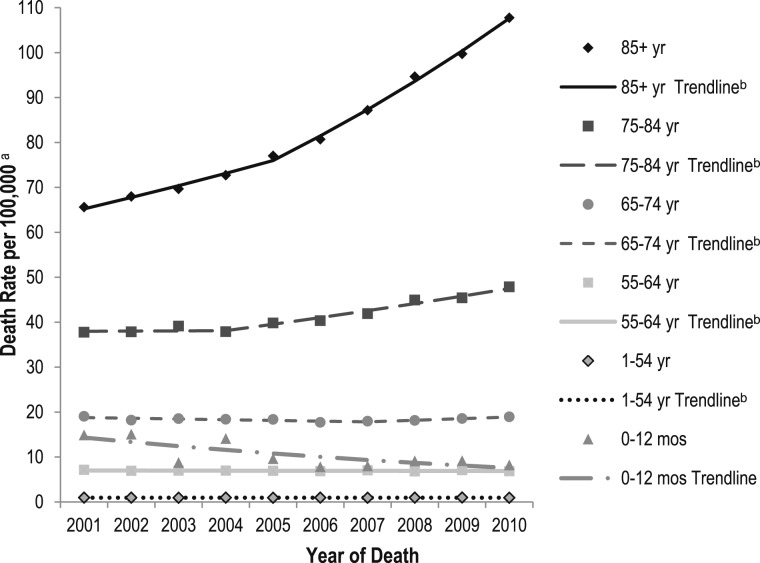
Death rates for PH were highest in the oldest age group (85 years and older) for every year (Tables 7, 8) and increased by > 65% between 2001 and 2010 (65.6 per 100,000 to 108.7 per 100,000). PH death rates in the 0- to 12-month age group were higher in 2001 (14.9 per 100,000) than in 2010 (8.2 per 100,000). The PH death rate trend analysis by age group for 2001 to 2010 shows differences in the direction of the slope segments (Fig 4, Table 9). Neonates through 12 months had the only statistically significant decrease in rates (APC, −6.9; P < .05). The greatest increases in PH death rates were among the oldest age group (85 years and older) from 2001 to 2005 (APC, 3.9; P < .05) and from 2005 to 2010 (APC, 7.2; P < .05). The AAPC for 2001 to 2010 shows significant increases in PH death rates for those aged 75 to 84 years and for those aged 85 years and older. Both parallelism and coincident comparisons of trend lines were rejected.
TABLE 7 .
] Deaths and Age-Specific Death Rates (per 100,000) of Pulmonary Hypertension as Any Cause of Death Among Americans by Age: United States, 2001-2005
| Age Groups | 2001 | 2002 | 2003 | 2004 | 2005 | |||||
| Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | |
| Total | 15,596 | 5.5 (5.4-5.6) | 15,667 | 5.5 (5.4-5.6) | 15,910 | 5.5 (5.4-5.6) | 16,385 | 5.6 (5.5-5.6) | 16,880 | 5.6 (5.5-5.7) |
| 0-12 mo | 598 | 14.9 (13.7-16.1) | 596 | 15.1 (13.9-16.3) | 349 | 8.8 (7.9-9.7) | 566 | 14.1 (12.9-15.3) | 385 | 9.6 (8.7-10.6) |
| 1-34 y | 457 | 0.3 (0.3-0.4) | 430 | 0.3 (0.3-0.3) | 414 | 0.3 (0.3-0.3) | 412 | 0.3 (0.3-0.3) | 408 | 0.3 (0.3-0.3) |
| 35-44 y | 580 | 1.3 (1.2-1.4) | 529 | 1.2 (1.1-1.3) | 561 | 1.3 (1.2-1.4) | 528 | 1.2 (1.1-1.3) | 514 | 1.2 (1.1-1.3) |
| 45-54 y | 1,081 | 2.7 (2.6-2.9) | 1,122 | 2.8 (2.6-3.0) | 1,055 | 2.6 (2.4-2.7) | 1,184 | 2.8 (2.7-3.0) | 1,166 | 2.7 (2.6-2.9) |
| 55-64 y | 1,793 | 7.1 (6.8-7.5) | 1,848 | 6.9 (6.6-7.2) | 1,950 | 7.0 (6.7-7.3) | 2,039 | 7.0 (6.7-7.3) | 2,120 | 6.9 (6.6-7.2) |
| 65-74 y | 3,503 | 19.1 (18.4-19.7) | 3,343 | 18.2 (17.6-18.8) | 3,426 | 18.5 (17.5-19.1) | 3,430 | 18.4 (17.8-19) | 3,466 | 18.4 (17.7-19.0) |
| 75-84 y | 4,755 | 37.8 (36.7-38.8) | 4,830 | 37.8 (36.8-38.9) | 5,044 | 39.1 (38.0-40.2) | 4,921 | 37.9 (36.8-38.9) | 5,207 | 39.8 (38.7-40.9) |
| ≥ 85 y | 2,829 | 65.6 (63.2-68.0) | 2,969 | 68.0 (65.5-70.4) | 3,111 | 69.7 (67.2-72.1) | 3,305 | 72.7 (70.2-75.2) | 3,614 | 77.0 (74.5-79.5) |
See Table 3 legend for expansion of abbreviation.
TABLE 8 .
] Deaths and Age-Specific Death Rates (per 100,000) of Pulmonary Hypertension as Any Cause of Death Among Americans by Age: United States, 2006-2010
| Age Groups | 2006 | 2007 | 2008 | 2009 | 2010 | |||||
| Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | Deaths | ASDR (95% CI) | |
| Total | 17,214 | 5.6 (5.5-5.7) | 18,164 | 5.8 (5.7-5.9) | 19,373 | 6.1 (6.0-6.2) | 20,330 | 6.3 (6.2-6.4) | 21,292 | 6.5 (6.4-6.5) |
| 0-12 mo | 316 | 7.8 (7.0-8.7) | 332 | 8.0 (7.1-8.9) | 376 | 9.1 (8.2-10.0) | 367 | 9.2 (8.2-10.1) | 325 | 8.2 (7.3-9.1) |
| 1-34 y | 415 | 0.3 (0.3-0.3) | 429 | 0.3 (0.3-0.3) | 422 | 0.3 (0.3-0.3) | 434 | 0.3 (0.3-0.3) | 386 | 0.3 (0.2-0.3) |
| 35-44 y | 488 | 1.1 (1.0-1.2) | 447 | 1.0 (0.9-1.1) | 509 | 1.2 (1.1-1.3) | 491 | 1.2 (1.1-1.3) | 465 | 1.1 (1.0-1.2) |
| 45-54 y | 1,210 | 2.8 (2.6-3.0) | 1,212 | 2.8 (2.6-2.9) | 1,233 | 2.8 (2.6-2.9) | 1,334 | 3.0 (2.8-3.1) | 1,238 | 2.8 (2.6-2.9) |
| 55-64 y | 2,179 | 6.8 (6.5-7.1) | 2,331 | 7.0 (6.8-7.3) | 2,325 | 6.8 (6.5-7.1) | 2,504 | 7.1 (6.8-7.3) | 2,516 | 6.9 (6.6-7.2) |
| 65-74 y | 3,401 | 17.7 (17.1-18.3) | 3,538 | 18.0 (17.4-18.6) | 3,717 | 18.1 (17.5-18.7) | 3,938 | 18.5 (18.0-19.1) | 4,133 | 19 (18.5-19.6) |
| 75-84 y | 5,279 | 40.3 (39.2-41.4) | 5,482 | 41.9 (40.8-43.0) | 5,873 | 44.9 (43.8-46.1) | 5,911 | 45.4 (44.2-46.5) | 6,256 | 47.9 (46.7-49.1) |
| ≥ 85 y | 3,926 | 80.7 (78.2-83.2) | 4,393 | 87.2 (84.6-89.7) | 4,918 | 94.7 (92.0-97.3) | 5,351 | 99.7 (97.0-102.4) | 5,973 | 108.7 (106.0-111.5) |
See Table 3 legend for expansion of abbreviation.
Figure 4 .
– Age-specific death rates for pulmonary hypertension as a contributing cause of death and trend lines among individuals of all ages, by six age groups: United States, 2001-2010. The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). aRates are per 100,000 population and age-specific death rates were calculated for each of six age groups; ball age-specific trend lines are compared with the referent age group of 0 to 12 mo, P < .05. Both parallelism and coincident comparison of trend lines were rejected. See Table 9 for additional data. See Figure 2 legend for expansion of abbreviation.
TABLE 9 .
] Death and Age-Specific Death Rates for Pulmonary Hypertension as Contributing Cause of Death Among Individuals of All Ages, by Six Age Groups: United States, 2001-2010
| Joinpoint Segment | Age Group, y | APC | 2001-2010 AAPC (95% CI) |
| 2001-2010 | 0-12a | −6.9b | −6.9c (−11.1 to −2.5) |
| 2001-2010 | 1-54 | 0.1 | 0.1 (−0.6 to 0.7) |
| 2001-2010 | 55-64 | −0.2 | −0.2 (−0.6 to 0.2) |
| 2001-2007 | 65-74 | −0.9 | 0.1 (−0.7 to 0.9) |
| 2007-2010 | 65-74 | 2.0 | … |
| 2001-2004 | 75-84 | 0.1 | 2.5c (1.6 to 3.4) |
| 2004-2010 | 75-84 | 3.7b | … |
| 2001-2005 | 85+ | 3.9b | 5.7c (5.3 to 6.2) |
| 2005-2010 | 85+ | 7.2b | … |
The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). Rates are per 100,000 population and age-specific death rates were calculated for each of six age groups. See Table 2 and 5 legends for expansion of abbreviations.
Mo.
APC is significantly different from zero at α = 0.05.
AAPC is significantly different from zero at α = 0.05.
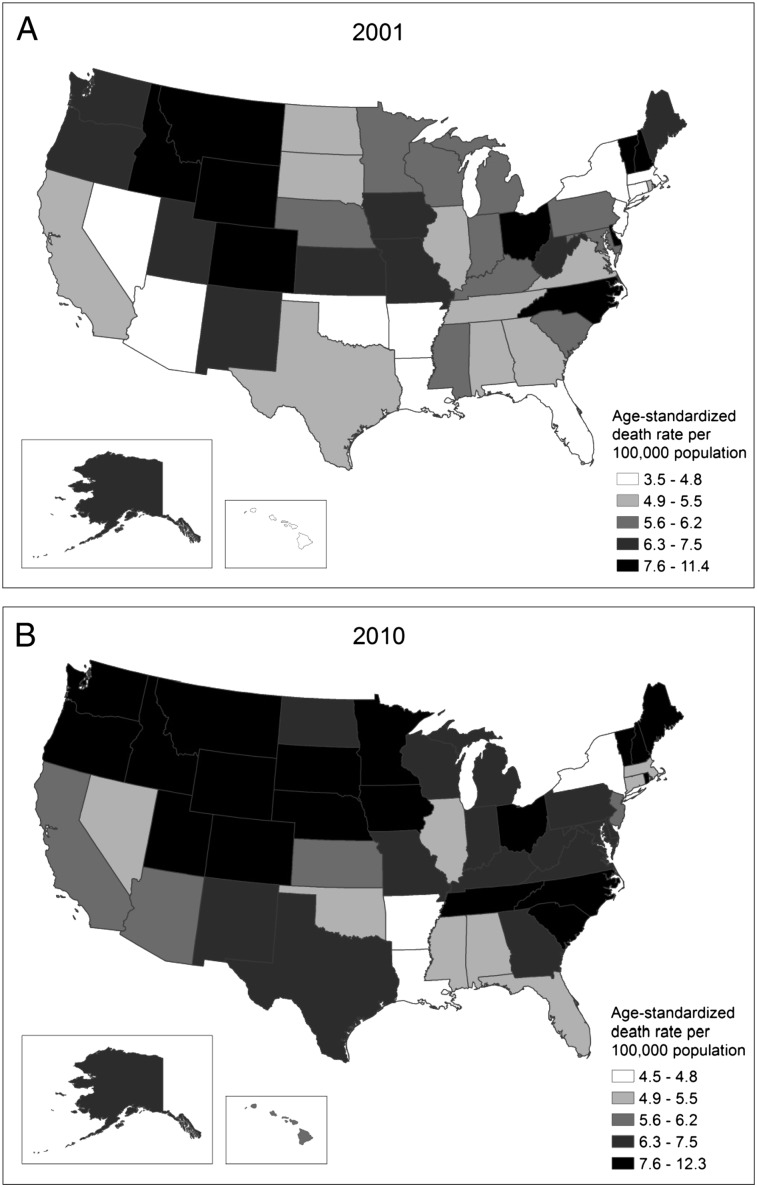
In 2001, the northern mountain states plus the District of Columbia, Vermont, New Hampshire, Delaware, North Carolina, and Ohio experienced the highest PH age-standardized death rates (Fig 5). By 2010, several other western and midwestern states, including Iowa, Minnesota, Nebraska, Oregon, and Washington, had experienced significant increases in death rates (P < .05) and had moved into the highest rate category. South Carolina and Tennessee also experienced significant increases in death rates between 2001 and 2010 (P < .05).
Figure 5 .
– Age-standardized death rates for pulmonary hypertension as any cause of death among all ages by state. A, Death rates in 2001. B, Death rates in 2010. The National Vital Statistics System was used to ascertain deaths due to pulmonary hypertension, which were considered those with decedents having ICD-10 codes I27.0, I27.2, I27.8, or I27.9 reported as any contributing cause of death (ie, any of the possible 20 conditions, including the underlying cause of death). Rates are per 100,000 population and are age standardized to the 2000 US standard population (eight age groups). See Figure 2 legend for expansion of abbreviation.
In 2001, the most common UCOD among decedents with PH listed as any contributing cause of death was PH (29.3%), followed by chronic lower respiratory disease (26.2%), coronary heart disease (8.8%), and interstitial lung disease (4.5%) (Table 10). Within the category of chronic lower respiratory disease, emphysema was indicated in 4.2% of deaths (Table 10). The most commonly reported underlying causes of death for all race/sex groups were PH and chronic lower respiratory disease.
TABLE 10 .
] Distribution of Underlying Cause of Death Among Decedents With Reported Pulmonary Hypertension by Race/Sex Groups, 2001
| Disease Category: ICD-10 Codes | Total | NH White Men | NH Black Men | Hispanic Men | NH White Women | NH Black Women | Hispanic Women |
| Diseases of the circulatory system (49.6%) | |||||||
| Pulmonary hypertension: I27.0, I27.2, I27.8, I27.9 | 29.3 (4,573) | 24.6 (1,318) | 33.5 (254) | 31.4 (86) | 30.4 (2,219) | 37.5 (474) | 35.0 (130) |
| Coronary heart disease: I20-I25 | 8.8 (1,380) | 10.6 (570) | 6.7 (51) | 8.4 (23) | 8.4 (616) | 5.6 (71) | 6.7 (25) |
| Valvular heart disease, nonrheumatic: I34-I38 | 2.6 (405) | 1.7 (92) | 2.1 (16) | 2.2 (6) | 3.4 (247) | 1.9 (24) | 3.5 (13) |
| Aortic stenosis: I06.0, I35.0, I35.2 | 0.9 (147) | 0.8 (43) | a | a | 1.1 (80) | 0.6 (8) | 2.4 (9) |
| Hypertension: I10-I15 | 1.0 (156) | 0.8 (41) | 1.7 (13) | a | 0.9 (68) | 2.1 (27) | a |
| Rheumatic heart disease: I00-I09 | 1.4 (219) | 0.5 (28) | a | a | 2.1 (155) | 1.5 (19) | 1.3 (5) |
| Pulmonary embolism: I26 | 1.3 (200) | 0.9 (49) | 2.0 (15) | a | 1.5 (112) | 1.1 (14) | 1.3 (5) |
| Heart failure: I50 | 0.6 (99) | 0.8 (41) | 0.7 (5) | a | 0.6 (44) | 0.6 (7) | a |
| Other cardiovascular/cerebrovascular disease: I27.1, I28-I33, I40-I49, I51-I78 | 3.6 (560) | 3.4 (181) | 5.3 (40) | 3.3 (9) | 3.5 (256) | 3.5 (44) | 5.7 (21) |
| Respiratory disorder or infection (37.5%) | |||||||
| Chronic lower respiratory disease: J40-J47 | 26.2 (4,080) | 33.3 (1,782) | 17.1 (130) | 14.6 (40) | 26.0 (1,896) | 12.9 (163) | 8.6 (32) |
| Emphysema only: J43 | 4.2 (654) | 6.0 (322) | 2.4 (18) | 1.8 (5) | 3.8 (276) | 1.7 (21) | a |
| Interstitial lung disease: J84 | 4.5 (701) | 4.6 (248) | 3.7 (28) | 5.5 (15) | 4.3 (311) | 5.6 (71) | 4.9 (18) |
| Influenza and pneumonia: J09-J18 | 0.9 (143) | 0.9 (49) | a | a | 0.9 (69) | 0.8 (10) | a |
| All other respiratory diseases: J00-06, J20-J39, J60-J70, J85-J98 | 1.7 (266) | 1.9 (101) | 2.9 (22) | 3.3 (9) | 1.4 (101) | 1.7 (21) | 1.3 (5) |
| Autoimmune diseases: M05-M06, M08, M30-35 | 1.3 (209) | 0.3 (16) | 0.8 (6) | a | 1.6 (119) | 3.2 (40) | 5.1 (19) |
| Congenital malformations, deformation, and chromosomal abnormalities: Q00-Q99 | 3.0 (473) | 2.5 (133) | 4.7 (36) | 10.6 (29) | 2.6 (190) | 3.3 (42) | 5.4 (20) |
| Malignant neoplasms of the trachea, bronchus, and lung: C34 | 1.8 (278) | 2.5 (132) | 1.8 (14) | a | 1.6 (116) | 0.6 (7) | a |
| Diabetes mellitus: E10-E14 | 1.0 (160) | 0.9 (47) | 1.1 (8) | a | 1.0 (74) | 1.1 (14) | 2.7 (10) |
| Nephritis, nephrotic syndrome, and nephrosis: N00-N07, N17-N19, N25-N27 | 0.7 (112) | 0.6 (33) | 1.3 (10) | a | 0.7 (52) | 1.0 (12) | a |
| Chronic liver disease and cirrhosis: K70, K73-74 | 0.3 (50) | 0.4 (20) | a | a | 0.3 (19) | a | 1.6 (6) |
| Malignant neoplasms of lymphoid, hematopoietic, and related tissue: C81-C96 | 0.4 (70) | 0.4 (23) | a | a | 0.4 (30) | 0.6 (7) | a |
| Sleep apnea: G47.3 | 0.1 (17) | 0.2 (10) | a | a | a | a | a |
| Certain conditions originating in the perinatal period: P00-P96 | 1.0 (160) | 0.9 (47) | 3.4 (26) | 6.9 (19) | 0.5 (37) | 1.6 (20) | 1.9 (7) |
| All other causes | 7.3 (1,139) | 6.6 (353) | 8.8 (67) | 5.5 (15) | 6.6 (479) | 12.9 (163) | 8.6 (32) |
| Total | 15,597 | 5,357 | 759 | 274 | 7,292 | 1,263 | 371 |
In 2010, PH (at 30.2%) was the most common UCOD among decedents with PH listed as any cause of death, followed by chronic lower respiratory disease (19.0%), coronary heart disease (10.3%), and interstitial lung disease (4.9%) (Table 11). Interestingly, from 2001 to 2010, the percentage of PH deaths caused by emphysema dropped from 4.2% to 1.4% of all PH deaths, whereas PH deaths caused by nonrheumatic valvular heart disease rose from 2.6% in 2001 to 4.1% in in 2010. Autoimmune diseases listed as the UCOD were higher for women than for men regardless of race or Hispanic origin.
TABLE 11 .
] Distribution of Underlying Cause of Death Among Decedents With Reported Pulmonary Hypertension by Race/Sex Groups, 2010
| Disease Category: ICD-10 codes | Total | NH White Men | NH Black Men | Hispanic Men | NH White Women | NH Black Women | Hispanic Women |
| Diseases of the circulatory system (54.2%) | |||||||
| Pulmonary hypertension: I27.0, I27.2, I27.8, I27.9 | 30.2 (6,436) | 26.1 (1,741) | 31.4 (322) | 23.2 (82) | 31.8 (3,273) | 35.9 (659) | 32.5 (201) |
| Coronary heart disease: I20-I25 | 10.3 (2,184) | 13.8 (921) | 8.5 (87) | 9.3 (33) | 9.2 (945) | 6.6 (121) | 5.2 (32) |
| Valvular heart disease, nonrheumatic: I34-I38 | 4.1 (879) | 3.5 (235) | 1.7 (17) | 2.8 (10) | 5.4 (551) | 1.6 (29) | 3.1 (19) |
| Aortic stenosis: I06.0, I35.0, I35.2 | 1.6 (350) | 1.4 (92) | a | a | 2.2 (230) | 0.5 (10) | 0.8 (5) |
| Hypertension: I10-I15 | 1.6 (336) | 1.1 (70) | 2.8 (29) | 2.0 (7) | 1.6 (168) | 2.7 (50) | 1.3 (8) |
| Rheumatic heart disease: I00-I09 | 1.5 (332) | 0.8 (54) | a | a | 2.2 (225) | 0.9 (16) | 1.6 (10) |
| Pulmonary embolism: I26 | 0.7 (145) | 0.7 (45) | 0.8 (8) | a | 0.7 (68) | 1.0 (18) | a |
| Heart failure: I50 | 0.4 (95) | 0.5 (31) | a | a | 0.5 (48) | 0.5 (9) | a |
| Other cardiovascular/cerebrovascular disease: I27.1, I28-I33, I40-I49, I51-I78 | 3.7 (784) | 3.4 (226) | 5.8 (60) | 3.7 (13) | 3.7 (381) | 4.0 (74) | 2.7 (17) |
| Respiratory disorder or infection (28.4%) | |||||||
| Chronic lower respiratory disease: J40-J47 | 19.0 (4,041) | 23.2 (1,547) | 15.4 (158) | 12.2 (43) | 18.9 (1939) | 11.8 (216) | 11.1 (69) |
| Emphysema only: J43 | 1.4 (295) | 1.8 (123) | 1.1 (11) | a | 1.3 (138) | 0.8 (15) | a |
| Interstitial lung disease: J84 | 4.9 (1,043) | 6.2 (413) | 4.6 (47) | 11.0 (39) | 3.9 (401) | 4.0 (73) | 6.1 (38) |
| Influenza and pneumonia: J09-J18 | 1.2 (254) | 0.9 (63) | 1.7 (17) | 1.7 (6) | 1.2 (127) | 0.9 (17) | 2.1 (13) |
| All other respiratory diseases: J00-06, J20-J39, J60-J70, J85-J98 | 1.9 (408) | 2.4 (159) | 3.6 (37) | 3.4 (12) | 1.3 (135) | 2.5 (45) | 1.8 (11) |
| Autoimmune diseases: M05-M06, M08, M30-35 | 2.4 (514) | 0.9 (61) | 1.5 (15) | a | 2.8 (283) | 4.9 (90) | 6.9 (43) |
| Congenital malformations, deformation, and chromosomal abnormalities: Q00-Q99 | 1.6 (334) | 1.3 (89) | 2.3 (24) | 5.4 (19) | 1.1 (114) | 2.2 (41) | 4.0 (25) |
| Malignant neoplasms of the trachea, bronchus, and lung: C34 | 1.5 (320) | 1.8 (121) | 1.7 (17) | a | 1.4 (141) | 0.8 (15) | 1.6 (10) |
| Diabetes mellitus: E10-E14 | 1.5 (312) | 1.5 (97) | 1.8 (18) | 1.4 (5) | 1.3 (132) | 1.7 (32) | 2.6 (16) |
| Nephritis, nephrotic syndrome, and nephrosis: N00-N07, N17-N19, N25-N27 | 1.1 (238) | 0.9 (63) | 1.2 (12) | 1.4 (5) | 1.1 (117) | 1.3 (23) | 1.3 (8) |
| Chronic liver disease and cirrhosis: K70, K73-74 | 0.8 (175) | 0.8 (56) | 1.0 (10) | 2.8 (10) | 0.7 (69) | 0.5 (9) | 2.4 (15) |
| Malignant neoplasms of lymphoid, hematopoietic, and related tissue: C81-C96 | 0.7 (150) | 0.8 (50) | 0.9 (9) | a | 0.7 (69) | 0.7 (13) | a |
| Sleep apnea: G47.3 | 0.6 (134) | 0.7 (48) | 1.0 (10) | a | 0.5 (48) | 1.0 (18) | a |
| Certain conditions originating in the perinatal period: P00-P96 | 0.1 (27) | a | 0.5 | a | a | a | a |
| All other causes | 8.5 (1,811) | 7.1 (474) | 11.4 (117) | 13.6 (48) | 7.9 (808) | 13.8 (254) | 10.0 (62) |
| Total | (21,292) | (6,660) | (1,026) | (353) | (1,027) | (1,836) | (619) |
The UCOD associated with PH showed several interesting trends (Table 12). The AAPC increased significantly for several UCOD conditions, including coronary heart disease, aortic stenosis, hypertension, autoimmune disease, influenza, and pneumonia; it showed no significant change for malignancies of the trachea and bronchus; and it decreased for heart failure, chronic lower respiratory disease (emphysema in particular), and congenital malformations.
TABLE 12 .
] Trends Over Time by Underlying Cause of Death Among Decedents With Reported PH
| Disease Category: ICD-10 Codes | 2001 | 2010 | AAPC | ||
| Rate/100,000 | % of Total PH Deaths | Rate/100,000 | % of Total PH Deaths | ||
| Diseases of the circulatory system | |||||
| Pulmonary hypertension: I27.0, I27.2, I27.8, I27.9 | 1.63 | 29.3 | 1.95 | 30.2 | 1.5 |
| Coronary heart disease: I20-I25 | 0.48 | 8.8 | 0.66 | 10.3 | 3.8a |
| Valvular heart disease, nonrheumatic: I34-I38 | 0.14 | 2.6 | 0.25 | 4.1 | 6.7a |
| Aortic stenosis: I06.0, I35.0, I35.2 | 0.04 | 0.9 | 0.10 | 1.6 | 10.8a |
| Hypertension: I10-I15 | 0.04 | 1.0 | 0.10 | 1.6 | 9.2a |
| Rheumatic heart disease: I00-I09 | 0.07 | 1.4 | 0.10 | 1.5 | 3.2a |
| Pulmonary embolism: I26 | 0.07 | 1.3 | 0.04 | 0.7 | −7.2a |
| Heart failure: I50 | 0.03 | 0.6 | 0.02 | 0.4 | −3.6a |
| Other cardiovascular/cerebrovascular disease: I27.1, I28-I33, I40-I49, I51-I78 | 0.21 | 3.6 | 0.23 | 3.7 | 2.0 |
| Respiratory disorder or infection | |||||
| Chronic lower respiratory disease: J40-J47 | 1.46 | 26.2 | 1.24 | 19.0 | −1.3a |
| Emphysema only: J43 | 0.24 | 4.2 | 0.08 | 1.4 | −8.7a |
| Interstitial lung disease: J84 | 0.24 | 4.5 | 0.31 | 4.9 | 4.5a |
| Influenza and pneumonia: J09-J18 | 0.04 | 0.9 | 0.07 | 1.2 | 5.5a |
| All other respiratory diseases: J00-06, J20-J39, J60-J70, J85-J98 | 0.08 | 1.7 | 0.12 | 1.9 | 3.3a |
| Autoimmune diseases: M5-M6, M8, M30-35 | 0.07 | 1.3 | 0.16 | 2.4 | 10.9a |
| Congenital malformations, deformation, and chromosomal abnormalities: Q00-Q99 | 0.17 | 3.0 | 0.11 | 1.6 | −5.2a |
| Malignant neoplasms of the trachea, bronchus, and lung: C34 | 0.09 | 1.8 | 0.09 | 1.5 | 0.1 |
| Diabetes mellitus: E10-E14 | 0.05 | 1.0 | 0.09 | 1.5 | 7.6a |
| Nephritis, nephrotic syndrome, and nephrosis: N00-N07, N17-N19, N25-N27 | 0.03 | 0.7 | 0.07 | 1.1 | 9.2a |
| Chronic liver disease and cirrhosis: K70, K73-74 | 0.02 | 0.3 | 0.05 | 0.8 | 17.0a |
| Malignant neoplasms of lymphoid, hematopoietic, and related tissue: C81-C96 | 0.02 | 0.4 | 0.04 | 0.7 | 6.4a |
| Sleep apnea: G47.3 | … | 0.1 | 0.04 | 0.6 | NA |
| Certain conditions originating in the perinatal period: P00-P96 | 0.05 | 1.0 | 0.01 | 0.1 | −12.5a |
| Total | 5.5 | … | 6.5 | … | … |
Hospitalizations
From 2001/2002 to 2009/2010, the age-adjusted rate of hospitalizations associated with PH increased by 44%, from 91 per 100,000 discharges to 131 per 100,000 discharges (P < .001). The rate of hospitalization for women increased by 52%, from 97 per 100,000 to 147 per 100,000 (P < .001). The rate of hospitalization for men increased by 33%, from 83 per 100,000 to 110 per 100,000 (P < .01). Crude and age-adjusted hospitalization rates were both higher for women than for men at each time interval, except for 2007 to 2008 (P < .05) (Table 13). The proportion of total PH hospitalizations among women remained consistently higher than among men: Women accounted for 61% of all PH hospitalizations in 2001/2002 and 63% in 2009/2010 (Table 14).
TABLE 13 .
] Hospitalization Rates (per 100,000) With Any Listed Pulmonary Hypertension Diagnosis by Age and Sex: United States, 2001/2002 Through 2009/2010
| Sex and Age Groups | 2001/2002 | 2003/2004 | 2005/2006 | 2007/2008 | 2009/2010 |
| Age-standardizeda rate | |||||
| Total | 91 (85-97) | 87 (81-93) | 94 (88-100) | 112 (100-124) | 131 (115-147) |
| Female | 97 (89-105) | 93 (85-101) | 100 (92-108) | 115 (101-129) | 147 (129-165) |
| Male | 83 (75-91) | 80 (70-90) | 85 (77-93) | 108 (94-122) | 110 (92-128) |
| Age-specific rate | |||||
| Total | 91 (82-101) | 89 (78-99) | 96 (85-108) | 117 (95-140) | 140 (106-173) |
| < 35 y | 7 (6-9) | 10 (7-12) | 11 (8-14) | 10 (7-13) | 17b |
| 35-44 y | 35 (25-46) | 27 (21-33) | 40 (31-49) | 40 (25-54) | 38 (24-52) |
| 45-54 y | 58 (48-69) | 69 (52-87) | 68 (55-82) | 85 (63-107) | 85 (61-110) |
| 55-64 y | 178 (151-206) | 158 (130-187) | 137 (111-163) | 154 (114-194) | 166 (122-210) |
| 65-74 y | 314 (271-357) | 303 (259-348) | 297 (246-348) | 383 (301-465) | 418 (308-529) |
| 75-84 y | 553 (478-629) | 529 (444-614) | 606 (521-691) | 711 (552-869) | 872 (658-1,086) |
| ≥ 85 y | 834 (647-1,020) | 728 (559-897) | 890 (746-1,035) | 1,182 (896-1,468) | 1,527 (1,158-1,896) |
| Female | 109 (96-122) | 104 (92-117) | 115 (100-129) | 134 (108-161) | 175 (133-216) |
| < 35 y | 8 (6-11) | 9 (6-11) | 14 (9-19) | 11 (7-15) | 19 (9-28) |
| 35-44 y | 38 (19-57) | 34 (25-43) | 41 (29-54) | 42 (24-59) | 42 (25-60) |
| 45-54 y | 62 (47-78) | 73 (55-91) | 72 (55-89) | 92 (64-120) | 108 (71-146) |
| 55-64 y | 203 (160-246) | 160 (120-199) | 141 (113-169) | 142 (110-175) | 173 (118-228) |
| 65-74 y | 334 (275-394) | 309 (259-360) | 329 (259-400) | 405 (302-508) | 458 (329-587) |
| 75-84 y | 545 (464-626) | 589 (491-686) | 620 (526-713) | 710 (530-891) | 986 (726-1,247) |
| ≥ 85 y | 931 (704-1,159) | 787 (581-992) | 967 (789-1,144) | 1,223 (915-1,531) | 1,697 (1,290-2,104) |
| Male | 73 (65-82) | 72 (61-83) | 77 (67-88) | 100 (79-121) | 104 (77-131) |
| < 35 y | 7 (4-9) | 11 (8-14) | 8 (5-12) | 9 (5-12) | 16b |
| 35-44 y | 33 (22-43) | 20 (13-27) | 38 (23-53) | 38 (16-60) | 33 (16-50) |
| 45-54 y | 54 (41-67) | 65 (43-88) | 64 (45-82) | 77 (51-104) | 62 (41-82) |
| 55-64 y | 151 (116-187) | 157 (117-196) | 134 (102-165) | 167 (104-229) | 159 (110-208) |
| 65-74 y | 290 (234-346) | 296 (233-358) | 259 (210-308) | 357 (277-438) | 373 (260-486) |
| 75-84 y | 566 (460-672) | 440 (312-569) | 586 (467-705) | 711 (539-884) | 713 (505-921) |
| ≥ 85 y | 606 (425-787) | 595 (406-785) | 725 (531-920) | 1,095 (707-1,483) | 1,173 (794-1,551) |
Data are presented as rate (95% CI). (Adapted with permission from Centers for Disease Control and Prevention, National Center for Health Statistics.11)
To the 2000 US standard population.
Estimate does not meet standards of reliability or precision. The National Center for Health Statistics considers an estimate to be reliable if it is based on at least 30 discharge records and it has a relative SE of ≤ 30% (ie, the SE is ≤ 30% of the estimate).
TABLE 14 .
] Average Annual Estimates of Hospitalizations per 2 Years for Pulmonary Hypertension by Age Group and Sex: United States, 2001/2002 Through 2009/2010
| Sex and Age Groups | 2001/2002 | 2003/2004 | 2005/2006 | 2007/2008 | 2009/2010 | |||||
| Estimate (95% CI)a | % | Estimate (95% CI)a | % | Estimate (95% CI)a | % | Estimate (95% CI)a | % | Estimate (95% CI)a | % | |
| Total | 261 (234-288) | … | 258 (227-289) | … | 286 (252-320) | … | 354 (286-422) | … | 429 (326-531) | … |
| < 35 y | 10 (8-13) | 4 | 14 (11-17) | 5 | 16 (11-20) | 6 | 14 (10-18) | 4 | 25b | 6 |
| 35-44 y | 16 (11-20) | 6 | 12 (9-15) | 5 | 17 (13-21) | 6 | 17 (11-23) | 5 | 15 (10-21) | 4 |
| 45-54 y | 23 (19-27) | 9 | 29 (21-36) | 11 | 29 (23-35) | 10 | 37 (28-47) | 11 | 38 (27-49) | 9 |
| 55-64 y | 46 (39-53) | 18 | 45 (37-53) | 17 | 43 (34-51) | 15 | 51 (38-64) | 14 | 59 (43-74) | 14 |
| 65-74 y | 58 (50-65) | 22 | 56 (48-64) | 22 | 56 (46-65) | 20 | 76 (59-92) | 21 | 88 (65-112) | 21 |
| 75-84 y | 70 (61-80) | 27 | 68 (57-79) | 26 | 79 (68-90) | 28 | 93 (72-113) | 26 | 115 87-143) | 27 |
| ≥ 85 y | 38 (29-46) | 14 | 35 (27-43) | 13 | 46 (39-54) | 16 | 66 (50-82) | 19 | 88 (67-109) | 20 |
| Female | 159 (140-177) | 61 | 155 (137-173) | 60 | 173 (152-194) | 61 | 206 (165-246) | 58 | 272 (207-337) | 63 |
| < 35 y | 6 (4-7) | 54 | 6 (4-8) | 44 | 10 (6-13) | 62 | 8 (5-10) | 54 | 13 (7-20) | 53 |
| 35-44 y | 9 (4-13) | 54 | 8 (6-9) | 63 | 9 (6-12) | 52 | 9 (5-13) | 53 | 9 (5-12) | 57 |
| 45-54 y | 13 (9-16) | 55 | 15 (11-19) | 54 | 16 (12-19) | 54 | 21 (14-27) | 55 | 24 (16-33) | 64 |
| 55-64 y | 27 (22-33) | 59 | 24 (18-29) | 52 | 23 (18-27) | 53 | 24 (19-30) | 48 | 32 (22-42) | 54 |
| 65-74 y | 33 (27-39) | 58 | 31 (26-36) | 56 | 34 (26-41) | 60 | 43 (32-54) | 57 | 52 (37-67) | 59 |
| 75-84 y | 42 (35-48) | 59 | 46 (38-53) | 67 | 48 (41-55) | 61 | 55 (41-69) | 59 | 76 (56-96) | 66 |
| ≥ 85 y | 29 (22-37) | 78 | 26 (19-33) | 75 | 34 (28-41) | 74 | 46 (35-58) | 70 | 66 (50-82) | 75 |
| Male | 102 (90-114) | 39 | 103 (88-119) | 40 | 113 (97-128) | 39 | 148 (117-179) | 42 | 157 (116-198) | 37 |
| < 35 y | 5 (3-6) | 46 | 8 (5-10) | 56 | 6 (4-9) | 38 | 6 (4-9) | 46 | 12b | 47 |
| 35-44 y | 7 (5-10) | 46 | 4 (3-6) | 37 | 8 (5-12) | 48 | 8 (3-13) | 47 | 7 (3-10) | 43 |
| 45-54 y | 10 (8-13) | 45 | 13 (9-18) | 46 | 13 (10-17) | 46 | 17 (11-23) | 45 | 13 (9-18) | 36 |
| 55-64 y | 19 (14-23) | 41 | 21 (16-27) | 48 | 20 (15-25) | 47 | 27 (17-37) | 52 | 27 (19-36) | 46 |
| 65-74 y | 24 (19-29) | 42 | 25 (20-30) | 44 | 22 (18-27) | 40 | 32 (25-40) | 43 | 36 (25-47) | 41 |
| 75-84 y | 29 (23-34) | 41 | 23 (16-30) | 33 | 31 (25-37) | 39 | 38 (29-47) | 41 | 39 (28-51) | 34 |
| ≥ 85 y | 8 (6-11) | 22 | 9 (6-12) | 25 | 12 (9-15) | 26 | 20 (13-27) | 30 | 22 (15-29) | 25 |
Estimate and 95% CI data are presented in thousands. (Adapted with permission from Centers for Disease Control and Prevention, National Center for Health Statistics.11)
Estimate does not meet standards of reliability or precision. The National Center for Health Statistics considers an estimate to be reliable if it is based on at least 30 discharge records and it has a relative SE of ≤ 30% (ie, the SE is ≤ 30% of the estimate).
Rates of PH hospitalization increased over the time period for those aged < 35 years, 45 to 54 years, 75 to 84 years, and ≥ 85 years (Table 13). There was no significant change in hospitalization rates for those aged 35 to 44 years, 55 to 64 years, or 75 to 84 years. The rate of PH hospitalization for those aged ≥ 85 years had the greatest increase, rising by 83%, from 834 per 100,000 to 1,527 per 100,000 (P < .001). The 75- to 84-year-old age group had the highest proportion of hospitalizations of all age groups throughout the period (27% in 2001-2002 and in 2009-2010) (Table 14). Among men, the only significant increase in the PH hospitalization rate from 2001 to 2002 to 2009 to 2010 was for those aged ≥ 85 years (606 in 2001 to 1,173 in 2010; P < .01) (Table 13). Among women, the rate of hospitalization increased for almost all age groups (< 35 years, 45-54 years, 75-84 years, and ≥ 85 years) (Table 13). The hospitalization rate for women < 35 years old was the lowest among all women and had the greatest percentage increase over time. For both men and women, rates of hospitalization increased as age increased. Hospitalizations of those ≥ 85 years old were predominately among women from 2001 to 2002 through 2009 to 2010; in fact, in 2009 to 2010, 75% of hospitalizations in this age group were of women (Table 14).
The three most commonly reported principal diagnoses at inpatient discharges with any listed PH remained the same throughout the 10-year period (Table 15). The most common principal diagnosis was congestive heart failure, followed by other heart diseases (which included PH) and chronic and unspecified bronchitis. Congestive heart failure was the primary diagnosis for 19% of the PH hospitalizations in 2001 to 2002 and for 18% in 2009 to 2010. The top three diagnoses accounted for 43% of the hospitalizations in 2001 to 2002 and for 37% in 2009 to 2010. Pneumonia and cardiac dysrhythmias rounded out the five leading principal diagnoses, except in 2007 to 2008, when respiratory failure was the fifth most common principal diagnosis. Acute myocardial infarction moved from the sixth most frequent principal diagnosis in 2001 to 2002 to the 10th most frequent in 2009 to 2010. Nephritis, nephrotic syndrome, and nephrosis first appeared as the 10th most common primary diagnosis in 2007 to 2008 and moved up to the seventh most common primary diagnosis in 2009 to 2010.
TABLE 15 .
] Primary Diagnoses for Hospitalizations with Any Listed Pulmonary Hypertension Diagnosis: United States, 2001/2002 Through 2009/2010
| Primary Diagnosis (ICD-9-CM Diagnosis Codes) | 2001/2002 | 2003/2004 | 2005/2006 | 2007/2008 | 2009/2010 |
| Congestive heart failure: 428.0,428.2-428.4 | 19.2 | 19.9 | 19.5 | 17.0 | 17.6 |
| Other heart disease (includes pulmonary hypertension): 391.0-392.0, 393-398, 415-416, 420-426, 428.1, 428.9-429.9 | 12.7 | 16.0 | 13.1 | 12.9 | 12.0 |
| Chronic and unspecified bronchitis: 490-491 | 10.6 | 7.8 | 9.2 | 6.9 | 7.3 |
| Pneumonia: 480-486 | 7.3 | 6.3 | 5.9 | 4.4 | 6.2 |
| Cardiac dysrhythmias: 427 | 4.3 | 4.8 | 5.9 | 5.8 | 5.1 |
| Acute myocardial infarction: 410 | 3.9 | 3.0 | 2.4 | 3.4 | 2.3 |
| Respiratory failure: 518.81, 518.83, 518.84 | 3.5 | 3.8 | 4.2 | 4.7 | 4.3 |
| Coronary atherosclerosis: 414.0, 414.2, 414.3 | 3.4 | 4.1 | 3.4 | 3.3 | 2.5 |
| Other diseases of the respiratory system: 416.2, 471-472, 475-478, 487, 488.0, 488.1, 500-511.1, 511.8-518.6, 518.82, 518.89-519 | 2.8 | 2.9 | 2.1 | 2.5 | 2.1 |
| Hypertensive heart disease: 402, 404 | 2.3 | 1.6 | 1.2 | 0.7 | 1.5 |
| Other COPD and allied conditions: 492, 494-496 | 1.8 | 2.1 | 0.9 | 0.3 | 0.5 |
| Cerebrovascular disease: 430-438 | 1.3 | 1.3 | 2.0 | 1.8 | 2.1 |
| Nephritis, nephrotic syndrome, and nephrosis: 580-589 | 0.6 | 0.8 | 0.8 | 2.3 | 2.7 |
| Asthma: 493 | 1.4 | 1.6 | 1.9 | 1.1 | 2.4 |
| Anemias: 280-285 | 1.0 | 1.5 | 1.8 | 0.9 | 1.6 |
| Complications of medical and surgical care, not elsewhere classified: 996-999 | 1.2 | 1.5 | 1.5 | 1.4 | 1.6 |
| Other diseases of the circulatory system: 390, 392.9, 403, 405, 417, 451-454, 456-459 | 0.7 | 1.4 | 1.5 | 1.5 | 1.9 |
| All other diagnoses | 21.8 | 19.8 | 22.6 | 29.2 | 26.6 |
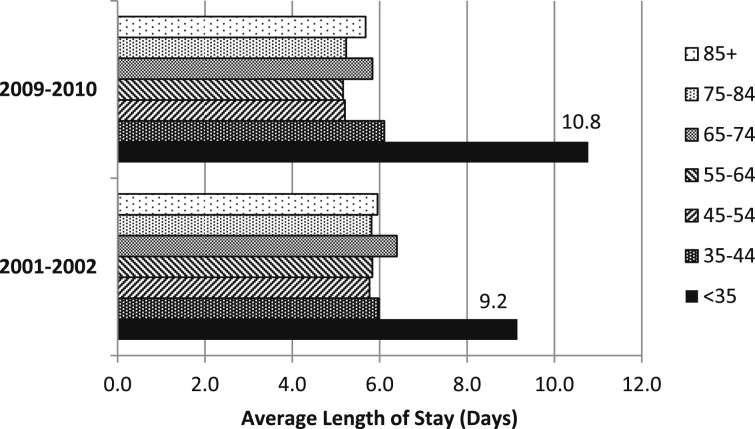
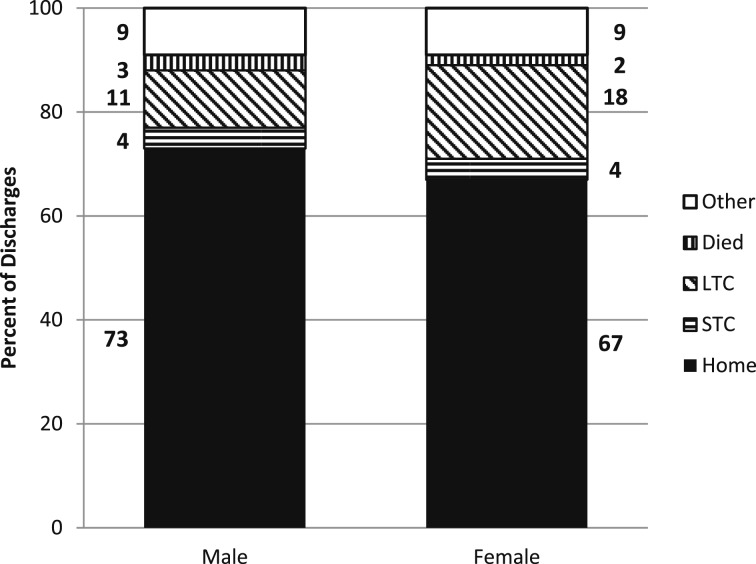
There were no significant trends in the average length of stay or the discharge status for any age group or for men or women from 2001 to 2002 to 2009 to 2010 (Fig 6). However, in 2001 to 2002, and again in 2009 to 2010, the average length of stay for hospitalizations for those aged < 35 years was significantly longer than for any other age group. In 2009 to 2010, significantly more men than women were discharged home (P < .02), and significantly more women than men were discharged to long-term care (P < .001) (Fig 7). As age increased, the percentage discharged to home decreased.
Figure 6 .
– Comparison of average length of hospital stay by age groups for discharges for pulmonary hypertension (PH) as any listed International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis: United States, 2001/2002 and 2009/2010. In both 2001/2002 and 2009/2010, the average length of stay for PH hospitalizations for those aged ≤ 35 y was longer than for all other age groups (P < .05). (Adapted with permission from Centers for Disease Control and Prevention, National Center for Health Statistics.11)
Figure 7 .
– Hospital discharge status by sex for pulmonary hypertension as any listed ICD-9-CM diagnosis: United States, 2009/2010. In 2009/2010, more men than women were discharged home (P < .03), and more women than men were discharged to LTC (P < .01). LTC = long-term care; STC = short-term care. See Figure 6 legend for expansion of other abbreviation. (Adapted with permission from Centers for Disease Control and Prevention, National Center for Health Statistics.11)
Discussion
The findings in our report indicate significant increases in death rates associated with PH for women, men, all racial/ethnic groups, and especially among those aged 75 years and older. Considering all PH deaths from 2001 to 2010, we identified significant decreases from PH associated with chronic lower respiratory disease (including emphysema specifically), conditions arising in the perinatal period, congenital malformations, and pulmonary embolism. At the same time, we identified increases from 2001 to 2010 in death rates for PH associated with aortic stenosis, hypertension, coronary heart disease, autoimmune diseases, diabetes, renal disease, and chronic liver disease. Given that PH mortality due to chronic lower respiratory disease represents a significant proportion of total PH mortality, the decline from 2001 to 2010 in these death rates may be affecting the increasing rates identified with other PH-associated conditions; therefore, the significant rates associated with other conditions such as aortic stenosis, hypertension, diabetes, renal disease, and chronic liver disease should be interpreted with caution, given their individually smaller contributions to total PH mortality.
Death rates among those ≥ 85 years old have increased rapidly since 2001. The Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry) compared demographics from their data on patients with group 1 PAH with those from a National Institutes of Health (NIH) registry from 1982. They found that their current registry patients had a mean age of 45 years compared with 36 years in the NIH registry in 1982, and a higher prevalence of women (78% vs 63%).18
Differences in death rates by sex and race/ethnicity were reported previously,2,19,20 and this report adds important new information about those trends. A previous report2 showed that the trend in the PH mortality rate for men actually declined from 1980 through 2002; information in this report shows that the rates for men continued to decline until 2006 and then began increasing. In general, between 2001 and 2010, mortality rates from COPD declined among men12 and this may explain, in part, the long-term decline in mortality among men with PH that was trending from 1980 to 2006. Reasons for the reversal in trend for men are unclear, but the previous decline may have been associated with reductions in COPD and emphysema, which the current analysis identifies as well. On the other hand, we identified increases in the rates of PH mortality in association with coronary heart disease and hypertension as well as autoimmune disease in men. Few studies have found male sex to be a risk factor for death among patients with PH associated with autoimmune disease.21
Death rates associated with PH for women from 2001 to 2010 were consistently higher than those for men. These sex differences are consistent with data from the REVEAL Registry, in which the patients were more likely to be women.18 Women have higher rates of connective tissue disease than do men.22 The French National Pulmonary Hypertension registry found that women have higher rates of idiopathic and inherited PH compared with men.23 In the REVEAL Registry, which includes only patients with PAH, it was found that roughly one-half of patients with PAH had idiopathic PAH and one-half had associated PAH, with connective tissue disease accounting for roughly 25% of the patients in the registry.24 With current ICD-10 mortality codes, there are challenges in separating PAH as idiopathic vs associated (Fig 1, Table 2). Indeed, most causes of death from PH in the multiple cause of death files are listed as “other secondary PH” or “PH, not otherwise specified.” Few cases are listed as “primary PH.” In a retrospective cohort study of 55 patients with PAH, Kawut et al25 found that patients with systemic sclerosis associated with PH had a higher risk of death despite having similar demographic and hemodynamic characteristics. Research is also ongoing regarding a hormonal mechanism that may explain the sex differences.9,26 Differential sex exposure to anorexigens may also help explain some sex differences,18 although it is unclear to what extent this applies to the US population.
Mortality rates for blacks are increasing at a greater rate than they are for whites. The race/sex differences found in these data are consistent with data from the REVEAL Registry, which noted that the greatest ratios of female to male patients in the registry by race/ethnicity were among blacks and the lowest were among whites.18 In addition to increased connective tissue disease among black women, blacks in general have higher rates of connective tissue disease, hypertension, and hemoglobinopathies in association with PH than do whites.22
Mortality data for all decedents with PH listed on the death certificate are consistent with a widening race/sex gap in recent years, although age-adjusted race/sex mortality gaps are less prominent than are patient ratios in the REVEAL Registry, which is not age adjusted and only includes PAH. Differential race/ethnicity changes over time may also be related to changing population demographics for PH mortality over the past decades. Previous studies have reported that blacks have a shorter survival from the time of diagnosis of PH,27 and have higher rates of PH associated with connective tissue disease. Our findings of increasing rates of PH mortality associated with hypertension, diabetes, and renal failure may also contribute to the racial gap in PH mortality. Poms et al28 found that those with PAH and diabetes have decreased survival.
Although they did not report on race/ethnicity associations of PH with chronic kidney disease, Bolignano et al29 suggested that renal failure itself may be a trigger for PH. Overall mortality from diabetes (listed as the UCOD) declined from 2001 to 2010,30 whereas deaths from diabetes in patients with PH have increased.
Interestingly, the geographic pattern of PH death rates by state does not mirror the patterns seen in maps showing all heart disease deaths, where the highest rates are typically located in the deep south and the lowest rates in the northwest.31 This highlights the differences in risk factors and populations at risk of PH.
We also found increasing hospitalization rates for both men and women, and there has been some shift in the most common primary reason for admission. Although heart failure is the most common underlying reason for admission associated with PH, the proportion of hospitalizations for heart failure associated with PH has declined, as have most major reasons for hospitalization associated with PH. Yet hospitalizations for asthma, renal conditions, and the category of less common conditions associated with PH have increased. This may reflect better documentation, but it may also suggest important changes in the underlying conditions associated with PH.
The fact that we found that women have twice as many hospitalizations associated with PH than do men likely reflects the difference in life expectancy but also the disproportionate burden of PH disease experienced by women. Compared with the previous report covering 1980 to 2002, women continue to have higher rates of hospitalizations than do men, and the greatest increase in rates over time is in the oldest age group. For women, increases in the PH hospitalization rate were noted across most age groups, whereas for men, the rate increased only among those ≥ 85 years old. Most hospitalizations for those ≥ 85 years old were among women. Discharge status is likely influenced by these demographic differences, with more women discharged to long-term care and more men discharged to their home.
Although some of the increase in mortality from PH-associated conditions may be explained by a heightened awareness of PH and referral to specialists able to make the diagnosis, the significant declines in mortality from congenital malformations, and conditions originating in the perinatal period, may be explained in part by both increased awareness and improved treatment. Although there has been a significant increase in the overall mortality rate for PH since 1980, it is interesting to note that the REVEAL Registry found similar severity at diagnosis (as evidenced by New York Heart Association and World Health Organization functional classes) between the NIH registry and the REVEAL Registry for PAH, and yet one in five patients diagnosed with PAH presented with a history of having symptoms for > 2 years prior to diagnosis.3 In the United Kingdom and Ireland cohort study from 2001 to 2009, patients were still presenting with severe symptoms late in the course of disease.32 Given the accelerating rate of mortality among those aged ≥ 85 years, the overall increase in mortality from PH may reflect different characteristics of disease manifestation among the older patients with additional comorbid conditions. Canadian researchers found that patients ≥ 65 years old were more likely to have PH associated with scleroderma than were younger patients, although they also noted that younger patients with PH associated with scleroderma were more likely to die during a median follow-up of 3 years.33
This report is subject to several limitations related to both mortality and hospitalization data. First, death certificates may not accurately capture the UCOD or contributing causes of death because of inaccuracy of diagnosis or limited knowledge of the deceased’s medical history by the person completing the death certificate. This limitation may result in underreporting of PH on death certificates. Second, race and ethnicity may not be accurately captured on death certificates or hospitalization data, especially for Hispanics and races other than black or white. This may affect the observed mortality and hospitalization rates for Hispanics and other nonwhite/nonblack individuals, especially AI/AN decedents.14 Third, comparisons of mortality data with epidemiologic data from disease registries are limited by the fact that some disease registries are prevalence registries, whereas others are incident registries, or they separate incident and prevalent cases, and by the fact that disease registries are predominately for PAH rather than for all PH causes. Fourth, although trends in UCOD over time can be identified, secular changes in the incidence or prevalence of the diseases reported as the UCOD over time are not reflected in the mortality trends shown here. Therefore, these trends must be interpreted with caution. Fifth, the NHDS does not include patients admitted to federal, military or Veterans Affairs hospitals; therefore, results underestimate the total number of hospitalizations for PH among adults. Sixth, unfortunately, the terminology used in the ICD and ICD-CM does not crosswalk easily with the Dana Point classification of PH, and although using the identical fourth digits in both the ICD-9-CM and the ICD-10, the descriptions do not crosswalk well with each other, nor do they crosswalk well with the Dana Point PH classification of PH. This contributes to challenges in correct classification of causes of death and hospital diagnoses. Lastly, the NHDS increased the number of diagnoses collected in 2010 from seven to 15. The number of diagnoses collected has a significant impact on estimates, particularly for conditions that are typically secondary diagnoses such as PH. Larger percentage differences can be seen in hospitalizations for older people, who are more likely to carry multiple diagnoses. The estimated number of PH hospitalizations in 2010 using seven diagnoses was 471,864, but the estimate increased to 728, 983 when using 15 diagnoses (a 54% increase in the estimated number of hospitalizations [Table 16]). For those aged 85 years and older, there is a 66% increase in the estimated number of hospitalizations if 15 diagnoses are used, compared with seven diagnoses. This analysis helps explain differences in previously reported hospitalizations associated with PH from the Healthcare Cost and Utilization Project on hospital stays related to PH.34
TABLE 16 .
] Difference in Hospital Discharges for Pulmonary Hypertension as Any Listed ICD-9-CM Diagnosis Using Seven vs 15 Diagnoses by Age Group: United States, 2010
| Age Groups | Pulmonary Hypertension Discharges 2010 (Seven Diagnoses) | Pulmonary Hypertension Discharges 2010 (15 Diagnoses) | Percent Difference |
| Total | 472 | 729 | 54 |
| < 35 y | 27a | 30a | 10 |
| 35-44 y | 19 | 25 | 33 |
| 45-54 y | 41 | 58 | 43 |
| 55-64 y | 61 | 93 | 52 |
| 65-74 y | 99 | 155 | 57 |
| 75-84 y | 130 | 210 | 62 |
| ≥ 85 y | 96 | 158 | 66 |
Numbers are presented in thousands. See Table 2 legend for expansion of abbreviations. (Adapted with permission from Centers for Disease Control and Prevention, National Center for Health Statistics.11)
Estimate does not meet standards of reliability or precision. The National Center for Health Statistics considers an estimate to be reliable if it is based on at least 30 discharge records and it has a relative SE of ≤ 30% (ie, the SE is ≤ 30% of the estimate).
Conclusions
Our report documents increases in mortality associated with PH among men, women, and all race and ethnic groups, and from several conditions commonly associated with PH (hypertension and coronary heart disease, aortic stenosis, liver disease and cirrhosis, and autoimmune disease) but also from renal disease and diabetes, while finding that PH-associated mortality decreased over time from deaths due to congenital malformations among newborns, and for emphysema, chronic lower respiratory disease, and pulmonary embolism. Approximately one-half of deaths associated with PH occur among those under 75 years of age. Although PH death rates have been stable for those aged 1 to 74 years over the past decade, the identified increases in PH death among those with a UCOD from hypertension, coronary heart disease, and valvular disease may be avoidable with improved public health efforts in the primary prevention of heart disease. The increased mortality from autoimmune conditions in association with PH requires further study. Increases in hospitalizations may reflect both improved recognition of PH as well as an increase in treatment options. The decline in PH mortality due to congenital malformations, chronic lower respiratory disease, and emphysema over time is encouraging. The ability to code mortality and hospitalizations using a modified diagnostic classification based on the five major classes in the Dana Point classification could improve the surveillance of PH, which would also aid in understanding age, race, and sex differences in PH mortality.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
ABBREVIATIONS
- AAPC
average annual percent change
- AI/AN
American Indian/Alaska native
- APC
annual percent change
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICD-10
International Classification of Diseases, 10th Revision
- NH
non-Hispanic
- NHDS
National Hospital Discharge Survey
- NIH
National Institutes of Health
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- REVEAL Registry
Registry to Evaluate Early and Long-term PAH Disease Management
- UCOD
underlying cause of death
Footnotes
References
- 1.Aronson JK. Rare diseases and orphan drugs. Br J Clin Pharmacol. 2006;61(3):243-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance—United States, 1980-2002. MMWR Surveill Summ. 2005;54(5):1-28 [PubMed] [Google Scholar]
- 3.Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140(1):19-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;173(10):887-893 [DOI] [PubMed] [Google Scholar]
- 5.Badesch DB, Champion HC, Sanchez MAG, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S55-S66 [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S43-S54 [DOI] [PubMed] [Google Scholar]
- 7.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(8):707-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert M, Simonneau G, Rubin LJ. A decade of achievement in pulmonary hypertension. Eur Respir Rev. 2011;20(122):215-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock A. Pulmonary hypertension. Eur Respir Rev. 2013;22(127):20-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923-931 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nhds.htm. Accessed March 31, 2014
- 12.Centers for Disease Control and Prevention, National Center for Health Statistics. About underlying cause of death 1999-2010. CDC WONDER website. http://wonder.cdc.gov/ucd-icd10.html. Accessed December 22, 2013
- 13.Link J, Glazer C, Torres F, Chin K. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest. 2011;139(3):497-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008; (148):1-13 [PubMed] [Google Scholar]
- 15.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. National Health Statistics Reports; No 29. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nhsr/nhsr029.pdf. Published 2010. Accessed December 15, 2013 [PubMed]
- 16.Klein RJ, Schoenborn CA. Age Adjustment Using the 2000 Projected US Population. Healthy People 2010 Statistics Notes, No. 20. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2001 [PubMed] [Google Scholar]
- 17.Gillum BS, Graves EJ, Kozak LJ. Trends in hospitalization: United States, 1988-92. National Center for Health Statistics website. http://www.cdc.gov/nchs/data/series/sr_13/sr13_124.pdf. Published 1996. Accessed December15, 2013
- 18.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139(1):128-137 [DOI] [PubMed] [Google Scholar]
- 19.Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979-1996. Chest. 2000;117(3):796-800 [DOI] [PubMed] [Google Scholar]
- 20.Davis KK, Lilienfeld DE, Doyle RL. Increased mortality in African Americans with idiopathic pulmonary arterial hypertension. J Natl Med Assoc. 2008;100(1):69-72 [DOI] [PubMed] [Google Scholar]
- 21.Yaqub A, Chung L. Epidemiology and risk factors for pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep. 2013;15(1):302. [DOI] [PubMed] [Google Scholar]
- 22.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886-895 [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan DS, Humbert M. A critical analysis of survival in pulmonary arterial hypertension. Eur Respir Rev. 2012;21(125):218-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376-387 [DOI] [PubMed] [Google Scholar]
- 25.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123(2):344-350 [DOI] [PubMed] [Google Scholar]
- 26.Austin ED. Gender, sex hormones, and pulmonary arterial hypertension. Advances in Pulmonary Hypertension. 2011;10(3):160-166 [Google Scholar]
- 27.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199-203 [DOI] [PubMed] [Google Scholar]
- 28.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144(1):169-176 [DOI] [PubMed] [Google Scholar]
- 29.Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013;61(4):612-622 [DOI] [PubMed] [Google Scholar]
- 30.Murphy SL, Xu JQ, Kochanek KD. Deaths: final data for 2010. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/copd/data.htm. Published 2013. Accessed December 9, 2013 [PubMed]
- 31.Centers for Disease Control and Prevention, Division for Heart Disease and Stroke Prevention. Interactive Atlas of Heart Disease and Stroke. Centers for Disease Control and Prevention website. http://www.cdc.gov/dhdsp/maps. Accessed December 22, 2013
- 32.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790-796 [DOI] [PubMed] [Google Scholar]
- 33.Shimony A, Fox BD, Afilalo J, Rudski LG, Hirsch A, Langleben D. Pulmonary arterial hypertension in the elderly-clinical characteristics and long-term survival. Lung. 2012;190(6):645-649 [DOI] [PubMed] [Google Scholar]
- 34.Merrill CT, Nagamine M, Elixhauser A. Hospital stays involving chronic pulmonary heart disease, 2005. HCUP Statistical Brief No. 43. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb43.pdf. Published December 2007. Accessed December 15, 2013 [PubMed]